CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
3. Organic Molecules—The Molecules of Life
3.3. Proteins
Proteins are polymers made up of monomers known as amino acids. An amino acid is a short carbon skeleton that contains an amino functional group (nitrogen and two hydrogens) attached on one end of the skeleton and a carboxylic acid group at the other end. In addition, the carbon skeleton may have one of several different “side chains” on it (figure 3.11). There are about 20 naturally occurring amino acids (Outlooks 3.2).
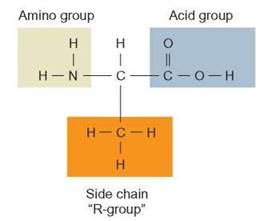
FIGURE 3.11. The Structure of an Amino Acid
An amino acid is composed of a short carbon skeleton with three functional groups attached: an amino group, a carboxylic acid group (acid group), and an additional group, the side chain that is different for each kind of amino acid.
The Structure of Proteins
Amino acids can bond together by dehydration synthesis reactions. When two amino acids undergo dehydration synthesis, the nitrogen of the amino group of one is bonded to the carbon of the acid group of another. This covalent bond is termed a peptide bond (figure 3.12).
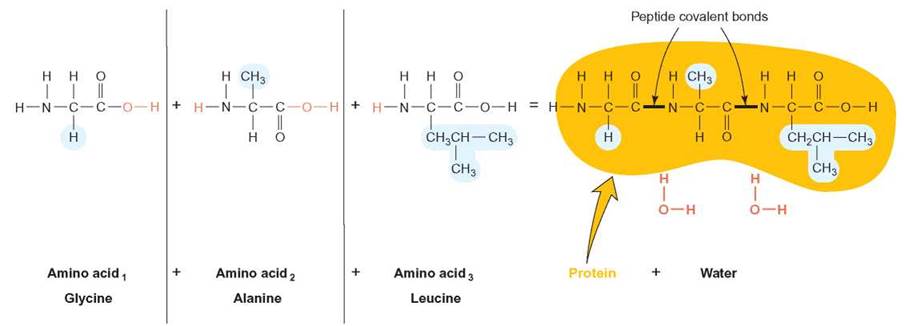
FIGURE 3.12. Peptide Covalent Bonds
The bond that results from a dehydration synthesis reaction between amino acids is called a peptide bond. This bond forms as a result of the removal of the hydrogen and hydroxide groups. In the formation of this bond, the nitrogen is bonded directly to the carbon. This tripeptide is made up of the amino acids glycine, alanine, and leucine. The side chain unique to each amino acid is shown in color.
You can imagine that, by using 20 different amino acids as building blocks, you can construct millions of combinations. Each of these combinations is termed a polypeptide chain. A specific polypeptide is composed of a specific sequence of amino acids bonded end to end. Protein molecules are composed of individual polypeptide chains or groups of chains forming a particular configuration. There are four levels, or degrees, of protein structure: primary, secondary, tertiary, and quaternary structure.
Primary Structure
A listing of the amino acids in their proper order within a particular polypeptide is its primary structure (figure 3.13a). The specific sequence of amino acids in a polypeptide is controlled by the genetic information of an organism. Genes are specific portions of DNA that serve as messages that tell the cell to link particular amino acids in a specific order; that is, they determine a polypeptide’s primary structure. The kinds of side chains on these amino acids influence the shape that the polypeptide forms, as well as its function.
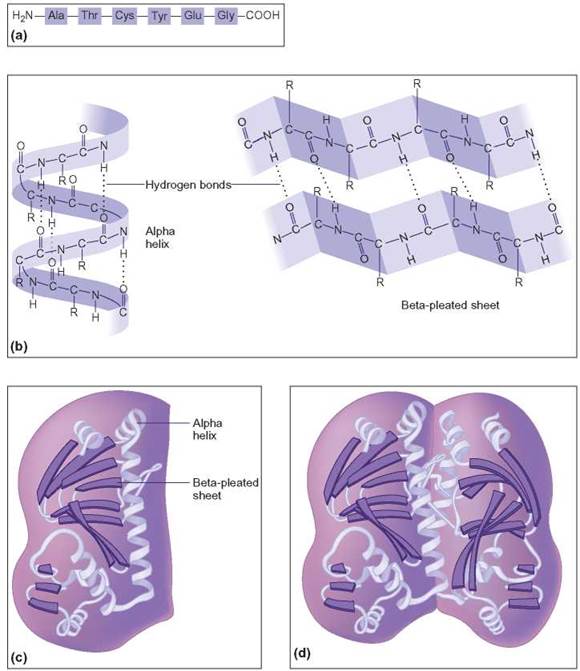
FIGURE 3.13. Levels of Protein Structure
(a) The primary structure of a protein molecule is simply a list of its amino acids in the order in which they occur, (b) This shows the secondary structure of protein molecules or how one part of the molecule is attached to another part of the same molecule. (c) If already folded parts of a single molecule attach at other places, the molecule is said to display tertiary (third-degree) structure, (d) Quaternary (fourth-degree) structure is displayed by molecules that are the result of two separate molecules (each with its own tertiary structure) combining into one large macromolecule.
