5 Steps to a 5: AP Biology 2017 (2016)
STEP 4
Review the Knowledge You Need to Score High
CHAPTER5 Chemistry
CHAPTER 6 Cells
CHAPTER 7 Respiration
CHAPTER 8 Photosynthesis
CHAPTER 9 Cell Division
CHAPTER 10 Heredity
CHAPTER 11 Molecular Genetics
CHAPTER 12 Evolution
CHAPTER 13 Taxonomy and Classification
CHAPTER 14 Plants
CHAPTER 15 Human Physiology
CHAPTER 16 Human Reproduction
CHAPTER 17 Behavioral Ecology and Ethology
CHAPTER 18 Ecology in Further Detail
CHAPTER 19 Laboratory Review
CHAPTER 5
Chemistry
IN THIS CHAPTER
Summary: This chapter introduces the chemical principles that are related to the AP Biology topics covered throughout the course.

Key Ideas
![]() Organic compounds contain carbon; important examples include lipids, proteins, and carbohydrates.
Organic compounds contain carbon; important examples include lipids, proteins, and carbohydrates.
![]() Enzymes are catalytic proteins that react in an induced-fit fashion with substrates to speed up a reaction.
Enzymes are catalytic proteins that react in an induced-fit fashion with substrates to speed up a reaction.
![]() The five types of chemical reactions you should learn include hydrolysis reactions, dehydration synthesis reactions, endergonic reactions, exergonic reactions, and redox reactions.
The five types of chemical reactions you should learn include hydrolysis reactions, dehydration synthesis reactions, endergonic reactions, exergonic reactions, and redox reactions.
Introduction
What is the name of the test you are studying for? The AP Biology exam. Then why in tarnation are we starting your review with a chapter titled Chemistry ?!?!? Because it is important that you have an understanding of a few chemical principles before we dive into the deeper biological material. We will keep it short, don’t worry. ![]()
Elements, Compounds, Atoms, and Ions
By definition, matter is anything that has mass and takes up space; an element is defined as matter in its simplest form; an atom is the smallest form of an element that still displays its particular properties. (Terms boldfaced in text are listed in the Glossary at the end of the book.) For example, sodium (Na) is an element mentioned often in this book, especially in Chapter 15 , Human Physiology. The element sodium can exist as an atom of sodium, in which it is a neutral particle containing an equal number of protons and electrons. It can also exist as an ion, which is an atom that has a positive or negative charge. Ions such as sodium that take on a positive charge are called cations, and are composed of more protons than electrons. Ions with a negative charge are called anions, and are composed of more electrons than protons.
Elements can be combined to form molecules, for example, an oxygen molecule (O2 ) or a hydrogen molecule (H2 ). Molecules that are composed of more than one type of element are called compounds, for example H2 O. The two major types of compounds you need to be familiar with are organic and inorganic compounds. Organic compounds contain carbon and usually hydrogen; inorganic compounds do not. Some of you are probably skeptical, at this point, as to whether any of what we have said thus far matters for this exam. Bear with me because it does. You will deal with many important organic compounds later on in this book, including carbohydrates, proteins, lipids, and nucleic acids .
John (11th grade): “My teacher wanted me to know these structures . . . she was right!”
Before moving onto the next section, where we discuss these particular organic compounds in more detail, we would like to cover a topic that many find confusing and therefore ignore in preparing for this exam. This is the subject of functional groups. These poorly understood groups are responsible for the chemical properties of organic compounds. They should not intimidate you, nor should you spend a million hours trying to memorize them in full detail. You should remember one or two examples of each group and be able to identify the functional groups on sight, as you are often asked to do so on the AP exam.
The following is a list of the functional groups you should study for this exam:
BIG IDEA 4.A.1
The subcomponents of a molecule determine its properties .
1. Amino group . An amino group has the following formula:
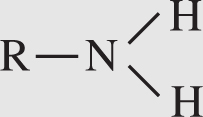
The symbol R stands for “rest of the compound” to which this NH2 group is attached. One example of a compound containing an amino group is an amino acid. Compounds containing amino groups are generally referred to as amines. Amino groups act as bases and can pick up protons from acids.
2. Carbonyl group . This group contains two structures:
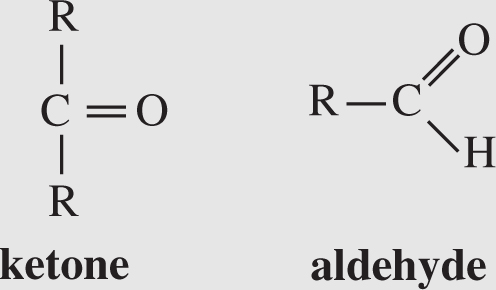
If the C=O is at the end of a chain, it is an aldehyde. Otherwise, it is a ketone. (Note: in aldehydes , there is an H at the end; there is no H in the word ketone .) A carbonyl group makes a compound hydrophilic and polar.Hydrophilic means water-loving, reacting well with water. A polar molecule is one that has an unequal distribution of charge, which creates a positive side and a negative side to the molecule.
3. Carboxyl group . This group has the following formula:
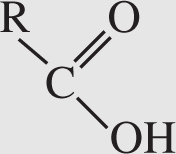
A carboxyl group is a carbonyl group that has a hydroxide in one of the R spots and a carbon chain in the other. This functional group shows up along with amino groups in amino acids. Carboxyl groups act as acids because they are able to donate protons to basic compounds. Compounds containing carboxyl groups are known as carboxylic acids .
4. Hydroxyl group . This group has the simplest formula of the bunch:
![]()
A hydroxyl group is present in compounds known as alcohols. Like carbonyl groups, hydroxyl groups are polar and hydrophilic.
5. Phosphate group . This group has the following formula:
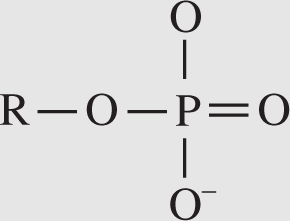
Phosphate groups are vital components of compounds that serve as cellular energy sources: ATP, ADP, and GTP. Like carboxyl groups, phosphate groups are acidic molecules.
6. Sulfhydryl group . This group also has a simple formula:
![]()
This functional group does not show up much on the exam, but you should recognize it when it does. This group is present in the amino acids methionine and cysteine and assists in structure stabilization in many proteins.
Lipids, Carbohydrates, and Proteins
BIG IDEA 4.C.1
These various molecules provide the cell with a wide range of functions .
Lipids
Lipids are organic compounds used by cells as long-term energy stores or building blocks. Lipids are hydrophobic and insoluble in water because they contain a hydrocarbon tail of CH2 S that is nonpolar and repellant to water. The most important lipids are fats, oils, steroids, and phospholipids.
