Cracking the AP Biology Exam
2
The Chemistry of Life
CARBOHYDRATES
Organic compounds that contain carbon, hydrogen, and oxygen are called carbohydrates. They usually contain these three elements in a ratio of 1 : 2 : 1, respectively. We can represent the proportion of elements within carbohydrate molecules by the formula CnH2nOn.
Most carbohydrates are categorized as either monosaccharides, disaccharides, or polysaccharides. The term saccharides is a fancy word for “sugar.” The prefixes mono-, di-, and poly- refer to the number of sugars in the molecule. Mono- means “one,” di- means “two,” and poly- means “many.” A monosaccharide is therefore a carbohydrate made up of a single type of sugar molecule.
Monosaccharides: The Simplest Sugars
Monosaccharides, the simplest sugars, serve as an energy source for cells. The two most common sugars are (1) glucose and (2) fructose.
Both of these monosaccharides are six-carbon sugars with the chemical formula C6H12O6. Glucose, the most abundant monosaccharide, is the most popular sugar around. Plants produce it by capturing sunlight for energy, while cells break it down to release stored energy. Glucose can come in two forms: α-glucose and β-glucose, which differ simply by a reversal of the H and OH on the first carbon. Fructose, the other monosaccharide you need to know for the test, is a common sugar in fruits.
One last thing. Glucose and fructose can be depicted as either “straight” or “rings.” Both of them are pretty easy to spot; just look for the six carbon molecules. Here are the two different forms:
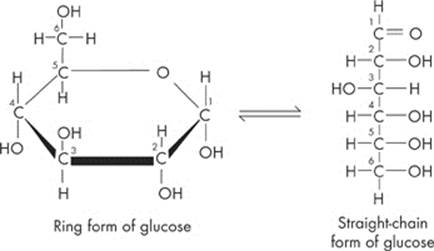
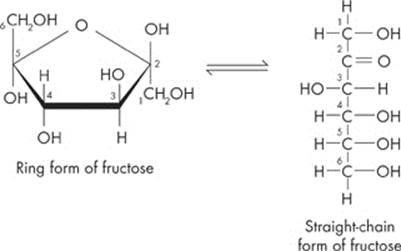
Disaccharides
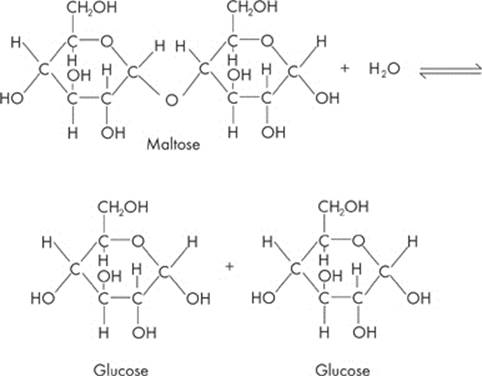
What happens when two monosaccharides are brought together? The hydrogen (–H) from one sugar molecule combines with the hydroxyl group (–OH) of another sugar molecule. What do H and OH add up to? Water (H2O)! So a water molecule is removed from the two sugars. The two molecules of monosaccharides are chemically linked and form a disaccharide.
Maltose is an example of a disaccharide:

Maltose is formed by linking two glucose molecules—forming a glycosidic bond. This process is called dehydration synthesis, or condensation. During this process, a water molecule is lost.
Now what if you want to break up the disaccharide and form two monosaccharides again? Just add water. That’s called hydrolysis.
Polysaccharides
Polysaccharides are made up of many repeated units of monosaccharides. Therefore, a polysaccharide is a kind of polymer, a molecule with repeating subunits of the same general type. The most common polysaccharides you’ll need to know for the test are starch, cellulose, andglycogen. Polysaccharides are often storage forms of sugar or structural components of cells. For instance, animals store glucose molecules in the form of glycogen in the liver and muscle cells. Plants “stockpile” α–glucose in the form of starch in structures called plastids. Cellulose, on the other hand, made up of β–glucose, is a major part of the cell wall in plants. Its function is to lend structural support. Chitin, a polymer of β–glucose molecules, serves as a structural molecule in the walls of fungus and in the exoskeletons of arthropods.
Here’s an AP question that may come up on the test: Why can’t humans digest cellulose? The glycosidic bond in polymers that have α–glucose can easily be broken down by humans but glycoside bond in polymers containing β–glucose polymers, such as cellulose, can not. This is because the bonds joining the glucose subunits in cellulose are different than those in starch. Starch is composed of α–glucose subunits held together by 1–4 glycoside linkages while cellulose contain β–glucose subunits held together by 1–4 linkages.