Cracking the AP Biology Exam
2
The Chemistry of Life
LIPIDS
Like carbohydrates, lipids consist of carbon, hydrogen, and oxygen atoms, but not in the 1:2:1 ratio typical of carbohydrates. The most common examples of lipids are fats, oils, phospholipids, and steroids. Let’s talk about the simple lipids—neutral fats. A typical fat consists of three fatty acids and one molecule of glycerol. If you see the word triglyceride on the test, it’s just a fancy word for “fat.” Let’s take a look:
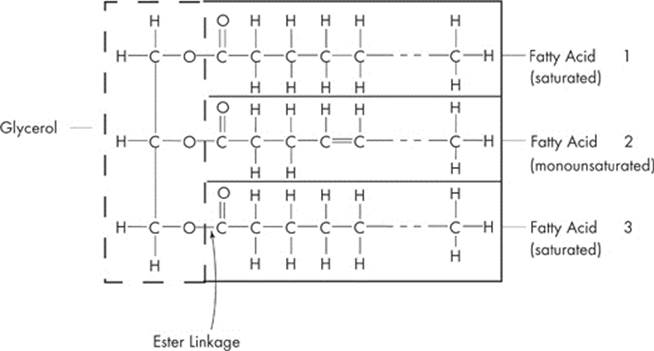
To make a triglyceride, each of the carboxyl groups (–COOH) of the three fatty acids must react with one of the three hydroxyl groups (–OH) of the glycerol molecule. This happens by the removal of a water molecule. So, the creation of a fat requires the removal of three molecules of water. Once again, what have we got? You probably already guessed it—dehydration synthesis! The linkage now formed between the glycerol molecule and the fatty acids are called ester linkage. A fatty acid can be saturated, which means it has a single covalent bond between each pair of carbon atoms or it can be unsaturated, which means adjacent carbons are joined by double bonds instead of single bonds. A polyunsaturated fatty acid has many double bonds within the fatty acid.
Lipids are important because they function as structural components of cell membranes, sources of insulation, and a means of energy storage.
Phospholipids
Another special class of lipids is known as phospholipids. Phospholipids contain two fatty acid “tails” and one negatively charged phosphate “head.” Take a look at a typical phospholipid:
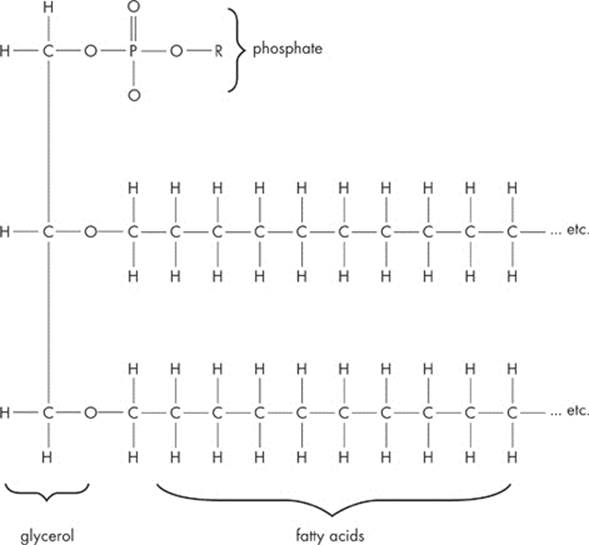
Phospholipids are extremely important, mainly because of some unique properties they possess, particularly with regard to water.
Interestingly enough, the two fatty acid tails are hydrophobic (“water–hating”). In other words, just like oil and vinegar, fatty acids and water don’t mix. The reason for this is that fatty acid tails are nonpolar, and nonpolar substances don’t mix well with polar ones, such as water.
On the other hand, the phosphate “head” of the lipid is hydrophilic (“water-loving”), meaning that it does mix well with water. Why? It carries a negative charge, and this charge draws it to the positively charged end of a water molecule. A molecule is amphipathic if it has both a hydrophilic region and a hydrophobic region. A phospholipid is amphipathic.
This arrangement of the fatty acid tails and the phosphate group head provides phospholipids with a unique shape. The two fatty acid chains orient themselves away from water, while the phosphate portion orients itself toward the water. Keep these properties in mind. We’ll see later how this orientation of phospholipids in water relates to the structure and function of cell membranes.
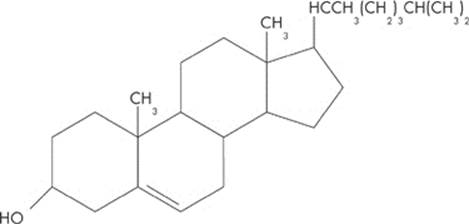
One class of lipids is known as steroids. All steroids have a basic structure of four linked carbon rings. This category includes cholesterol, vitamin D, and a variety of hormones. Take a look at a typical steroid: