CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
3. Organic Molecules—The Molecules of Life
3.5. Lipids
There are three main types of lipids: true fats (e.g., olive oil), phospholipids (the primary component of cell membranes), and steroids (some hormones). In general, lipids are large, nonpolar (do not have a positive end and a negative end), organic molecules that do not dissolve easily in polar solvents, such as water. For example, nonpolar vegetable oil molecules do not dissolve in polar water molecules; they separate. Molecules in this group are generally called fats. They are not polymers, as are carbohydrates, proteins, and nucleic acids. Fats are soluble in nonpolar substances, such as ether or acetone. Just like carbohydrates, lipids are composed of carbon, hydrogen, and oxygen. They do not, however, have the same ratio of carbon, hydrogen, and oxygen in their empirical formulas. Lipids generally have very small amounts of oxygen, compared with the amounts of carbon and hydrogen. Simple lipids are not able to be broken down into smaller, similar subunits. Complex lipids can be hydrolyzed into smaller, similar units.
True (Neutral) Fats
True (neutral) fats are important, complex organic molecules that are used to provide energy, among other things. The building blocks of a fat are a glycerol molecule and fatty acids. Glycerol is a carbon skeleton that has three alcohol groups attached to it. Its chemical formula is C3H5(OH)3. At room temperature, glycerol looks like clear, lightweight oil. It is used under the name glycerin as an additive to many cosmetics to make them smooth and easy to spread.
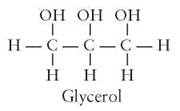
A fatty acid is a long-chain carbon skeleton that has a carboxyl functional group. True (neutral) fat molecules that form from a glycerol molecule and 3 attached fatty acids are called triglycerides; those with 2 fatty acids are diglycerides; those with 1 fatty acid are monoglyceride (figure 3.20). Triglycerides account for about 95% of the fat stored in human tissue.
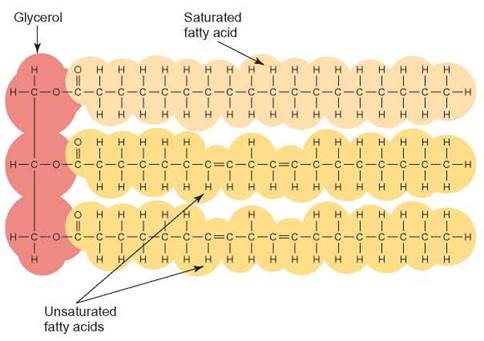
FIGURE 3.20. A Triglyceride Molecule
The arrangement of the 3 fatty acids (yellow) attached to a glycerol molecule (red) is typical of the formation of a fat. The structural formula of the fat appears to be very cluttered until you dissect the fatty acids from the glycerol; then, it becomes much more manageable. This example of a triglyceride contains a glycerol molecule, 2 unsaturated fatty acids (linoleic acid), and a third saturated fatty acid (stearic acid).
If the carbon skeleton of a fatty acid molecule has as much hydrogen bonded to it as possible, it is called saturated. The saturated fatty acid shown in figure 3.21a is stearic acid, a component of solid meat fats, such as bacon fat. Notice that, at every point in this structure, the carbon has as much hydrogen as it can hold. Saturated fats are generally found in animal tissues—they tend to be solids at room temperatures. Some other examples of saturated fats are butter, whale blubber, suet, lard, and fats associated with such meats as steak and pork chops.
A fatty acid is said to be unsaturated if the carbons are double-bonded to each other at one or more points. The occurrence of a double bond in a fatty acid is indicated by the Greek letter m (omega), followed by a number indicating the location of the first double bond in the molecule. Counting begins from the omega end, that is the end farthest from the carboxylic acid functional group. Oleic acid, one of the fatty acids found in olive oil, is comprised of 18 carbons with a single double bond between carbons 9 and 10. Therefore, it is chemically designated C18:1ω9 and is a monounsaturated fatty acid. This fatty acid is commonly referred to as an omega-9 fatty acid. The unsaturated fatty acid in figure 3.21b is linoleic acid, a component of sunflower and safflower oils. Notice that there are two double bonds between the carbons and fewer hydrogens than in the saturated fatty acid. Linoleic acid is chemically a polyunsaturated fatty acid with two double bonds and is designated C18:2ω6, an omega-6 fatty acid. This indicates that the first double bond of this 18-carbon molecule is between carbons 6 and 7. Because the human body cannot make this fatty acid and must be taken in as a part of the diet, it is called an essential fatty acid. The other essential fatty acid, linolenic acid (figure 3.21c ), is C18:3ω3; it has three double bonds. This fatty acid is commonly referred to as an omega-3 fatty acid. One key function of these essential fatty acids is the synthesis of the prostaglandin hormones that are necessary in controlling cell growth and specialization. Many food manufacturers are now adding omega-3 fatty acids to their products, based on evidence that these reduce the risk of cardiovascular disease.
