CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
2. The Basics of Life
2.8. Chemical Reactions
When compounds are broken or formed, new materials with new properties are produced. This kind of a change in matter is called a chemical change, and the process is called a chemical reaction. In a chemical reaction, the elements stay the same but the compounds and their properties change when the atoms are bonded in new combinations. All living things use energy and matter. In other words, they are constantly performing chemical reactions.
Chemical reactions produce new chemical substances with greater or smaller amounts of potential energy. Energy is absorbed to produce new chemical substances with more potential energy. Energy is released when the new chemical substances produced have less potential energy. For example, new chemical substances are produced in green plants through the process of photosynthesis. A green plant uses radiant energy (sunlight), carbon dioxide, and water to produce new chemical materials and oxygen. These new chemical materials, the stuff that makes up leaves, roots, and wood, contain more chemical energy than the carbon dioxide and water from which they were formed.
A chemical equation is a way of describing what happens in a chemical reaction. For example, the chemical reaction of photosynthesis is described by the equation
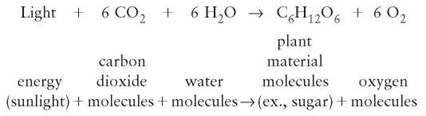
In chemical reactions, reactants are the substances that are changed (in photosynthesis, the carbon dioxide molecules and water molecules); they appear on the left side of the equation. The equation also indicates that energy is absorbed; the term energy appears on the left side. The arrow indicates the direction in which the chemical reaction is occurring; it means “yields." The new chemical substances are on the right side and are called products. Reading the photosynthesis reaction as a sentence, you would say, “Carbon dioxide and water use energy to react, yielding plant materials and oxygen."
Notice in the photosynthesis reaction that there are numbers preceding some of the chemical formulas and subscripts within each chemical formula. The number preceding each of the chemical formulas indicates the number of each kind of molecule involved in the reaction. The subscripts indicate the number of each kind of element in a single molecule of that compound. Chemical reactions always take place in whole number ratios. That is, only whole molecules can be involved in a chemical reaction. It is not possible to have half a molecule of water serve as a reactant or become a product. Half a molecule of water is not water. Furthermore, the numbers of atoms of each element on the reactant side must equal the numbers on the product side. Because the preceding equation has equal numbers of each element (C, H, O) on both sides, the equation is said to be “balanced."
Five of the most important chemical reactions that occur in organisms are (1) oxidation-reduction, (2) dehydration synthesis, (3) hydrolysis, (4) phosphorylation, and (5) acidbase reactions.
Oxidation-Reduction Reactions
An oxidation-reduction reaction is a chemical change in which electrons are transferred from one atom to another and, with it, the energy contained in its electrons. As implied by the name, such a reaction has two parts and each part tells what happens to the electrons. Oxidation describes what happens to the atom or molecule that loses an electron. Reduction describes what happens to the atom or molecule that gains an electron. When the term oxidation was first used, it specifically meant reactions involving the combination of oxygen with other atoms. But fluorine, chlorine, and other elements were soon recognized to participate in similar reactions, so the definition was changed to describe the shifts of electrons in the reaction. The name also implies that, in any reaction in which oxidation occurs, reduction must also take place. One cannot take place without the other. Cellular respiration is an oxidation-reduction reaction that occurs in all cells:
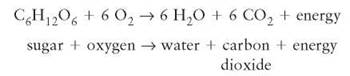
In this cellular respiration reaction, sugar is being oxidized (losing its electrons) and oxygen is being reduced (gaining the electrons from sugar). The high chemical potential energy in the sugar molecule is released, and the organism uses some of this energy to perform work. In the previously mentioned photosynthesis reaction, water is oxidized (loses its electrons) and carbon dioxide is reduced (gains the electrons from water). The energy required to carry out this reaction comes from the sunlight and is stored in the product, sugar.
Dehydration Synthesis Reactions
Dehydration synthesis reactions are chemical changes in which water is released and a larger, more complex molecule is made (synthesized) from smaller, less complex parts. The water is a product formed from its component parts (H and OH), which are removed from the reactants. Proteins, for example, consist of a large number of amino acid subunits joined together by dehydration synthesis:
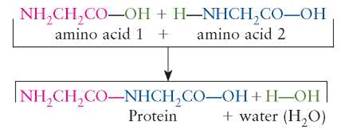
The building blocks of protein (amino acids) are bonded to one another to synthesize larger, more complex product molecules (i.e., protein). In dehydration synthesis reactions, water is produced as smaller reactants become chemically bonded to one another, forming fewer but larger product molecules.
Hydrolysis Reactions
Hydrolysis reactions are the opposite of dehydration synthesis reactions. In a hydrolysis reaction, water is used to break the reactants into smaller, less complex products:
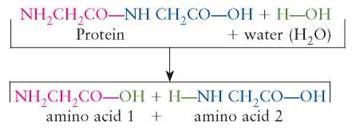
A more familiar name for this chemical reaction is digestion. This is the kind of chemical reaction that occurs when a protein food, such as meat, is digested. Notice in the previous example that the H and OH component parts of the reactant water become parts of the building block products.