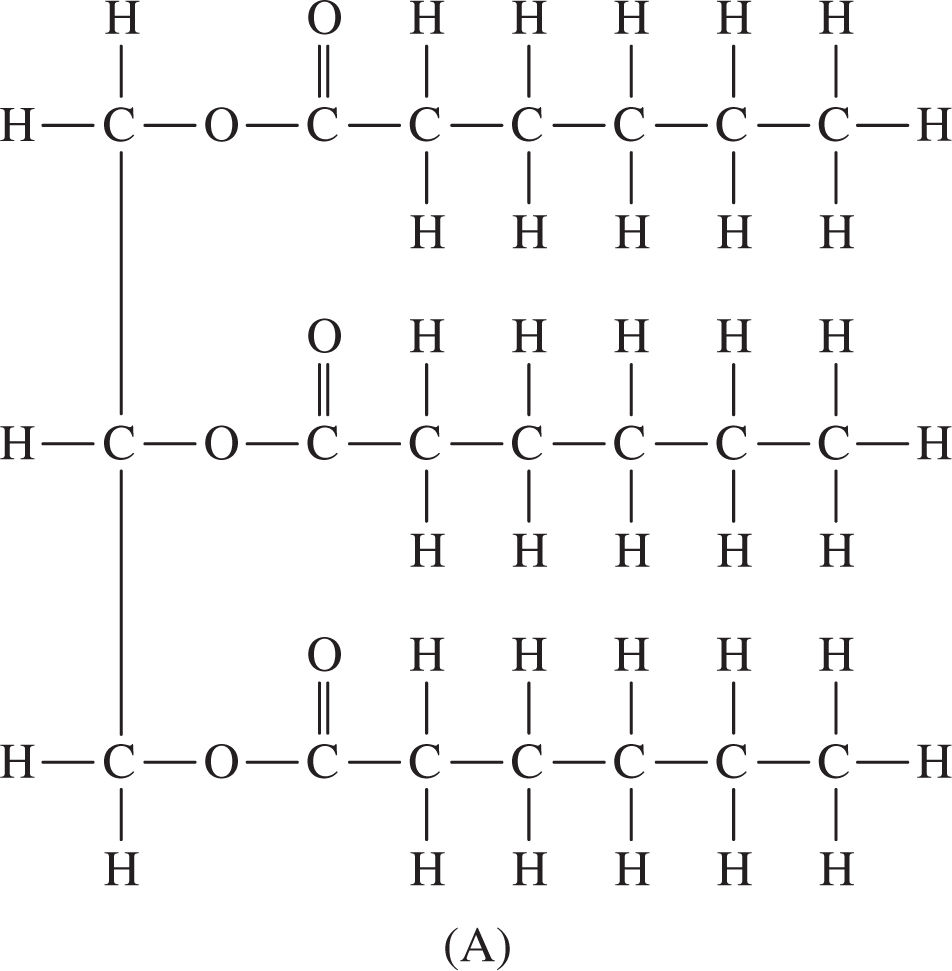
Fats, which are lipids made by combining glycerol and three fatty acids (Figure 5.1 ), are used as long-term energy stores in cells. They are not as easily metabolized as carbohydrates, yet they are a more effective means of storage; for instance, one gram of fat provides two times the energy of one gram of carbohydrate. Fats can be saturated or unsaturated . Saturated fat molecules contain no double bonds. Unsaturated fats contain one (mono-) or more (poly-) double bonds, which means that they contain fewer hydrogen molecules per carbon than do saturated fats. Saturated fats are the bad guys and are associated with heart disease and atherosclerosis. Most of the fat found in animals is saturated, whereas plants tend to contain unsaturated fats. Fat is formed when three fatty-acid molecules connect to the OH groups of the glycerol molecule. These connecting bonds are formed by dehydration synthesis reaction (Figure 5.2 ).
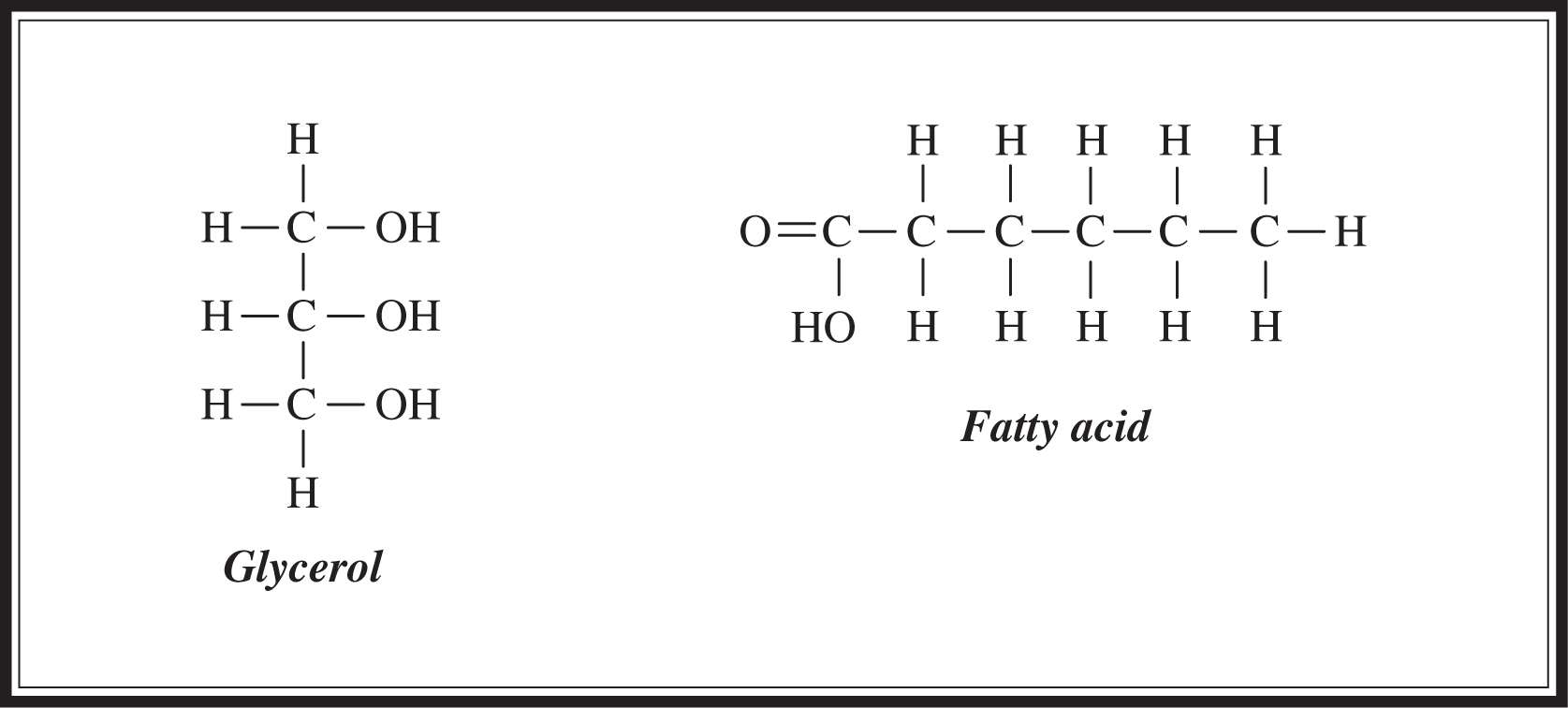
Figure 5.1 Structure of glycerol and fatty acids.
