Biology of Humans
20. Genetics and Human Inheritance
In the previous chapter we considered how chromosomes and the genes they carry are distributed during cell division. We learned that mitosis is necessary for growth and repair of body tissues. We also learned that, in humans, meiosis is necessary to prepare the gametes needed for reproduction. In this chapter, we will see that the genes we receive at the moment of conception influence all the biochemical reactions taking place inside our cells, our susceptibility to disease, our behavior patterns, and even our life span. Our environment is also an important influence, but our genes provide the basic blueprint for our possibilities and limitations. In this chapter, you will learn more about the genetic foundation that has been so important in shaping who you are. You will learn how heritable traits are passed to new generations and how to predict the distribution of traits from one generation to the next.
Principles of Inheritance
The understanding of meiosis gained in Chapter 19 helps us answer important questions about inheritance: Why is your brother the only sibling with Mom's freckles and widow's peak (a hairline that comes to a point on the forehead)? How can you have blue eyes when both your parents are brown eyed? Will you be bald at 40, like Dad? Let's delve deeper into how chromosomes, meiosis, and heredity are related.
Before beginning, you may want to review some of the terms that were introduced in Chapter 19 and that are summarized in Figure 20.1. Recall that somatic (body) cells have a pair of every chromosome. Thus, human cells have 23 pairs of chromosomes.
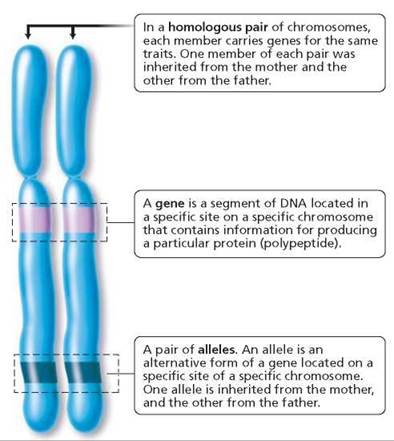
FIGURE 20.1. Important terms in genetics
One member of each pair was inherited from the female parent and the other from the male parent. The chromosomes that carry genes for the same traits are a homologous pair of chromosomes, or homologues. Chromosomes are made of DNA and protein. Certain segments of the DNA of each chromosome function as genes. A gene directs the synthesis of a specific protein that can play either a structural or a functional role in the cell.1 (In some cases, the gene directs the synthesis of a polypeptide that forms part of a protein.) In this way, the gene-determined protein can influence whether a certain trait, or characteristic, will develop. For instance, the formation of your brother's widow's peak was directed by a protein coded for by a gene that he inherited from Mom. Genes for the same trait are found at the same specific location on homologous chromosomes.
· Would you become a risk taker if you knew you had a genetic disease that causes death in middle age?
Different forms of a gene are called alleles. Alleles produce different versions of the trait they determine. For example, there is a gene that determines whether freckles will form. One allele of this gene causes freckles to form;the other does not. Somatic cells carry two alleles for each gene, one allele on each homologous chromosome. If a person has at least one allele for freckles, melanin will be deposited, and freckles will form. If neither homologue bears the freckle allele that leads to melanin deposition, for example, the person will not have freckles.
Individuals with two copies of the same allele of a gene are said to be homozygous (homo, same; zygo, joined together) for that trait. Those with different alleles of a given gene are said to be heterozygous (hetero, different; zygo, joined together). When the effects of a certain allele can be detected, regardless of whether an alternative allele is also present, the allele is described as a dominant allele. Dimples and freckles are human traits dictated by dominant alleles. An allele whose effects are masked in the heterozygous condition is described as a recessive allele. Because of this masking, only homozygous recessive alleles are expressed. Conditions such as cystic fibrosis, in which excessive mucus production impairs lung and pancreatic function; and albinism, in which the pigment melanin is missing in the hair, skin, and eyes, result from recessive alleles. It is customary to designate a dominant allele with a capital letter and a recessive allele with a lowercase letter—A and a, for example.
We observe the dominant form of the trait whether the individual is homozygous dominant (AA) or heterozygous (Aa) for that trait. As a consequence, we cannot always tell exactly which alleles are present. It is important to remember that an individual's genetic makeup is not always revealed by the individual's appearance. A genotype is the precise set of alleles a person possesses for a given trait or traits. It tells us whether the individual is homozygous or heterozygous for a given gene. A phenotype, on the other hand, is the observable physical trait or traits of an individual. Figure 20.2 shows the genotype or genotypes for several phenotypes in humans (for example, the freckled phenotype has two genotypes: FF and Ff). Table 20.1 summarizes terms commonly used in genetics as they apply to the inheritance of freckles, which is determined by a dominant allele.