Many polypeptides fold into globular shapes after they have been made as the molecule bends. Some of the amino acids in the chain can form bonds with their neighbors.
Secondary Structure
Some sequences of amino acids in a polypeptide are likely to twist, whereas other sequences remain straight. These twisted forms are referred to as the secondary structure of polypeptides (figure 3.13b). For example, at this secondary level some proteins (e.g., hair) take the form of an alpha helix: a shape like that of a coiled spring. Like most forms of secondary structure, the shape of the alpha helix is maintained by hydrogen bonds formed between different amino acid side chains at different locations within the polypeptide. Remember from chapter 2 that these forces of attraction do not form molecules but result in the orientation of one part of a molecule to another part within the same molecule. Other polypeptides form hydrogen bonds that cause them to make several flat folds that resemble a pleated skirt. This is called a beta-pleated sheet.
Tertiary Structure
It is possible for a single polypeptide to contain one or more coils and pleated sheets along its length. As a result, these different portions of the molecule can interact to form an even more complex globular structure. This occurs when the coils and pleated sheets twist and combine with each other. The complex, three-dimensional structure formed in this manner is the polypeptide’s tertiary (third-degree) structure (figure 3.13c). A good example of tertiary structure can be seen when a coiled electric cord becomes so twisted that it folds around and back on itself in several places. The oxygen-holding protein found in muscle cells, myoglobin, displays tertiary structure. It is composed of a single (153 amino acids) helical molecule folded back and bonded to itself in several places.
Quaternary Structure
Frequently, several different polypeptides, each with its own tertiary structure, twist around each other and chemically combine. The larger globular structure formed by these interacting polypeptides is referred to as the protein’s quaternary (fourth-degree) structure. The individual polypeptide chains are bonded to each other by the interactions of certain side chains, which can form disulfide covalent bonds (figure 3.13d). One group of proteins that form quaternary structure are immunoglobulins, also known as antibodies. They are involved in fighting infectious diseases such as the flu, the mumps, and chicken pox.
The Form and Function of Proteins
If a protein is to do its job effectively, it is vital that it has a particular three-dimensional shape. The protein’s shape can be altered by changing the order of the amino acids, which causes different cross-linkages to form. Figure 3.14 shows the importance of the protein’s three-dimensional shape, another emergent property.
For example, normal hemoglobin found in red blood cells consists of two kinds of polypeptide chains, called the alpha and beta chains. The beta chain is 146 amino acids long. If just one of these amino acids is replaced by a different one, the hemoglobin molecule may not function properly. A classic example of this results in a condition known as sickle-cell anemia. In this case, the sixth amino acid in the beta chain, which is normally glutamic acid, is replaced by valine. What might seem like a minor change causes the hemoglobin to fold differently. The red blood cells that contain this altered hemoglobin assume a sickle shape when the body is deprived of an adequate supply of oxygen.
In other situations, two proteins may have the same amino acid sequence but they do not have the same three-dimensional form. The difference in shape affects how they function. Mad cow disease (bovine spongiform encephalopathy—BSE), chronic wasting disease in deer and Creutzfeldt-Jakob disease (CJD) in humans are caused by rogue proteins called prions. The prions that cause these diseases have an amino acid sequence identical to a normal brain protein but are folded differently. The normal brain protein contains helical segments, whereas the corresponding segments of the prion protein are pleated sheets. When these malformed proteins enter the body, they cause normal proteins to fold differently. This causes the death of brain cells which causes loss of brain function and eventually death.

Changing environmental conditions also influence the shape of proteins. Energy in the form of heat or light may break the hydrogen bonds within protein molecules. When this occurs, the chemical and physical properties of the protein are changed and the protein is said to be denatured. (Keep in mind that a protein is a molecule, not a living thing, and therefore cannot be “killed.”) A common example of this occurs when the gelatinous, clear portion of an egg is cooked and the protein changes to a white solid. Some medications, such as insulin, are proteins and must be protected from denaturation so as not to lose their effectiveness. For protection, such medications may be stored in brown bottles to protect them from light or may be kept under refrigeration to protect them from heat.