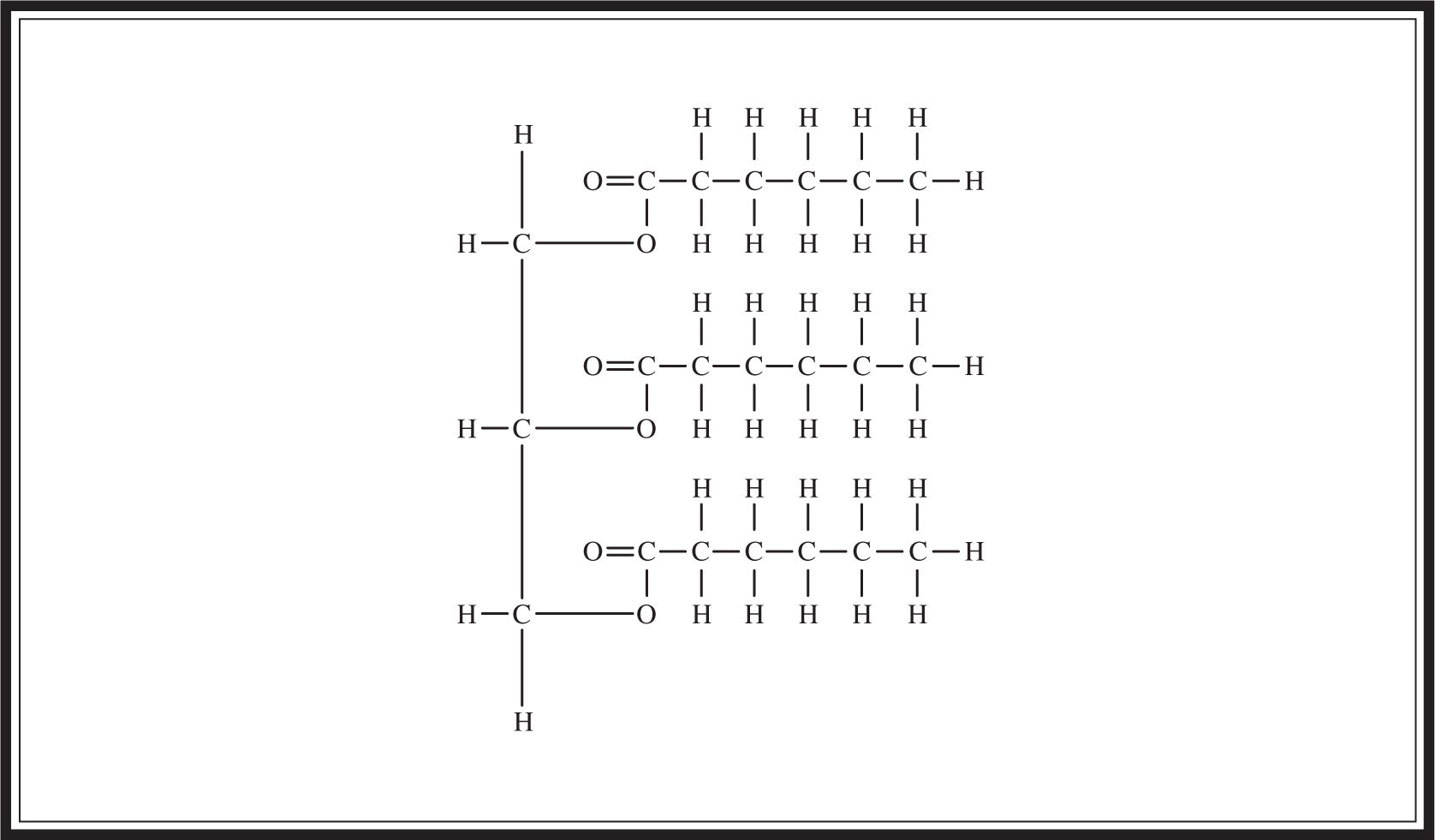
Figure 5.2 Fat structure (glycerol plus three fatty acids).
Steroids are lipids composed of four carbon rings that look like chicken-wire fencing in pictorial representations. One example of a steroid is cholesterol, an important structural component of cell membranes that serves as a precursor molecule for another important class of steroids: the sex hormones (testosterone, progesterone, and estrogen). You should be able to recognize the structures shown in Figure 5.3 for the AP exam.
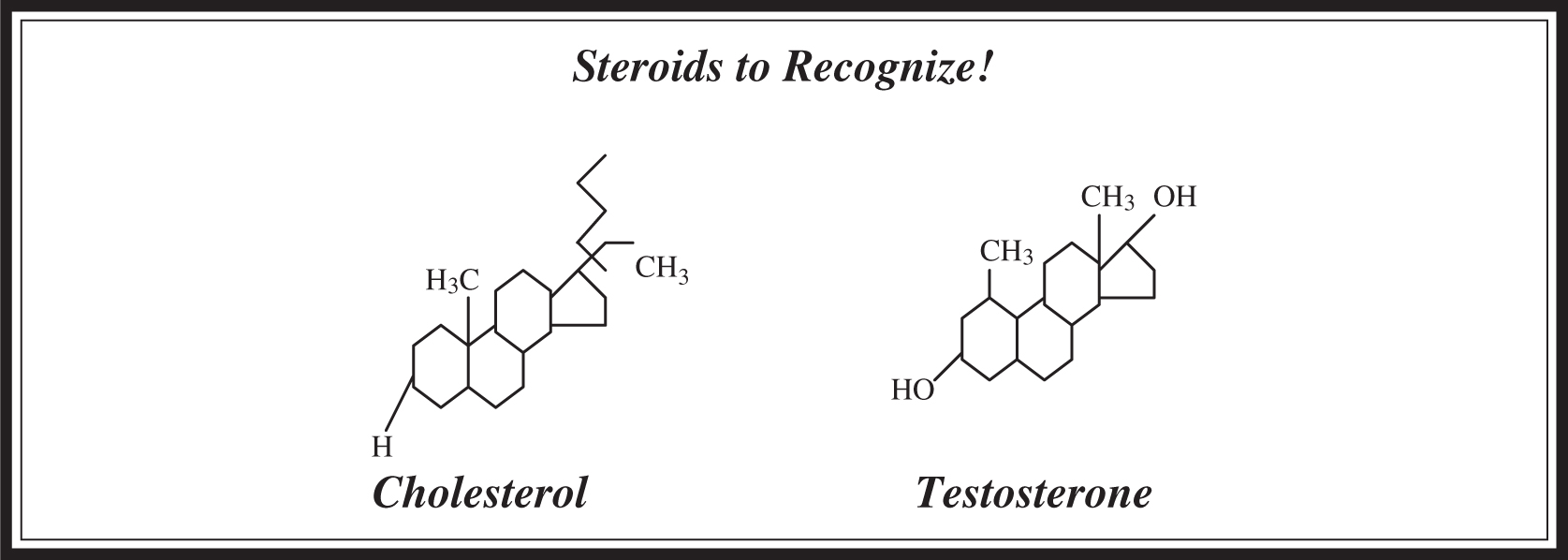
Figure 5.3 Steroid structures.
A phospholipid is a lipid formed by combining a glycerol molecule with two fatty acids and a phosphate group (Figure 5.4 ). Phospholipids are bilayered structures; they have both a hydrophobic tail (a hydrocarbon chain) and a hydrophilic head (the phosphate group) (Figure 5.5 ). They are the major component of cell membranes; the hydrophilic phosphate group forms the outside portion and the hydrophobic tail forms the interior of the wall.