Phosphorylation Reactions
A phosphorylation reaction takes place when a cluster of atoms known as a phosphate group
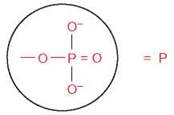
is added to another molecule. This cluster is abbreviated in many chemical formulas in a shorthand form as P, and only the P is shown when a phosphate is transferred from one molecule to another. This is a very important reaction, because the bond between a phosphate group and another atom contains the potential energy that is used by all cells to power numerous activities. Phosphorylation reactions result in the transfer of their potential energy to other molecules to power the activities of all organisms (figure 2.14).

FIGURE 2.14. Phosphorylation and Muscle Contractions
When the phosphate group is transferred between molecules, energy is released which powers muscle contractions.
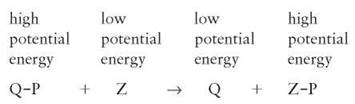
This type of reaction is commonly involved in providing the kinetic energy needed by all organisms. It can also take place in reverse. When this occurs, energy must be added from the environment (sunlight or another phosphorylated molecule) and is stored in the newly phosphorylated molecule.
Acid-Base Reactions
Acid-base reactions take place when the ions of an acid interact with the ions of a base, forming a salt and water (see section 2.9). An aqueous solution containing dissolved acid is a solution containing hydrogen ions. If a solution containing a second ionic basic compound is added, a mixture of ions results. While they are mixed together, a reaction can take place—for example,
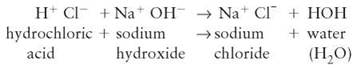
In an acid-base reaction, the H from the acid becomes chemically bonded to the OH of the base. This type of reaction frequently occurs in organisms and their environment. Because acids and bases can be very harmful, reactions in which they neutralize one another protect organisms from damage.
OUTLOOKS 2.1
Water and Life—The Most Common Compound of Living Things
1. Water has a high surface tension. Because water molecules are polar, hydrogen bonds form between water molecules, and they stick more to one another than to air molecules. Thus, water tends to pull together to form a smooth surface where water meets air. This layer can be surprisingly strong. For instance, some insects can walk on the surface of a pond. The tendency of water molecules to stick to each other and to some other materials explains why water can make things wet. It also explains why water climbs through narrow tubes, called capillary tubes. This capillary action also helps water move through soil, up the vessels in plants' stems, and through the capillaries (tiny blood vessels) in animals.
2. Water has unusually high heats of vaporization and fusion. Because polar water molecules stick to one another, an unusually large amount of heat energy is required to separate them. Water resists changes in temperature. It takes 540 calories of heat energy to convert 1 gram of liquid water to its gaseous state, water vapor. This means that large bodies of water, such as lakes and rivers, must absorb enormous amounts of energy before they will evaporate and leave the life within them high and dry. This also means that humans can get rid of excess body heat by sweating because, when the water evaporates, it removes heat from the skin. On the other hand, a high heat of fusion means that this large amount of heat energy must be removed from liquid water before it changes from a liquid to its solid state, ice. Therefore, water can remain liquid and a suitable home for countless organisms long after the atmospheric temperature has reached the freezing point, 0°C (32°F).
3. Water has unusual density characteristics. Water is most dense at 4°C. As heat energy is lost from a body of water and its temperature falls below 4°C, its density decreases and this less dense, colder water is left on top. As the surface water reaches the freezing point and changes from its liquid to its solid phase, the molecules form new arrangements, which resemble a honeycomb. The spaces between the water molecules make the solid phase, ice, less dense than the water beneath and the ice floats. It is the surface water that freezes to a solid, covering the denser, liquid water and the living things in it.
4. Water's specific gravity is also an important property. Water has a density of 1 gram/cubic centimeter at 4°C. Anything with a higher density sinks in water, and anything with a lower density floats. Specific gravity is the ratio of the density of a substance to the density of water. Therefore, the specific gravity of water is 1.00. Any substance with a specific gravity less than 1.00 floats. If you mix water and gasoline, the gasoline (specific gravity of 0.75) floats to the top. People also vary in the specific gravity of their bodies. Some persons find it very easy to float in water, whereas others find it impossible. This is directly related to each person's specific gravity, which is a measure of the person's ratio of body fat to muscle and bone.
5. Water is considered the universal solvent, because most other chemicals can be dissolved in water. This means that wherever water goes—through the ground, in the air, or through an organism—it carries chemicals. Water in its purest form is even capable of acting as a solvent for oils.
6. Water comprises 50-60% of the bodies of most living things. This is important, because the chemical reactions of all living things occur in water.
7. Water vapor in the atmosphere is known as humidity, which changes with environmental conditions. The ratio of how much water vapor is in the air to how much water vapor could be in the air at a certain temperature is called relative humidity. Relative humidity is closely associated with your comfort. When the relative humidity and temperature are high, it is difficult to evaporate water from your skin, so it is more difficult to cool yourself and you are uncomfortably warm.
8. Water's specific gravity changes with its physical phase. Ice is also more likely to change from a solid to a liquid (melt) as conditions warm. If the specific gravity of water did not decrease when it freezes, then the ice would likely sink and never thaw. Our life-giving water would be trapped in ocean-sized icebergs. Ice also provides a protective layer for the life under the ice sheet.

2.8. CONCEPT REVIEW
21. Give an example of an ion exchange reaction.
22. What happens during an oxidation-reduction reaction?
23. Explain the difference between a reactant and a product.