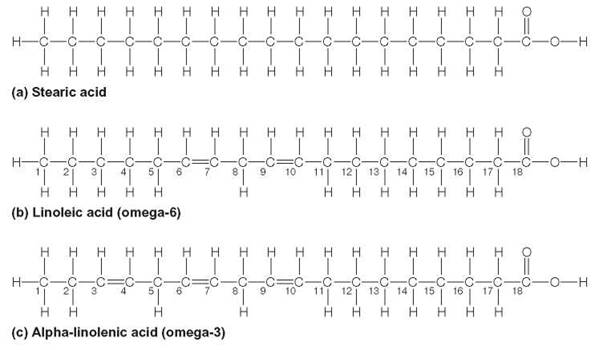
FIGURE 3.21. Structure of Saturated and Unsaturated Fatty Acids
(a) Stearic acid is an example of a saturated fatty acid. (b) Linoleic acid is an example of an unsaturated fatty acid. It is technically an omega-6 fatty acid, because the first double bond occurs at carbon number 6. (c) An omega-3 fatty acid, linolenic acid. Both linoleic and linolenic acids are essential fatty acids for humans.
|
Sources of Omega-3 Fatty Acids |
Sources of Omega-6 Fatty Acids |
|
Certain fish oils |
Corn oil |
|
(salmon, sardines, herring) |
Peanut oil |
|
Flaxseed oil |
Cottonseed oil |
|
Soybeans |
Soybean oil |
|
Soybean oil |
Sesame oil |
|
Walnuts |
Sunflower oil |
|
Canola oil Green, leafy vegetables |
Safflower oil |
Many unsaturated fat molecules are plant fats or oils— they are usually liquids at room temperatures. Peanut, corn, and olive oils are mixtures of true fats and are considered unsaturated because they have double bonds between the carbons of the carbon skeleton (Outlooks 3.3). A polyunsaturated fatty acid is one that has several double bonds in the carbon skeleton. When glycerol and 3 fatty acids are combined by three dehydration synthesis reactions, a fat is formed. That dehydration synthesis is almost exactly the same as the reaction that causes simple sugars to bond.
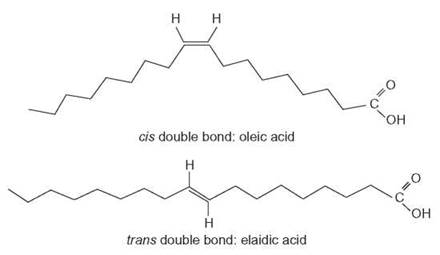
In nature, most unsaturated fatty acids have hydrogen atoms that are on the same side of the double-bonded carbons. These are called cis fatty acids. If the hydrogens are on opposite sides of the double bonds, they are called trans fatty acids. Trans fatty acids are found naturally in grazing animals, such as cattle and sheep. Therefore, humans acquire them in their diets in the form of meat and dairy products. Trans fatty acids are also formed during the hydrogenation of either vegetable or fish oils. The hydrogenation process breaks the double bonds in the fatty acid chain and adds more hydrogen atoms. This can change the liquid to a solid. Many product labels list the term hydrogenated. This process extends shelf life and allows producers to convert oils to other solids, such as margarine.
Clinical studies have shown that trans fatty acids tend to raise total blood cholesterol levels, but less than the more saturated fatty acids. Dietary trans fatty acids also tend to raise the so-called bad fats (low-density lipoproteins, LDLs) and lower the so-called good fats (high-density lipoproteins, HDLs) when consumed instead of cis fatty acids. Scientific evidence indicates that this increases the risk for heart disease (Outlooks 3.4). Because of the importance of trans fatty acids in cardiovascular health, the U.S. Department of Health and Human Services (HHS) requires that the amount of trans fatty acids in foods be stated under the listed amount of saturated fat. The HHS suggests that a person eat no more than 20 grams of saturated fat a day (about 10% of total calories), including trans fatty acids.
Fats are important molecules for storing energy. There is more than twice as much energy in a gram of fat as in a gram of sugar—9 Calories versus 4 Calories. This is important to an organism, because fats can be stored in a relatively small space yet yield a high amount of energy. Fats in animals also provide protection from heat loss; some animals have an insulating layer of fat under the skin. The thick layer of blubber in whales, walruses, and seals prevents the loss of internal body heat to the cold, watery environment in which they live. The same layer of fat and the fat deposits around some organs (such as the eyes, kidneys and heart) cushion the organs from physical damage.