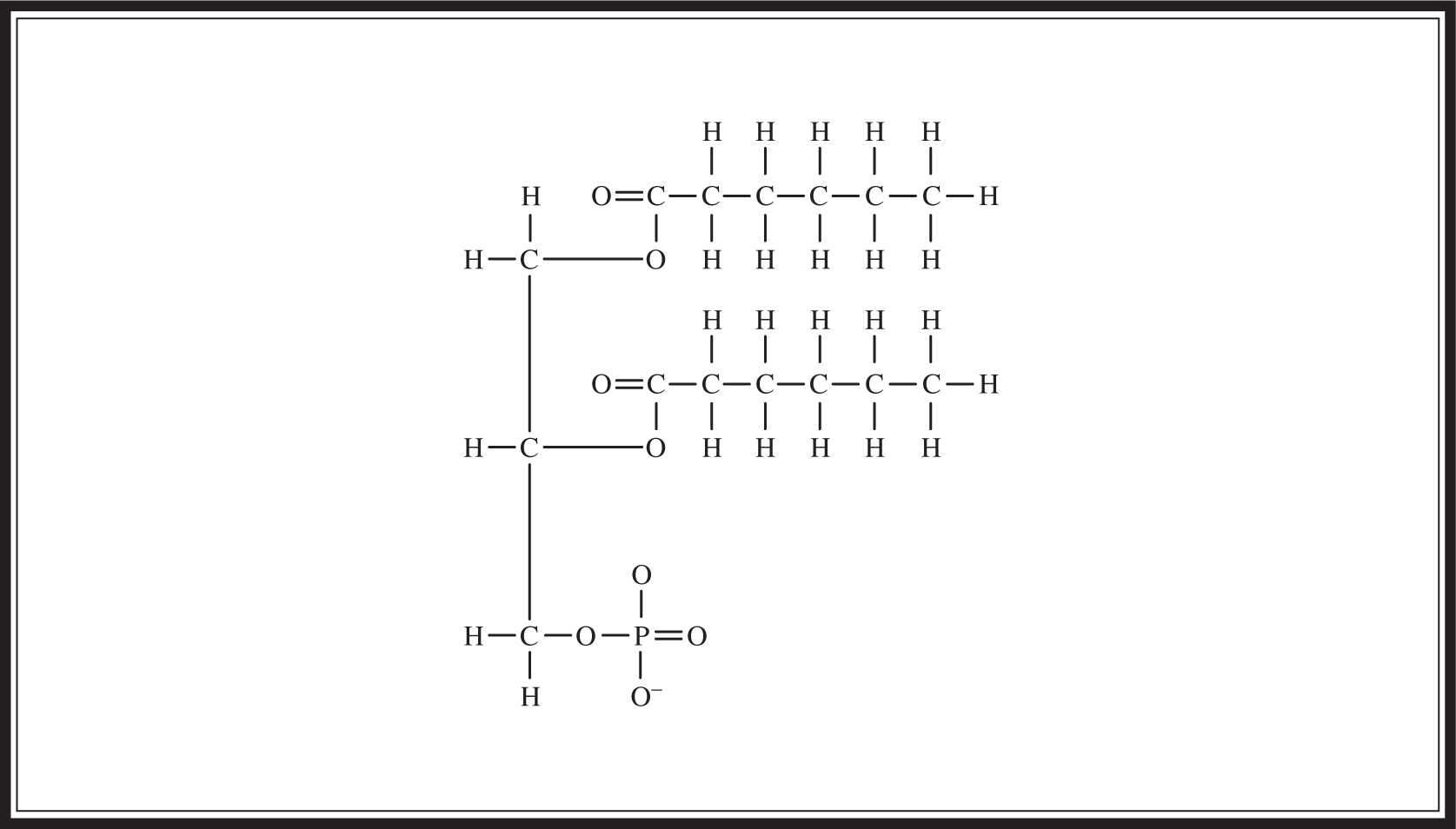
Figure 5.4 Structure of phospholipid.
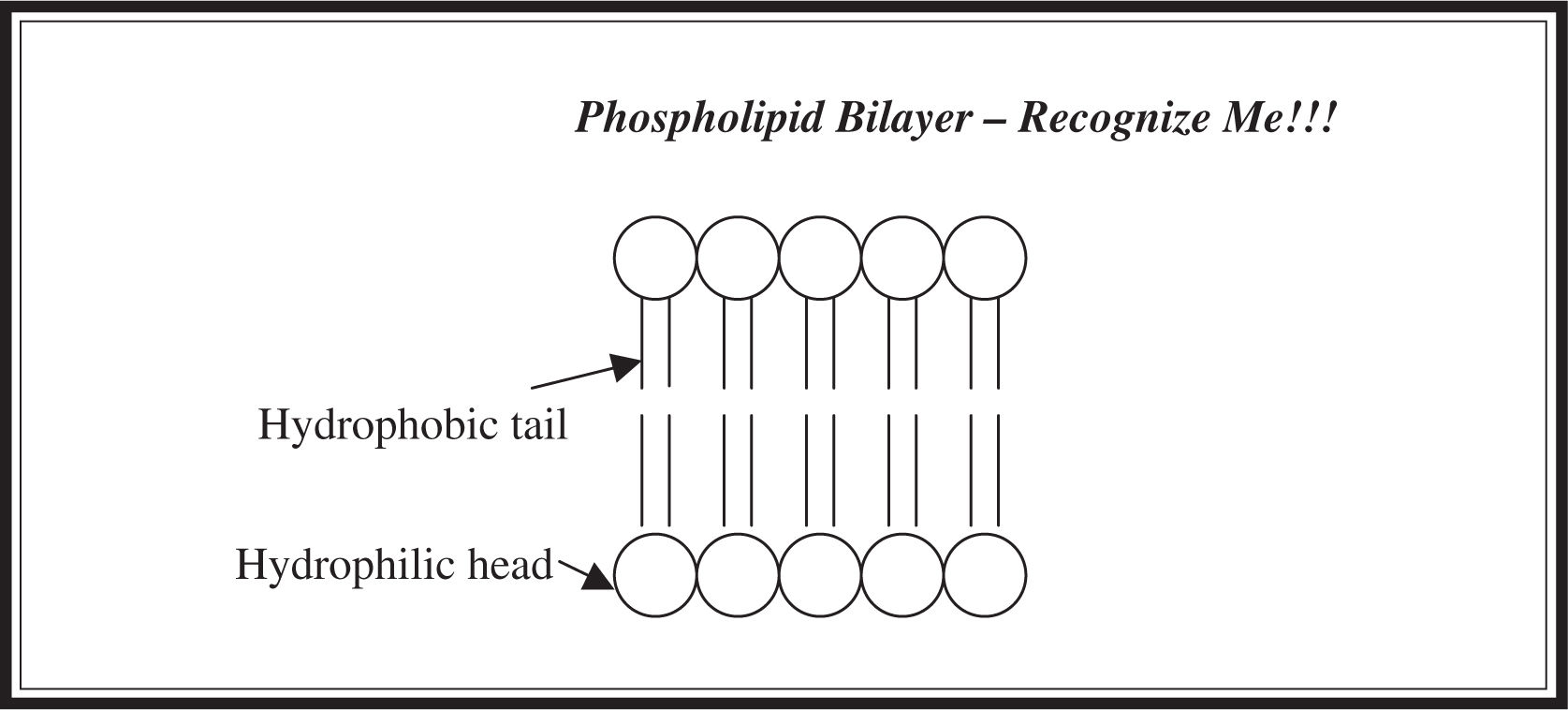
Figure 5.5 Bilayered structure of phospholipids.
Carbohydrates
Carbohydrates can be simple sugars or complex molecules containing multiple sugars. Carbohydrates are used by the cells of the body in energy-producing reactions and as structural materials. Carbohydrates have the elements C, H, and O. Hydrogen and oxygen are present in a 2:1 ratio. The three main types of carbohydrates you need to know are monosaccharides, disaccharides, and polysaccharides.
A monosaccharide, or simple sugar, is the simplest form of a carbohydrate. The most important monosaccharide is glucose (C6 H12 O6 ), which is used in cellular respiration to provide energy for cells. Monosaccharides with five carbons (C5 H10 O5 ) are used in compounds such as genetic molecules (RNA) and high-energy molecules (ATP). The structure of glucose is shown in Figure 5.6 .
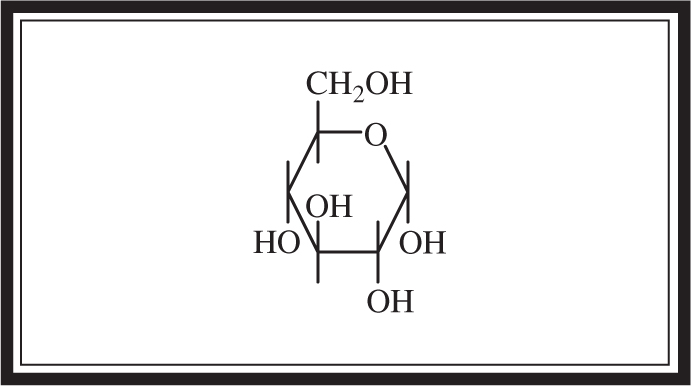
Figure 5.6 Glucose structure.
