CONCEPTS IN BIOLOGY
PART II. CORNERSTONES: CHEMISTRY, CELLS, AND METABOLISM
5. Enzymes, Coenzymes, and Energy
5.3. Cofactors, Coenzymes, and Vitamins
Certain enzymes need an additional molecule to help them function. Cofactors are inorganic ions or organic molecules that serve as enzyme helpers. Ions such as zinc, iron, and magnesium assist enzymes in their performance as catalysts. These ions chemically combine with the enzyme. A coenzyme is an organic molecule that functions as a cofactor. They are made from molecules such as certain amino acids, nitrogenous bases, and vitamins. Vitamins are a group of unrelated organic molecules used in the making of certain coenzymes; they also play a role in regulating gene action. Vitamins are either water-soluble (e.g., vitamin B complex) or fat-soluble (e.g., vitamin A). For example, the vitamin riboflavin (B2) is metabolized by cells and becomes flavin adenine dinucleotide (FAD). The vitamin niacin is metabolized by cells and becomes nicotinamide adenine dinucleotide (NAD+). Coenzymes such as NAD+ and FAD are used to carry electrons to and from many kinds of oxidation-reduction reactions. NAD+, FAD, and other coenzymes are bonded only temporarily to their enzymes and therefore can assist various enzymes in their reaction. Vitamins are required in your diet because cells are not able to manufacture these molecules (figure 5.4).
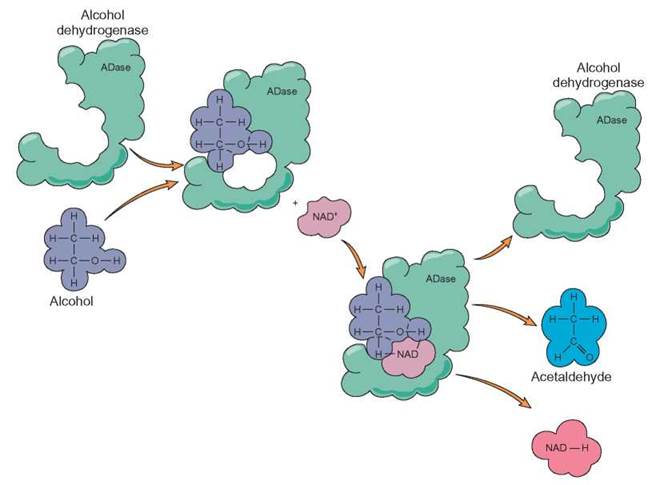
FIGURE 5.4. The Role of Coenzymes
NAD+ is a coenzyme that works with the enzyme alcohol dehydrogenase (ADase) during the breakdown of alcohol in the liver. This coenzyme helps by carrying the hydrogen from the alcohol molecule after it is removed by the enzyme. Notice that the hydrogen on the alcohol is picked up by the NAD+. The use of the coenzyme NAD+ makes the enzyme function more efficiently, because one of the end products of this reaction (hydrogen) is removed from the reaction site. If anything interferes with the formation of NAD+ (i.e., niacin deficiency or high temperatures), the breakdown of alcohol becomes less efficient, allowing the alcohol to cause cell damage.
Another vitamin, pantothenic acid, becomes coenzyme A (CoA), a molecule used to carry a specific 2-carbon functional group, acetyl (-COCH3), generated in one reaction to another reaction. Like enzymes, the cell uses inorganic cofactors, coenzymes, and vitamins repeatedly until these molecules are worn out and destroyed. Coenzymes play vital roles in metabolism. Without them, most cellular reactions would come to an end and the cell would die.
5.3. CONCEPT REVIEW
6. How do enzymes, coenzymes, and vitamins relate to one another?
7. Why must vitamins be a part of the human diet?
8. What is the relationship between vitamins and coenzymes?