Phospholipids
Phospholipids are a class of complex, water-insoluble organic molecules that resemble neutral fats but have a phosphate- containing group (PO4) in their structure (figure 3.22). Phospholipids are important because they are a major component of cell membranes. Without these lipids, the cell contents would not be separated from the exterior environment. Some of the phospholipids are better known as lecithins. Found in cell membranes, lecithins help in the emulsification of fats—that is, they help separate large portions of fat into smaller units. This allows the fat to mix with other materials. Lecithins are added to many types of food for this purpose (chocolate bars, for example). Some people take lecithin as nutritional supplements because they believe it leads to healthier hair and better reasoning ability. But once inside the intestines, lecithins are destroyed by enzymes, just as any other phospholipid is. Because phospholipids are essential components of the membranes of all cells, they will also be examined in chapter 4.
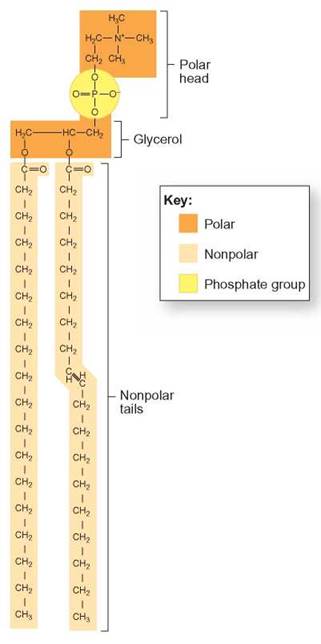
FIGURE 3.22. A Phospholipid Molecule
This molecule is similar to a fat but has a phosphate group (yellow) in its structure. You can think of phospholipid molecules as having a “head” with two strings dangling down. The head portion is the glycerol and phosphate group, which is polar and soluble in water. The strings are the fatty acid segments of the molecule and are nonpolar and not water-soluble.
OUTLOOKS 3.3
What Happens When You Deep-Fry Food?
You have probably noticed that deep-fried foods are covered with some sort of breading or batter. The coating forms a barrier and protects the underlying food (e.g., chicken or cheese) from being burned when it is placed in the hot oil. This means that your food is being cooked indirectly, not directly, as you would cook a hot dog on a grill. Deep-fried foods cook quickly because fats and oils can be heated to higher temperatures before they boil. Cooking at these higher temperatures keeps the cooking fats and oils from getting inside the food. If the fat or oil is not hot enough, the food will be greasy. If the oil is too hot, the coating will burn before the food inside can be cooked the way you like it. Even though there is some variation in oil temperature due to the thickness and kind of food being deep-fried, the general rule is to have your oil at 375°F (190°C). The best oils to use for stir-frying are those that can be heated to a high temperature without smoking (e.g., canola, peanut, or grape-seed oil).

OUTLOOKS 3.4
Fat and Your Diet
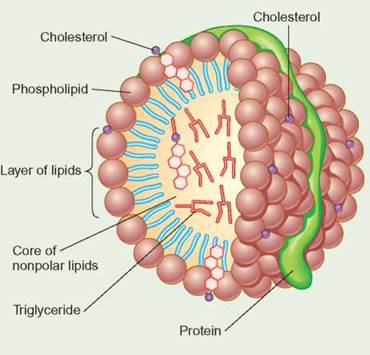
When triglycerides are eaten in fat-containing foods, digestive enzymes hydrolyze them into glycerol and fatty acids. These molecules are absorbed by the intestinal tract and coated with protein to form lipoprotein, as shown in the accompanying diagram. The combination allows the fat to dissolve better in the blood, so that it can move throughout the body in the circulatory system.
Five types of lipoproteins found in the body are
1. Chylomicrons
2. Very-low-density lipoproteins (VLDLs)
3. Low-density lipoproteins (LDLs)
4. High-density lipoproteins (HDLs)
5. Lipoprotein a [Lp(a)]
Chylomicrons are very large particles formed in the intestine; they are 80-95% triglycerides. As the chylomicrons circulate through the body, cells remove the triglycerides in order to make sex hormones, store energy, and build new cell parts. When most of the triglycerides have been removed, the remaining portions of the chylomicrons are harmlessly destroyed.