A disaccharide is a sugar consisting of two monosaccharides bound together. Common disaccharides include sucrose, maltose, and lactose. Sucrose, a major energy carbohydrate in plants, is a combination of fructose and glucose; maltose, a carbohydrate used in the creation of beer, is a combination of two glucose molecules; and lactose, found in dairy products, is a combination of galactose and glucose.
A polysaccharide is a carbohydrate containing three or more monosaccharide molecules. Polysaccharides, usually composed of hundreds or thousands of monosaccharides, act as a storage form of energy and as structural material in and around cells. The most important carbohydrates for storing energy are starch and glycogen . Starch, made solely of glucose molecules linked together, is the storage form of choice for plants. Animals store much of their carbohydrate energy in the form of glycogen, which is most often found in liver and muscle cells. Glycogen is formed by linking many glucose molecules together.
Two important structural polysaccharides are cellulose and chitin . Cellulose, a compound composed of many glucose molecules, is used by plants in the formation of their cell walls. Chitin is an important part of the exoskeletons of arthropods such as insects, spiders, and shellfish (see Chapter 13 , Taxonomy and Classification).
Julie (11th grade): “Remembering these four came in handy on the test!”
Proteins
A protein is a compound composed of chains of amino acids. Proteins have many functions in the body—they serve as structural components, transport aids, enzymes, and cell signals, to name only a few. You should be able to identify a protein or an amino acid by sight if asked to do so on the test.
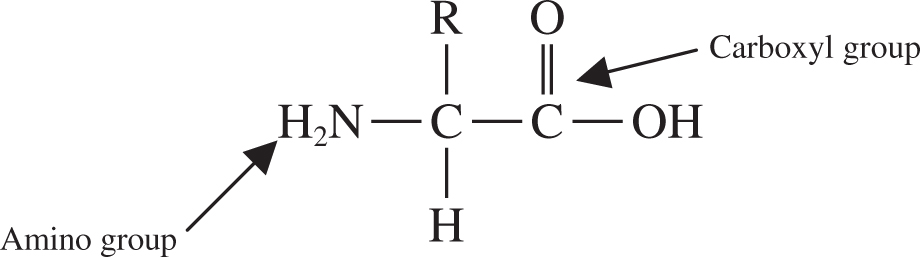
An amino acid consists of a carbon center surrounded by an amino group, a carboxyl group, a hydrogen, and an R group (See Figure 5.7 .) Remember that the R stands for “rest” of the compound, which provides an amino acid’s unique personal characteristics. For instance, acidic amino acids have acidic R groups, basic amino acids have basic R groups, and so forth.
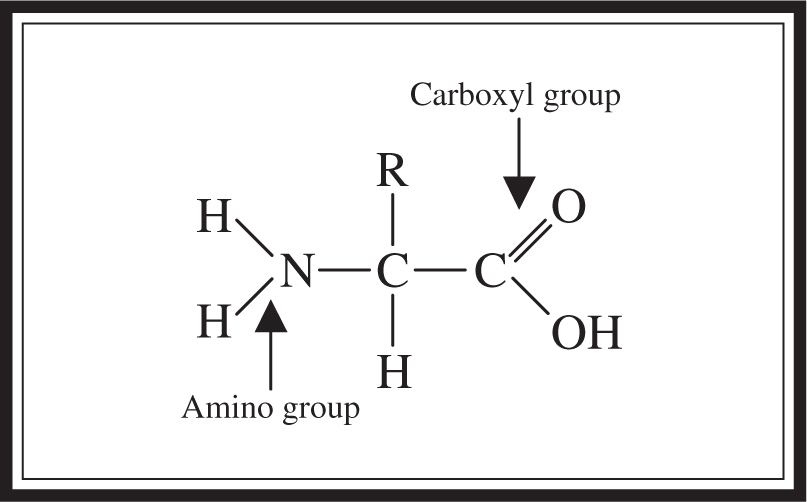
Figure 5.7 Structure of an amino acid.
Many students preparing for the AP exam wonder if they need to memorize the 20 amino acids and their structures and whether they are polar, nonpolar, or charged. This is a lot of effort for perhaps one multiple-choice question that you might encounter on the exam. We think that this time would be better spent studying other potential exam questions. If this is of any comfort to you, we have yet to see an AP Biology question that asks something to the effect of “Which of these 5 amino acids is nonpolar?” (Disclaimer: This does not mean that it will never happen ![]() .) It is more important for you to identify the general structure of an amino acid and know the process of protein synthesis, which we discuss in Chapter 15 .
.) It is more important for you to identify the general structure of an amino acid and know the process of protein synthesis, which we discuss in Chapter 15 .
A protein consists of amino acids linked together as shown in Figure 5.8 . They are most often much larger than that depicted here. Figure 5.8 is included to enable you to identify a peptide linkage on the exam. Most proteins have many more amino acids in the chain.
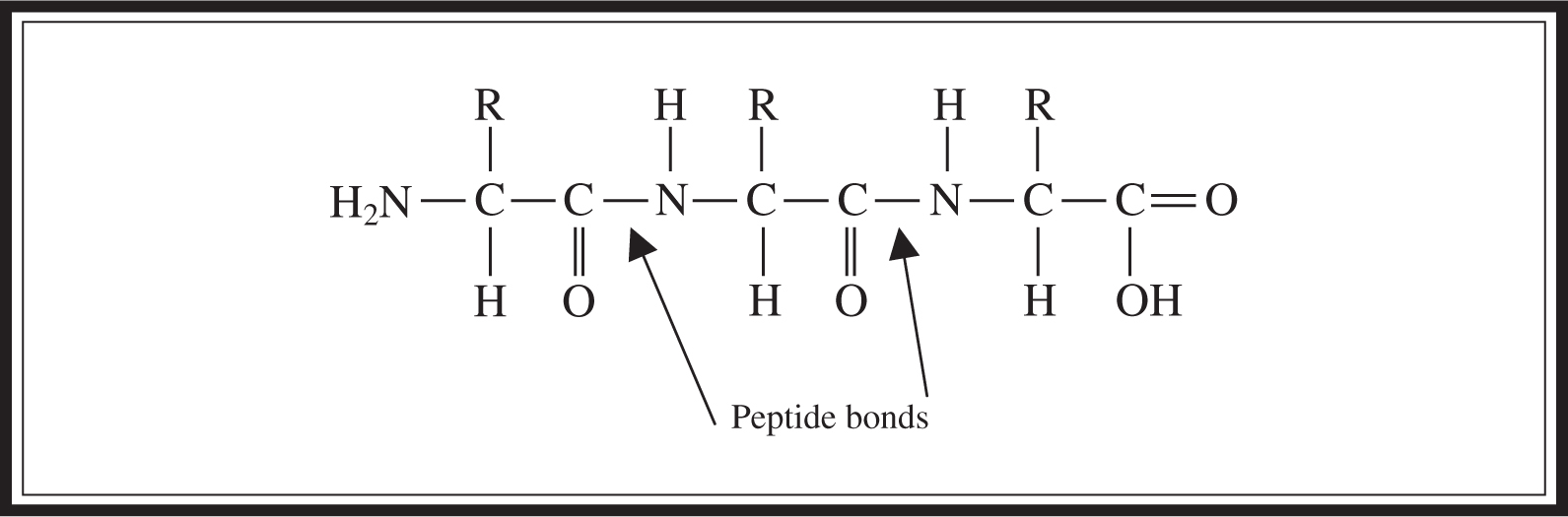
Figure 5.8 Amino acid structure exhibiting peptide linkage.
