Cracking the AP Biology Exam
2
The Chemistry of Life
WATER: THE VERSATILE MOLECULE
One of the most important substances in nature is water. Did you know that more than 60 percent of your body weight consists of water? Water is considered a unique molecule because it plays an important role in chemical reactions.
Let’s take a look at one of the properties of water. Water has two hydrogen atoms joined to an oxygen atom:
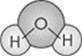
In water molecules, the hydrogen atoms have a partial positive charge and the oxygen atom has a partial negative charge. Molecules that have partially positive and partially negative charges are said to be polar. Water is therefore a polar molecule. The positively-charged ends of the water molecules strongly attract the negatively-charged ends of other polar compounds. Likewise, the negatively-charged ends strongly attract the positively-charged ends of neighboring compounds. These forces are most readily apparent in the tendency of water molecules to stick together, as in the formation of water beads or raindrops.
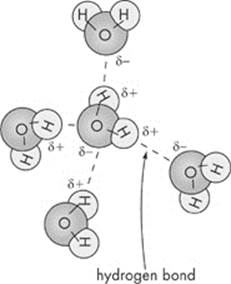
Another type of bond important in organisms is a hydrogen bond. Hydrogen bonds are weak chemical bonds that form when a hydrogen atom that is covalently bonded to one electronegative atom is also attracted to another electronegative atom. Water molecules are held together by hydrogen bonds. Although hydrogen bonds are individually weak, collectively, they are strong when present in large numbers. Because it can react with other polar substances, water makes a great solvent; it can dissolve many kinds of substances. The hydrogen bonds that hold water molecules together contribute to a number of special properties:
- As mentioned above, water molecules have a strong tendency to stick together. That is, water exhibits cohesive forces. These forces are extremely important to life. For instance, when water molecules evaporate from a leaf, they “pull” neighboring water molecules. These, in turn, draw up the molecules immediately behind them, and so on, all the way down the plant vessels. The resulting chain of water molecules enables water to move up the stem.
- Water molecules also like to stick to other substances—that is, they’re adhesive. Have you ever tried to separate two glass slides stuck together by a film of water? They’re difficult to separate because of the water sticking to the glass surfaces. These two forces taken together—cohesion and adhesion—account for the ability of water to rise up the roots, trunks, and branches of trees. Water has a high surface tension because of the cohesiveness of its molecules. Since this phenomenon occurs in thin vessels, it’s called capillary action.
- Another remarkable property of water is its high heat capacity. What’s heat capacity? Your textbook will give you a definition something like this: “Heat capacity is the quantity of heat required to change the temperature of a substance by 1 degree.” What does that mean? In plain English, heat capacity refers to the ability of a substance to store heat. For example, when you heat up an iron kettle, it gets hot pretty quickly. Why? Because it has a low specific heat. It doesn’t take much heat to increase the temperature of the kettle. Water, on the other hand, has a high heat capacity. You have to add a lot of heat to get an increase in temperature. Water’s ability to resist temperature changes is one of the things that helps keep the temperature in our oceans fairly stable. It’s also why organisms that are mainly made up of water, like us, are able to keep a constant body temperature.
So let’s review the unique properties of water:
- Water is polar and can dissolve other polar substances.
- Water has cohesive and adhesive properties.
- Water has a high heat capacity.
- Water has a high surface tension.