The VLDLs and LDLs are formed in the liver. VLDLs contain all types of lipid, protein, and 10-15% cholesterol, whereas the LDLs are about 50% cholesterol. As with the chylomicrons, the body uses these molecules for the fats they contain. However, in some people, high levels of LDL and lipoprotein a [Lp(a)] in the blood are associated with atherosclerosis, stroke, and heart attack. It appears that saturated fat disrupts the clearance of LDLs from the bloodstream. Thus, while in the blood, LDLs may stick to the insides of the vessels, forming deposits, which restrict blood flow and contribute to high blood pressure, strokes, and heart attacks. Even though they are 30% cholesterol, a high level of HDLs (made in the intestine), compared with LDLs and [Lp(a)], is associated with a lower risk for atherosclerosis. One way to reduce the risk of this disease is to lower your intake of LDLs and [Lp(a)]. This can be done by reducing your consumption of saturated fats. An easy way to remember the association between LDLs and HDLs is "L = Lethal" and "H = Healthy" or "Low = Bad" and "High = Good." The federal government's cholesterol guidelines recommend that all adults get a full lipoprotein profile (total cholesterol, HDL, LDL, and triglycerides) once every five years.
They also recommend a sliding scale for desirable LDL levels; however, recent studies suggest that one's LDL level should be as low as possible.
Taking certain drugs is one way to control the level of lipoproteins in the body. Statins are a group of medicines (e.g., simvastatin, atorvastatin) that work by blocking the action of enzymes that control the rate of cholesterol production in the body. Their use can lower cholesterol 20-60%. They also increase the liver's ability to remove low-density lipoproteins. An additional benefit is a slight increase in high-density lipoproteins and a decrease in triglycerides.
Total cholesterol goal values:
• 75-169 mg/dL (milligram per deciliter) for those age 20 and younger
• 100-199 mg/dL for those over age 21
Low-density lipoprotein (LDL) goal values:
• Less than 70 mg/dL for those with heart or blood vessel disease and for other patients at very high risk of heart disease (those with metabolic syndrome)
• Less than 100 mg/dL for high-risk patients (for example, some patients who have diabetes or multiple heart disease risk factors)
• Less than 130 mg/dL otherwise
Very-low-density lipoprotein (VLDL) goal values:
• Less than 40 mg/dL
High-density lipoprotein (HDL) goal value:
• Greater than 45 mg/dL (the higher the better)
Triglyceride goal value:
• Less than 150 mg/dL
Steroids
Steroids, another group of lipid molecules, are characterized by their arrangement of interlocking rings of carbon. Many steroid molecules are sex hormones. Some of them regulate reproductive processes, such as egg and sperm production (see chapter 27); others regulate such things as salt concentration in the blood. Figure 3.23 illustrates some of the steroid compounds, such as testosterone and progesterone, that are typically manufactured by organisms and also in the laboratory as pharmaceuticals. We have already mentioned one steroid molecule: cholesterol. Serum cholesterol (the kind found in your blood and associated with lipoproteins) has been implicated in many cases of atherosclerosis. However, your body makes this steroid for use as a component of cell membranes. Cholesterol is necessary for the manufacture of vitamin D, which assists in the proper development of bones and teeth. Cholesterol molecules in your skin react with ultraviolet light to produce vitamin D. The body also uses it to make bile acids. These products of the liver are channeled into your intestine to emulsify fats.
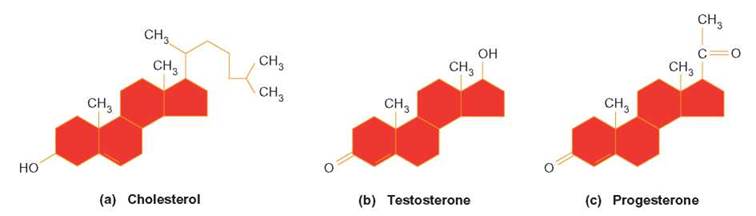
FIGURE 3.23. Steroids
(a) Cholesterol is produced by the human body and is found in cell membranes. (b) Testosterone increases during puberty, causing the male sex organs to mature. (c) Progesterone is a female sex hormone produced by the ovaries and placenta. Notice the slight structural differences among these molecules.
Regulating the amount of cholesterol in the body to prevent its negative effects can be difficult, because we consume it in our diet. Recall that diets high in saturated fats increase the risk for diseases such as atherosclerosis. By watching your diet, you can reduce the amount of cholesterol in your blood serum by about 20%, as much as taking a cholesterol-lowering drug. Therefore, it is best to eat foods that are low in cholesterol. Because many foods that claim to be low- or no-cholesterol have high levels of saturated fats, they should also be avoided in order to control serum cholesterol levels.