The AP exam may expect you to know about the structure of proteins:

Primary structure . The order of the amino acids that make up the protein.
Secondary structure . Three-dimensional arrangement of a protein caused by hydrogen bonding at regular intervals along the polypeptide backbone.
Tertiary structure . Three-dimensional arrangement of a protein caused by interaction among the various R groups of the amino acids involved.
Quaternary structure . The arrangement of separate polypeptide “subunits” into a single protein. Not all proteins have quaternary structure; many consist of a single polypeptide chain.
Proteins with only primary and secondary structure are called fibrous proteins. Proteins with only primary, secondary, and tertiary structures are called globular proteins. Either fibrous or globular proteins may contain a quaternary structure if there is more than one polypeptide chain.
Enzymes
CT teacher: “The topic of enzymes is full of essay material. Know it well.”
Enzymes are proteins that act as organic catalysts and will be encountered often in your review for this exam. Catalysts speed up reactions by lowering the energy (activation energy) needed for the reaction to take place, but are not used up in the reaction. The substances that enzymes act on are known as substrates .
Enzymes are selective; they interact only with particular substrates. It is the shape of the enzyme that provides the specificity. The part of the enzyme that interacts with the substrate is called the active site. The induced-fit model of enzyme-substrate interaction describes the active site of an enzyme as specific for a particular substrate that fits its shape. When the enzyme and substrate bind together, the enzyme is induced to alter its shape for a tighter active site–substrate attachment. This tight fit places the substrate in a favorable position to react, speeding up (accelerating) the rate of reaction. After an enzyme interacts with a substrate, converting it into a product, it is free to find and react with another substrate; thus, a small concentration of enzyme can have a major effect on a reaction.
BIG IDEA 4.B.1
The shape of enzymes and their active sites are important to their function .
Every enzyme functions best at an optimal temperature and pH. If the pH or temperature strays from those optimal values, the effectiveness of the enzyme will suffer. The effectiveness of an enzyme can be affected by four things:

1. The temperature
2. The pH
3. The concentration of the substrate involved
4. The concentration of the enzyme involved
You should be able to identify the basic components of an activation energy diagram if you encounter one on the AP exam. The important parts are identified in Figure 5.9 .
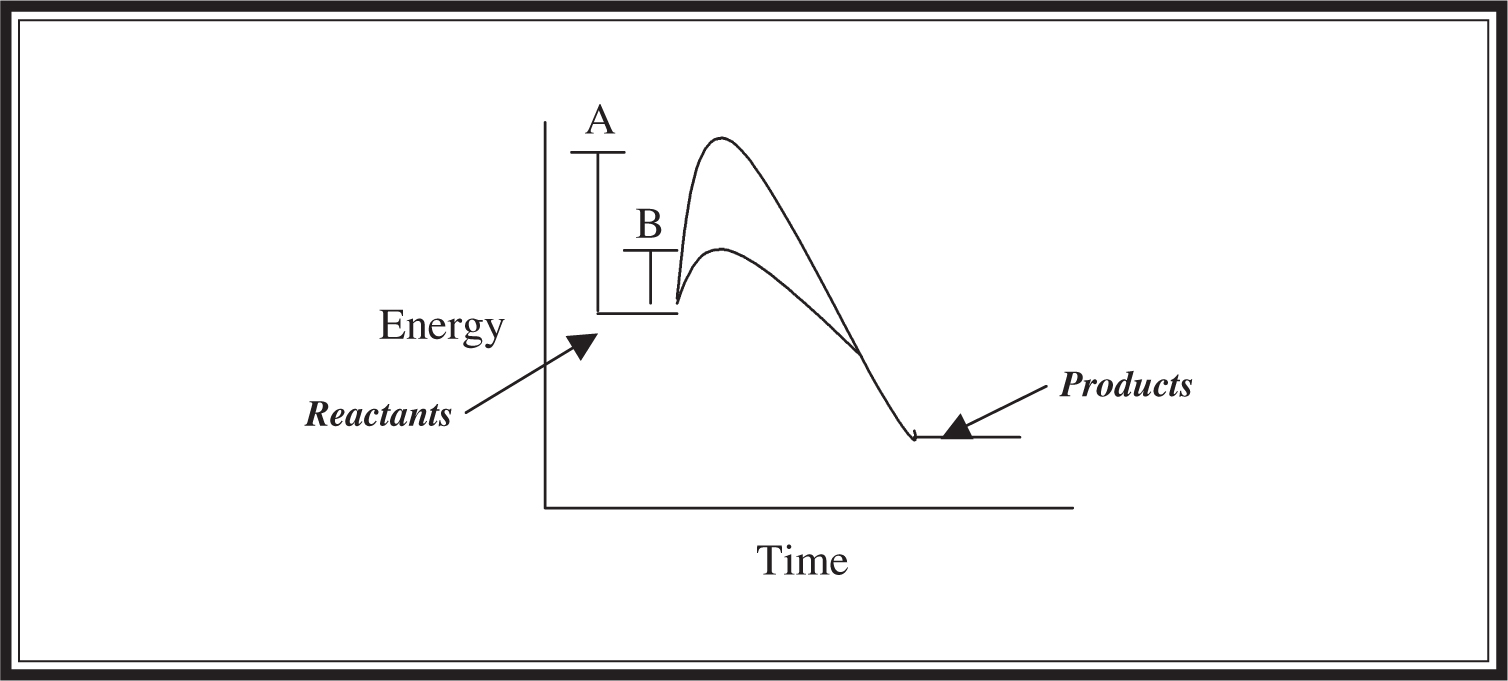
Figure 5.9 Plot showing energy versus time. Height A represents original activation energy; height B represents the lowered activation energy due to the addition of enzyme.
The last enzyme topic to cover is the difference between competitive and noncompetitive inhibition. In competitive inhibition (Figure 5.10 ), an inhibitor molecule resembling the substrate binds to the active site and physically blocks the substrate from attaching. Competitive inhibition can sometimes be overcome by adding a high concentration of substrate to outcompete the inhibitor. In noncompetitive inhibition (Figure 5.11 ), an inhibitor molecule binds to a different part of the enzyme, causing a change in the shape of the active site so that it can no longer interact with the substrate.
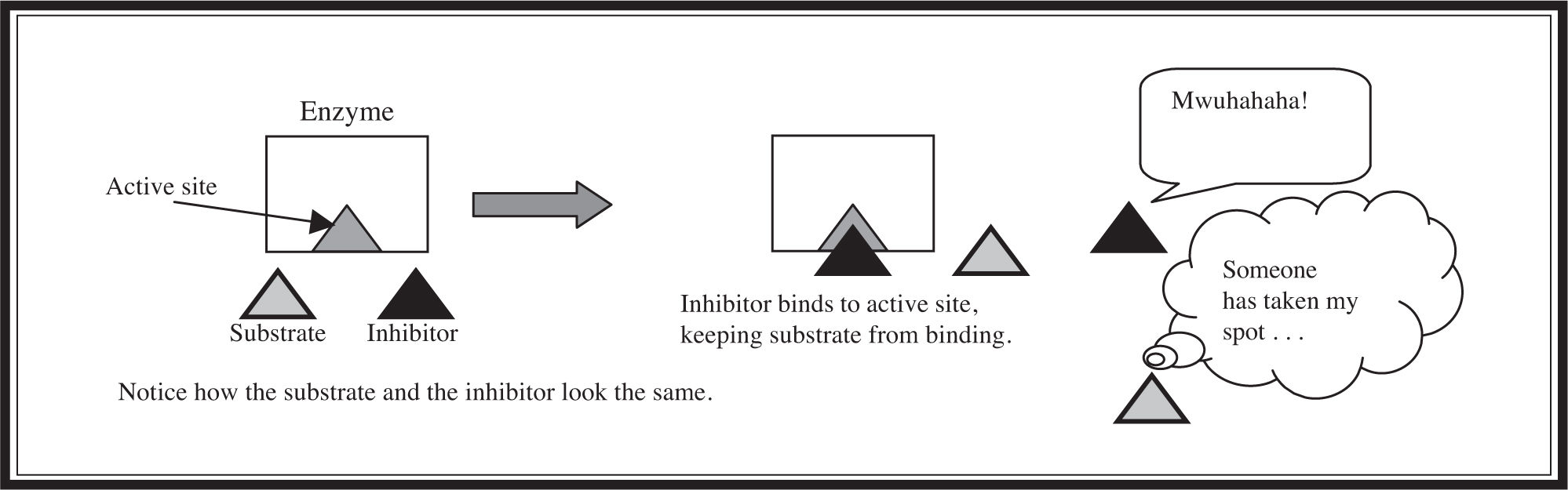
Figure 5.10 Competitive inhibition.
