CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.6. Chemical Changes—Forming New Kinds of Matter
Atoms interact with other atoms to fill their outermost energy level with electrons to become more stable. When these chemical reactions take place, the result is a change in matter in which different chemical substances are created by forming or breaking chemical bonds. When a chemical reaction occurs the interacting atoms may become attached, or bonded, to one another by a chemical bond. Two types of bonds are (1) ionic bonds and (2) covalent bonds.
Ionic Bonds and Ions
Any positively or negatively charged atom or molecule is called an ion. Ionic bonds are formed after atoms transfer electrons to achieve a full outermost energy level. Electrons are donated or received in the transfer, forming a positive and a negative ion, a process called ionization. The force of attraction between oppositely charged ions forms ionic bonds, and ionic compounds are the result. Ionic compounds are formed when an element from the left side of the periodic table (those eager to gain electrons) reacts with an element from the right side (those eager to donate electrons). This results in the formation of a stable group, which has an orderly arrangement and is a crystalline solid.
Ions and ionic compounds are very important in living systems. For example, sodium chloride is a crystal solid known as table salt. A positively charged sodium ion is formed when a sodium atom loses 1 electron. This results in a stable, outermost energy level with 8 electrons. When an atom of chlorine receives an electron to stabilize its outermost energy level, it becomes a negative ion. All positively charged ions are called cations and all negative charged ions are called anions (figure 2.8). When these oppositely charged ions are close to one another, the attractive force between them forms an ionic bond. Ionic crystals form by the addition of ions to the outer surface of a small cluster of starter ions or seeds (figure 2.9). The dots in the following diagram represent the electrons in the outermost energy levels of each atom. This kind of diagram is called an electron dot formula.
![]()
When many ionic compounds (crystals) are dissolved in water, the ionic bonds are broken and the ions separate, or dissociate, from one another. For example, solid sodium chloride dissociates in water to become ions in solution:
![]()
Any substance that dissociates into ions in water and allows the conduction of electric current is called an electrolyte.
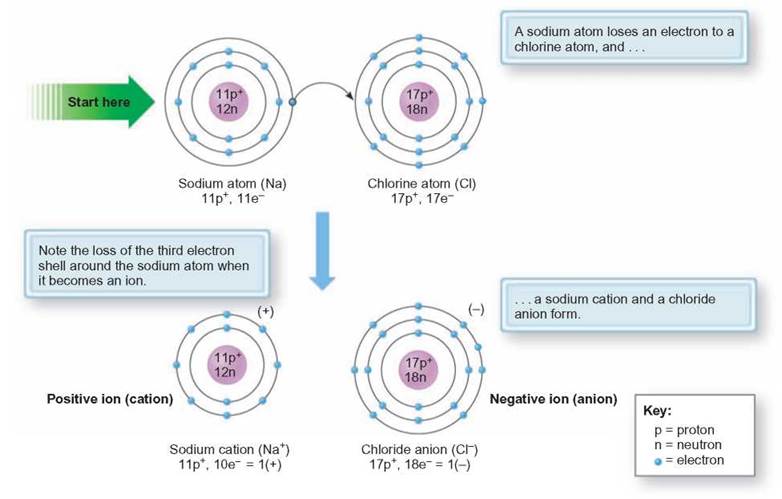
FIGURE 2.8. Ion Formation
A sodium atom has 2 electrons in the first energy level, 8 in the second energy level, and 1 in the third level. When it loses its 1 outer electron, it becomes a sodium cation.
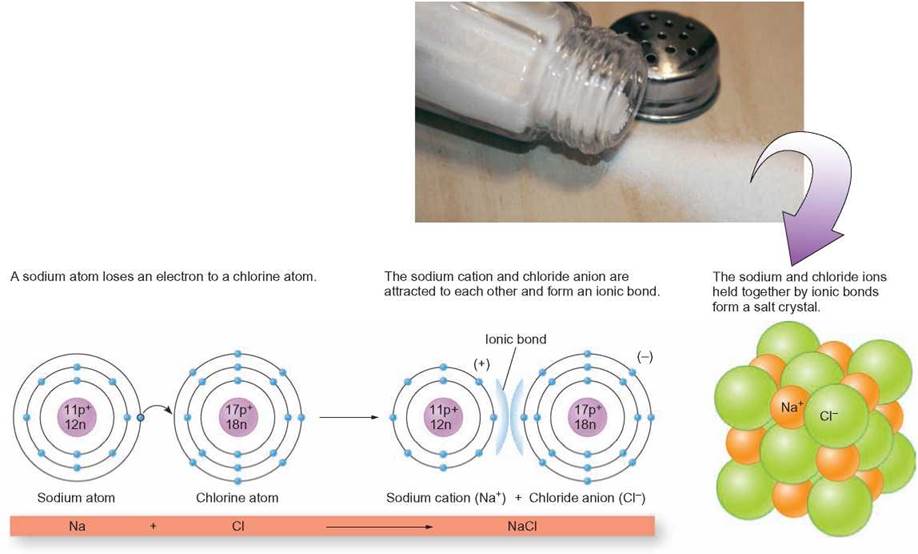
FIGURE 2.9. Crystals
A crystal is composed of ions that are bonded together and form a three-dimensional structure. Crystals grow with the addition of atoms to their outside surface.