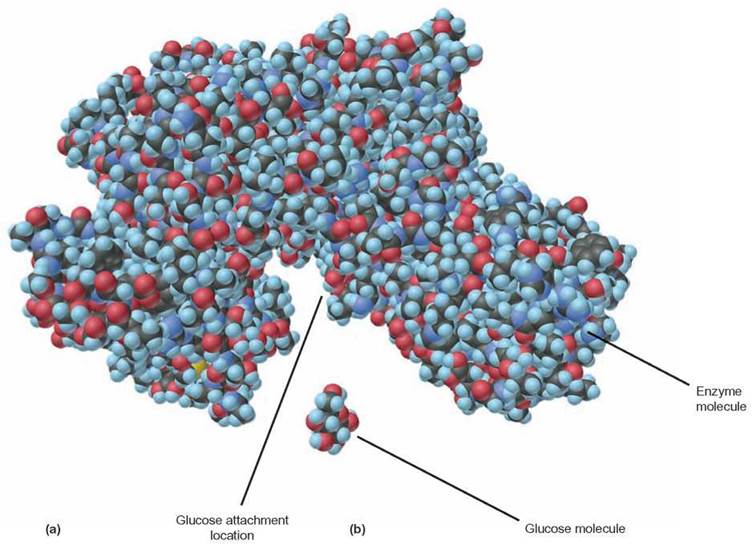
FIGURE 3.14. The Three-Dimensional Shape of Proteins
(a) The specific arrangement of amino acids in a polypeptide allows the amino acid side chains to bond with other amino acids. These stabilizing interactions result in a protein with a specific surface geometry. The large molecule pictured is an enzyme, a protein molecule that acts as a tool to speed the rate of a chemical reaction. Without having this specific shape, this protein would not be able to attach to the smaller (b) glucose molecule and chemically change the glucose molecule.
What Do Proteins Do?
There are thousands of kinds of proteins in living things, and they can be placed into three categories based on the functions they serve. Structural proteins are important for maintaining the shape of cells and organisms. The proteins that make up cell membranes, muscle cells, t endons, and blood cells are examples of structural proteins. The protein collagen, found throughout the human body, gives tissues shape, support, and strength.
Regulator proteins, the second category of proteins, help determine what activities will occur in the organism. Regulator proteins include enzymes, chaperones, and some hormones. These molecules help control the chemical activities of cells and organisms. Enzymes speed the rate of chemical reactions and will be discussed in detail in chapter 5. Some examples of enzymes are the digestive enzymes in the intestinal tract. The job of a chaperone is to help other proteins fold into their proper shape. For example, some chaperones act as heat shock proteins—that is, they help repair heat-damaged proteins. Three hormones that are regulator proteins are insulin, glucagon, and oxytocin. Insulin and glucagon, produced by different cells of your pancreas, control the amount of glucose in the blood. If insulin production is too low, or if the molecules are improperly constructed, glucose molecules are not removed from the bloodstream at a fast enough rate. The excess sugar is then eliminated in the urine. Other symptoms of excess “sugar” in the blood include excessive thirst and even loss of consciousness. When blood sugar is low, glucagon is released from the pancreas to stimulate the breakdown of glycogen. The disease caused by improperly functioning insulin is known as diabetes. Oxytocin, a third protein hormone, stimulates the contraction of the uterus during childbirth. It is an organic molecule that has been produced artificially (e.g., Pitocin), and used by physicians to induce labor.
Carrier proteins are the third category. These pick up molecules at one place and transport them to another. For example, proteins from your food attach to cholesterol circulating in your blood to form lipoproteins, which are carried from the digestive system throughout the body.
OUTLOOKS 3.2
So You Don't Eat Meat! How to Stay Healthy
Humans require nine amino acids in their diet: threonine, tryptophan, methionine, lysine, phenylalanine, isoleucine, valine, histidine, and leucine. They are called essential amino acids because the body is not able to manufacture them. The body uses these essential amino acids in the synthesis of the proteins required for good health. For example, the sulfur-containing amino acid methionine is essential for the absorption and transportation of the elements selenium and potassium. It also prevents excess fat buildup in the liver, and it traps heavy metals, such as lead, cadmium, and mercury, bonding with them so that they can be excreted from the body. Because essential amino acids are not readily available in most plant proteins, they are most easily acquired through meat, fish, and dairy products.
If this is the case, how do people avoid nutritional deficiency if for economic or personal reasons do not eat meat, poultry, fish, meat products, dairy products, and honey? People who exclude all animal products from their diet are called vegans. Those who include only milk are called lacto-vegetarians; those who include eggs are ovo-vegetarians, and those who include both eggs and milk are lacto-ovo-vegetarians. For anyone but a true vegan, the essential amino acids can be provided in even a small amount of milk and eggs. True vegans can get all their essential amino acids by eating certain combinations of plants or plant products. Even though there are certain plants that contain all of these amino acids (soy, lupin, hempseed, chia seed, amaranth, buckwheat, and quinoa) most plants contain one or more of the essential amino acids. However, by eating the right combination of different plants, it is possible to get all the essential amino acids in one meal. These combinations are known as complementary foods.

3.3. CONCEPT REVIEW
8. How do the primary, secondary, tertiary, and quaternary structures of proteins differ?
9. List the three categories of proteins and describe their functions.