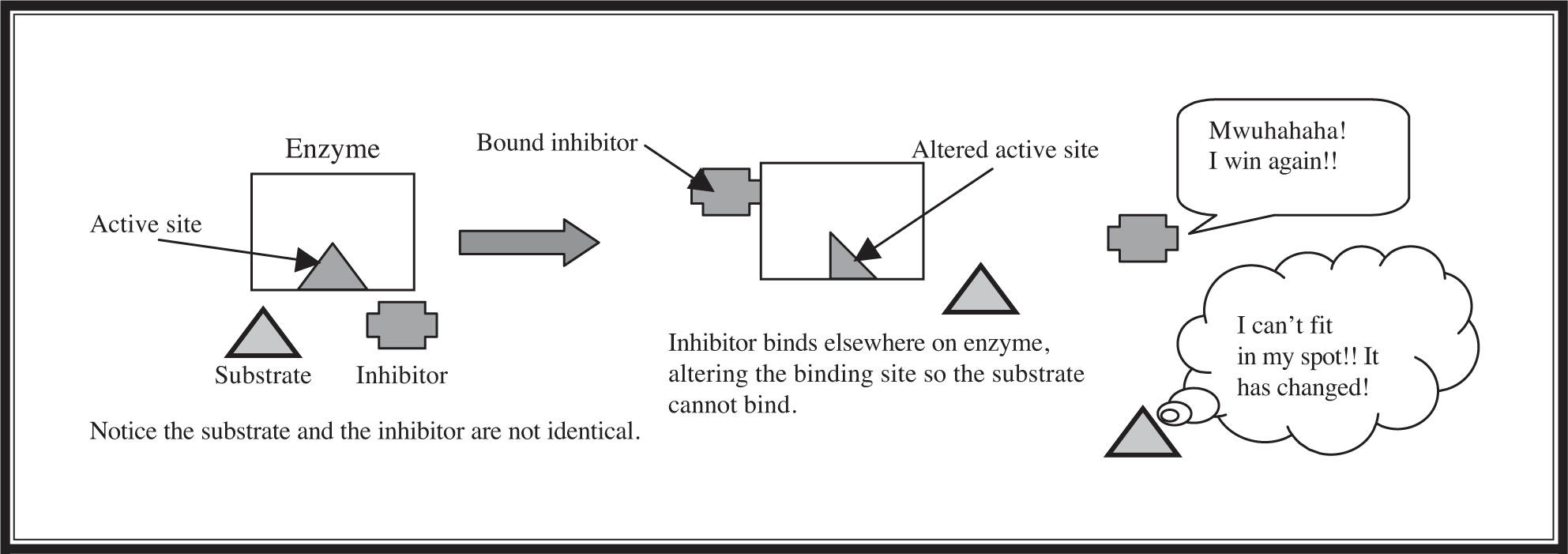
Figure 5.11 Noncompetitive inhibition.
pH: Acids and Bases
The pH scale is used to indicate how acidic or basic a solution is. It ranges from 0 to 14; 7 is neutral. Anything less than 7 is acidic; anything greater than 7 is basic. The pH scale is a logarithmic scale and as a result, a pH of 5 is 10 times more acidic than a pH of 6. Following the same logic, a pH of 4 is 100 times more acidic than a pH of 6. Remember that as the pH of a solution decreases , the concentration of hydrogen ions in the solution increases, and vice versa. For the most part, chemical reactions in humans function at or near a neutral pH. The exceptions to this rule are the chemical reactions involving some of the enzymes of the digestive system. (See Chapter 15 , Human Physiology.)
Reactions

There are five types of reactions you should know for this exam:
1. Hydrolysis reaction . A reaction that breaks down compounds by the addition of H2 O.
2. Dehydration synthesis reaction . A reaction in which two compounds are brought together with H2 O released as a product.
3. Endergonic reaction . A reaction that requires input of energy to occur.
A + B + energy → C
4. Exergonic reaction . A reaction that gives off energy as a product.
A + B → energy + C
5. Redox reaction . A reaction involving the transfer of electrons. Such reactions occur along the electron transport chain of the mitochondria during respiration (Chapter 7 ).
![]() Review Questions
Review Questions
For questions 1–4, please use the following answer choices:
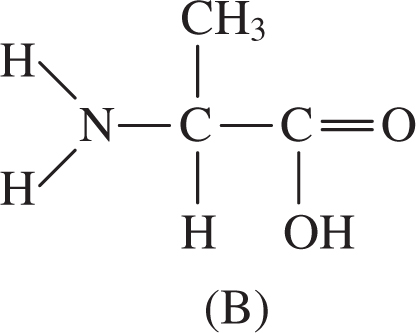
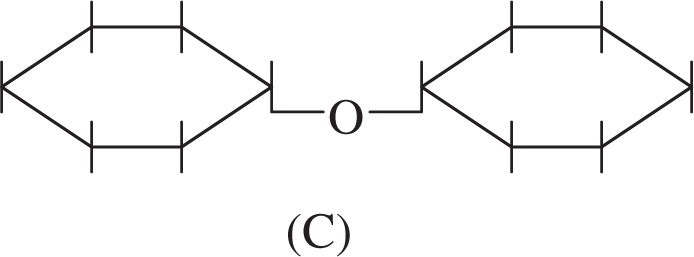
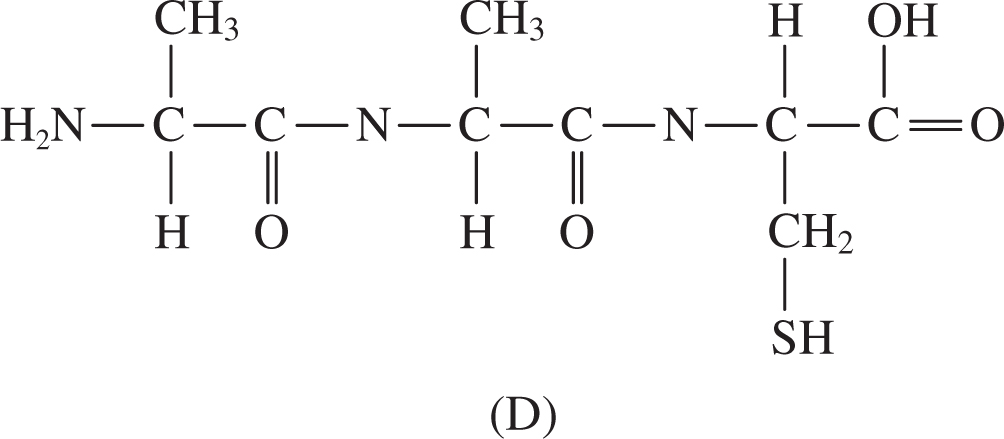
1 . Which of the structures shown above is a polypeptide?