Covalent Bonds
Most substances do not have the properties of ionic compounds, because they are not composed of ions. Most substances are composed of electrically neutral groups of atoms that are tightly bound together. As noted earlier, many gases are diatomic, occurring naturally as two of the same kinds of atoms bound together as an electrically neutral molecule. Hydrogen, for example, occurs as molecules of H2 and no ions are involved. The hydrogen atoms are held together by a covalent bond, a chemical bond formed by the sharing of a pair of electrons. In the diatomic hydrogen molecule, each hydrogen atom contributes a single electron to the shared pair. Hydrogen atoms both share one pair of electrons, but other elements might share more than one pair.
Consider how the covalent bond forms between two hydrogen atoms by imagining two hydrogen atoms moving toward one another. Each atom has a single electron. As the atoms move closer and closer together, their outer energy levels begin to overlap. Each electron is attracted to the oppositely charged nucleus of the other atom and the overlap tightens. Then, the repulsive forces from the like-charged nuclei stop the merger. A state of stability is reached between the 2 nuclei and 2 electrons, because the outermost energy level is full and an H2 molecule has been formed. The electron pair is now shared by both atoms, and the attraction of each nucleus for the electron of the other holds the atoms together (figure 2.10).
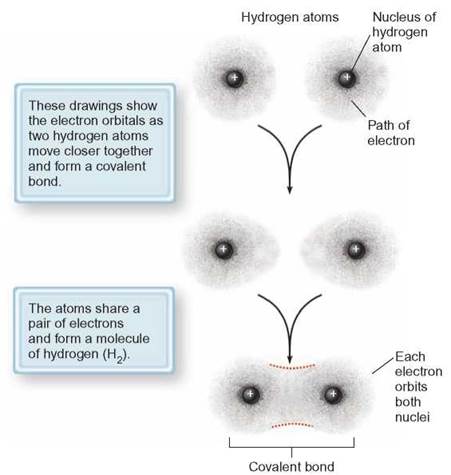
FIGURE 2.10. Covalent Bond Between Atoms
When two hydrogen atoms come so close to each other that the locations of the outermost electrons overlap, an electron from each one can be shared to “fill” the outermost energy levels. After the hydrogen atoms have bonded, a new electron distribution pattern forms around the entire molecule, and both electrons share the outermost molecular energy level.
Dots can be used to represent the electrons in the outer energy levels of atoms. If each atom shares one of its electrons with the other, the two dots represent the bonding pair of electrons shared by the two atoms. Bonding pairs of electrons are often represented by a simple line between two atoms, as in the following example:
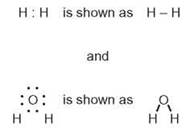
A covalent bond in which a single pair of electrons is shared by two atoms is called a single covalent bond or, simply, a single bond. Some atoms can share more than one electron pair. A double bond is a covalent bond formed when two pairs of electrons are shared by two atoms. This happens mostly in compounds involving atoms of the elements C, N, O, and S. For example, ethylene, a gas given off by ripening fruit, has a double bond between the two carbons (figure 2.11). The electron dot formula for ethylene is

A triple bond is a covalent bond formed when three pairs of electrons are shared by two atoms. Triple bonds occur mostly in compounds with atoms of the elements C and N. Atmospheric nitrogen gas, for example, forms a triple covalent bond:
![]()
2.6. CONCEPT REVIEW
15. Why are the outermost electrons of an atom important?
16. Name two kinds of chemical bonds that hold atoms together. How do these bonds differ from one another?
HOW SCIENCE WORKS 2.2
Greenhouse Gases and Their Relationship to Global Warming
What actually causes global warming? An explanation is relatively straightforward: several greenhouse gases. Carbon dioxide (CO2), chlorofluorocarbons (CCl3F), methane (CH4), and nitrous oxide (N2O) are called greenhouse gases because they let sunlight enter the atmosphere to warm the Earth's surface. When this energy is reradiated as infrared radiation (heat), it is absorbed by these gases in the atmosphere. Because the effect is similar to what happens in a greenhouse (the glass allows light to enter but retards the loss of heat), these gases are called greenhouse gases, and the warming thought to occur from their increase is called the greenhouse effect. What do we know about these gases?
Carbon dioxide (CO2)
• The most abundant of the greenhouse gases
• Sources include
- Cellular respiration
- Burning of fossil fuels (i.e., gasoline, coal) o Deforestation (i.e., the loss of plants using CO2 in photosynthesis)
Chlorofluorocarbons (CCl3F)
• Sole source is from human activities
• Used as coolants in refrigerators and air conditioners, as cleaning solvents, propellants in aerosol containers, and as expanders in foam products
• 15,000 times more efficient than the greenhouse gas, CO2
• Use of chlorofluorocarbons and similar compounds is being phased out worldwide.

Methane (CH4)
• Small amount found naturally in the atmosphere
• Sources include
- Burning of fossil fuels
- Most from biological sources (i.e., wetlands, rice felds, livestock, bacteria)
Nitrous oxide (N2O)
• Minor part of atmosphere
• Sources include
- Burning of fossil fuels
- Nitrogen-containing fertilizers
- Deforestation
|
Greenhouse Gas |
Pre-1750 Concentration (ppm) |
Concentration (ppm) (2007) |
Contribution to Global Warming (percent) |
|
Carbon dioxide (CO2) |
280 |
382 |
60 |
|
Methane (CH4) |
0.608 |
1.78 |
20 |
|
Chlorofluoro-carbons (CCl3F) |
0 |
0.00088 |
14 |
|
Nitrous oxide (N2O) |
0.270 |
0.321 |
6 |
Source: Data from Intergovernmental Panel on Climate Change, with updates from Oak Ridge National Laboratory.