3.5. CONCEPT REVIEW
12. Describe three kinds of lipids.
13. What is meant by HDL, LDL, and VLDL? Where are they found? How do they relate to disease?
Summary
The chemistry of living things involves a variety of large and complex molecules. This chemistry is based on the carbon atom and the fact that carbon atoms can connect to form long chains or rings. This results in a vast array of molecules. The structure of each molecule is related to its function. Changes in the structure may result in abnormal functions, called disease. Some of the most common types of organic molecules found in living things are carbohydrates, lipids, proteins, and nucleic acids. Table 3.2 summarizes the major types of biologically important organic molecules and how they function in living things.
TABLE 3.2. Types of Organic Molecules Found in Living Things
|
Type of Organic Molecule |
Basic Subunit |
Function |
Examples |
|
Carbohydrates |
Simple sugar/monosaccharides |
Provide energy |
Glucose Cellulose, starch, glycogen |
|
Proteins |
Amino acid |
Maintain the shape |
Cell membrane |
|
of cells and parts |
Hair |
||
|
of organisms |
Antibodies Clotting factors Enzymes Muscle |
||
|
As enzymes, regulate the rate of cell reactions |
Ptyalin in the mouth |
||
|
As hormones, affect physiological activity, such as growth or metabolism |
Insulin |
||
|
Serve as molecules that carry other molecules to distant places in the body |
Lipoproteins, hemoglobin |
||
|
Nucleic acids |
Nucleotide |
Store and transfer genetic information that controls the cell |
DNA |
|
Are involved in protein synthesis |
RNA |
||
|
Lipids 1. Fats |
Glycerol and fatty acids |
Provide energy |
Lard |
|
Provide insulation |
Olive oil |
||
|
Serve as shock absorbers |
Linseed oil Tallow |
||
|
2. Phospholipids |
Glycerol, fatty acids, and phosphate group |
Form a major component of the structure of the cell membrane |
Cell membrane |
|
3. Steroids and prostaglandins |
Structure of interlocking carbon rings |
Often serve as hormones that control the body processes |
Testosterone |
|
Vitamin D |
|||
|
Cholesterol |
Basic Review
1. A(n) _____ formula indicates the number of each kind of atom within a molecule.
2. The name of this functional group, -NH2, is
a. amino.
b. alcohol.
c. carboxylic acid.
d. aldehyde.
3. Molecules that have the same empirical formula but different structural formulas are called
a. ions.
b. isomers.
c. icons.
d. radicals.
4. Which is not a macromolecule?
a. carbohydrate
b. protein
c. sulfuric acid
d. steroid
5. Which is not a polymer?
a. insulin
b. DNA
c. fatty acid
d. RNA
6. The monomer of a complex carbohydrate is
a. an amino acid.
b. a monosaccharide.
c. a nucleotide.
d. a fatty acid.
7. When blood sugar is low, this protein hormone is released from the pancreas to stimulate the breakdown of glycogen.
a. glucagon
b. estrogen
c. oxytocin
d. glycine
8. Mad cow disease is caused by a
a. virus.
b. bacteria.
c. prion.
d. hormone.
9. _____ occurs when the shape of a macromolecule altered as a result of exposure to excess heat or light.
10. By watching your diet it is possible to reduce the amount of cholesterol in your blood serum by about _____ %.
11. Organic compounds that do not have their proper _____ are not likely to function properly in a cell.
12. The genetic material of many organisms belongs to which major category of organic compounds?
a. carbohydrate
b. protein
c. nucleic acid
d. lipid
13. “Oh no! The doctor said my cholesterol was too high.” I guess I’ll have to keep an eye on the amount of _____ I eat.
14. Many types of birth control pills contain compounds called _____, one of which is estrogen.
15. Beta-pleated sheets found in protein molecules is an example of _____ structures.
Answers
1. empirical 2. a 3. b 4. c 5. c 6. b 7. a 8. c 9. Denaturation 10. 20% 11. 3-D shape 12. c 13. lipids 14. steroids 15. Secondary
Thinking Critically
Archaeologists, anthropologists, chemists, biologists, and healthcare professionals agree that the drinking of alcohol dates back thousands of years. Evidence also exists that this practice has occurred in most cultures around the world. Use the Internet to search out answers to the following questions:
1. What is the earliest date for which there is evidence for the production of ethyl alcohol?
2. In which culture did alcohol drinking first occur?
3. What is the molecular formula and structure of ethanol?
4. Do alcohol and water mix?
5. How much ethanol is consumed in the form of beverages in the United States each year?
6. What is the legal limit to be considered intoxicated in your state?
7. How is the legal limit in your state measured?
8. Why is there a tax on alcoholic beverages?
9. How do the negative effects of drinking alcohol compare for men and women?
10. Have researchers demonstrated any beneficial effects of drinking alcohol?
Compare what you thought you knew to what you can now support with scientific evidence.