2 . Which of these structures is a disaccharide?
3 . Which of these structures is a fat?
4 . Which of these structures is an amino acid?
5 . Which of the following has both a hydrophobic portion and a hydrophilic portion?
A. Starch
B. Phospholipids
C. Proteins
D. Steroids
E. Chitin
6 . A solution that has a pH of 2 is how many times more acidic than one with a pH of 5?
A. 2
B. 5
C. 10
D. 100
E. 1,000
7 . The structure below contains which functional group?
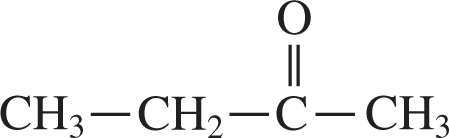
A. Aldehyde
B. Ketone
C. Amino
D. Hydroxyl
E. Carboxyl
8 . Which of the following will least affect the effectiveness of an enzyme?
A. Temperature
B. pH
C. Concentration of substrate
D. Concentration of enzyme
E. Original activation energy of system
9 . Which of the following is similar to the process of competitive inhibition?
A. When you arrive at work in the morning, you are unable to park your car in your (assigned) parking spot because the car of the person who parks next to you has taken up just enough space that you cannot fit your own car in.
B. When you arrive at work in the morning, you are unable to park your car in your parking spot because someone with a car exactly like yours has already taken your spot, leaving you nowhere to park your car.
C. As you are about to park your car in your spot at work, a giant bulldozer comes along and smashes your car away from the spot, preventing you from parking your car in your spot.
D. When you arrive at work in the morning, you are unable to park your car in your parking spot because someone has placed a giant cement block in front of your spot.
10 . All the following are carbohydrates except
A. starch.
B. glycogen.
C. chitin.
D. glycerol.
E. cellulose.
11 . An amino acid contains which of the following functional groups?
A. Carboxyl group and amino group
B. Carbonyl group and amino group
C. Hydroxyl group and amino group
D. Carboxyl group and hydroxyl group
E. Carbonyl group and carboxyl group
![]() Answers and Explanations
Answers and Explanations
1 . D
2 . C
3 . A
4 . B
5 . B— A phospholipid has both a hydrophobic portion and a hydrophilic portion. The hydrocarbon portion, or tail, of the phospholipid dislikes water, and the phosphate portion, the head, is hydrophilic.
6 . E— Because the pH scale is logarithmic, 2 is 1,000 times more acidic than 5.
7 . B— This functional group is a carbonyl group. The two main types of carbonyl groups are ketones and aldehydes. In this case, it is a ketone because there are carbon chains on either side of the carbon double-bonded to the oxygen.
8 . E— The four main factors that affect enzyme efficiency are pH, temperature, enzyme concentration, and substrate concentration.
9 . B— Competitive inhibition is the inhibition of an enzyme–substrate reaction in which the inhibitor resembles the substrate and physically blocks the substrate from attaching to the active site. This parking spot represents the active site, your car is the substrate, and the other car already in the spot is the competitive inhibitor. Examples A and D more closely resemble noncompetitive inhibition.
10 . D— Glycerol is not a carbohydrate. It is an alcohol. Starch is a carbohydrate stored in plant cells. Glycogen is a carbohydrate stored in animal cells. Chitin is a carbohydrate used by arthropods to construct their exoskeletons. Cellulose is a carbohydrate used by plants to construct their cell walls.
11 . A
![]() Rapid Review
Rapid Review
Try to rapidly review the following material:
Organic compounds: contain carbon; examples include lipids, proteins, and carbs (carbohydrates).
Functional groups: amino (NH2 ), carbonyl (RCOR), carboxyl (COOH), hydroxyl (OH), phosphate (PO4 ), sulfhydryl (SH).
Fat: glycerol + 3 fatty acids.
Saturated fat: bad for you; animals and some plants have it; solidifies at room temperature.
Unsaturated fat: better for you, plants have it; liquifies at room temperature.
Steroids: lipids whose structures resemble chicken-wire fence; include cholesterol and sex hormones.
Phospholipids: glycerol + 2 fatty acids + 1 phosphate group; make up membrane bilayers of cells; have hydrophobic interiors and hydrophilic exteriors.
Carbohydrates: used by cells for energy and structure; monosaccharides (glucose), disaccharides (sucrose, maltose, lactose), storage polysaccharides (starch [plants], glycogen [animals]), structural polysaccharides (chitin [fungi], cellulose [arthropods]).
Proteins: made with the help of ribosomes out of amino acids; serve many functions (e.g., transport, enzymes, cell signals, receptor molecules, structural components, and channels).
Enzymes: catalytic proteins that react in an induced-fit fashion with substrates to speed up the rate of reactions by lowering the activation energy; effectiveness is affected by changes in pH, temperature, and substrate and enzyme concentrations.
Competitive inhibition: inhibitor resembles substrate and binds to active site.
Noncompetitive inhibition: inhibitor binds elsewhere on enzyme; alters active site so that substrate cannot bind.
pH: logarithmic scale <7 acidic, 7 neutral, >7 basic (alkaline); pH 4 is 10 times more acidic than pH 5.
Reaction types:
Hydrolysis reaction: breaks down compounds by adding water.
Dehydration reaction: two components brought together, producing H2 O.
Endergonic reaction: reaction that requires input of energy.
Exergonic reaction: reaction that gives off energy.
Redox reaction: electron transfer reactions.
CHAPTER 5
Chemistry
1 . A change in pH from 8 to 11 indicates a change in concentration of H+ ions by a factor of
(A) 3.
(B) 30.
(C) 300.
(D) 1,000.
2 . A nonspontaneous reaction has a free energy change that is
(A) greater than zero.
(B) equal to zero.
(C) less than zero.
(D) dependent on the initial energy level.
3 . Which of the following does not represent a functional group found in organic compounds?
(A) Sulfoxyl group
(B) Carboxyl group
(C) Hydroxyl group
(D) Carbonyl group
4 . Leafy green vegetables are healthier to consume than red meats because
(A) most of the fat found in animals is unsaturated.
(B) red meats do not contain steroids.
(C) most of the fat found in plants is unsaturated.
(D) plants tend to contain higher levels of estrogen, which leads to a reduction in cholesterol.
![]() Answers and Explanations
Answers and Explanations
1 . D —This question tests your understanding of the pH scale, which measures the amount of H+ ions in a solution. The scale is a logarithmic scale, which means each step represents a tenfold change in the concentration of H+ . Therefore, a change from 8 to 11, which is a three-step change, would represent a 103 change in the H+ concentration, or a 1,000 times change.
2 . A —Nonspontaneous reactions occur when the free energy change (ΔG) is greater than zero.
3 . A —The sulfoxyl group is not a real group. A carboxyl group has a hydroxide ion at one of the R spots and a carbon chain in the other, and it often shows up along with amino groups in amino acids. Hydroxyl groups are present in compounds known as alcohols. Carbonyl groups contain carbons double-bonded to oxygen atoms. They are known to make compounds hydrophilic and polar.
4 . C —Fats can be saturated or unsaturated. Unsaturated fat molecules contain one or more double bonds, which means they contain fewer hydrogen molecules per carbon than do saturated fats. Saturated fats are the bad guys and are associated with heart disease and atherosclerosis. Most of the fat found in animals is saturated, whereas plants tend to contain unsaturated fats.