FIGURE 20.2. Genotypes for selected human phenotypes
TABLE 20.1. Review of Common Terms in Genetics
|
Genotypes: The Alleles That Are Present |
Description |
Phenotype: The Observable Trait (Examples) |
|
FF |
Homozygous dominant: • Two dominant alleles present. • Dominant phenotype expressed. |
Freckles |
|
Ff |
Heterozygous: • Different alleles present. • Dominant phenotype expressed. |
Freckles |
|
ff |
Homozygous recessive: • Two recessive alleles present. • Recessive phenotype expressed. |
No freckles |
Gamete Formation
Recall from Chapter 19 that the members of each homologous pair of chromosomes segregate during meiosis I, with each homologue going to a different daughter cell. Thus, an egg or a sperm has only one member of each homologous pair. Consider what this fact means for inheritance. Because the alleles for each gene segregate during gamete formation, half the gametes bear one allele, and half bear the other. This principle, known as the law of segregation, explains how, for every gene in our chromosomes, one of the alleles comes from our mother and one comes from our father.
Furthermore, each pair of homologous chromosomes lines up at the midline of the cell during meiosis I independently of the other pairs. The orientation of the paternal and maternal homologues relative to the poles of the cell (that is, with regard to which homologue is closer to which pole) is entirely random. What this fact means for inheritance is that pairs of alleles for genes on different chromosomes segregate into gametes independently. This principle, known as the law of independent assortment, explains why the mixture of alleles that came from the mother and alleles that came from the father is different in every gamete.
Mendelian Genetics
During the nineteenth century, Gregor Mendel, a monk who grew up in a region of what was then Austria and is now part of the Czech Republic, worked out much of what we know today about the laws of heredity by performing specific crosses of pea plants. Although Mendel knew nothing about chromosomes, his ideas about the inheritance of traits are consistent with what we now know about the movement of chromosomes during meiosis. Mendel's ideas are used today to predict the outcome of hereditary crosses.
One-trait crosses. We begin our discussion by using Mendel's ideas to consider the inheritance of a single trait, using the example of freckles, a characteristic determined by a dominant allele. Suppose a freckled female who is homozygous dominant (FF) had a child with a homozygous recessive male with no freckles (ff; Figure 20.3). The following sequence of steps allows us to predict the degree of likelihood that their child will have freckles.
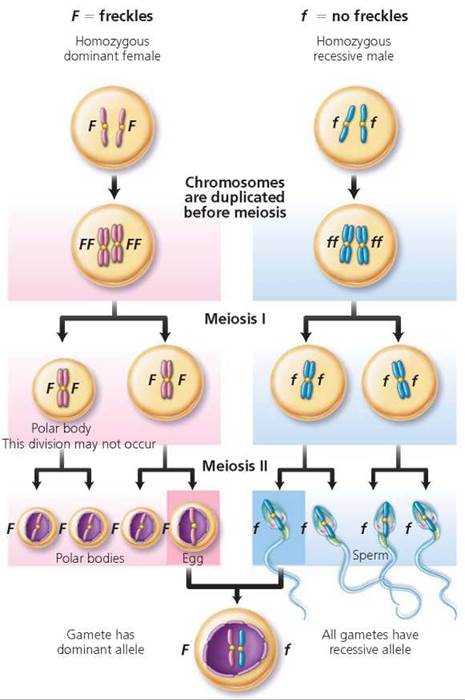
FIGURE 20.3. Gamete formation by a female who is homozygous dominant for freckles (FF), a male who is homozygous recessive for no freckles (ff), and the heterozygous (Ff) individual resulting from the union of these gametes.
1. Identify the possible gametes that each parent can produce. We know that alleles segregate during meiosis, but in this example, both parents are homozygous. Therefore, each can produce only one type of gamete (as far as freckles are concerned). The female produces gametes (eggs) with the dominant allele (F), and the male produces gametes (sperm) with the recessive allele (f).
2. Use a Punnett square to determine the probable outcome of the genetic cross. A Punnett square is a diagram that is used to predict the genetic makeup of the offspring of individuals of particular genotypes. In a Punnett square, columns are set up and labeled to represent each of the possible gametes of one parent, let's say the father (Figure 20.4). Remember, the alleles of each gene segregate during meiosis. In this case, then, there would be two columns to represent the gametes of the male without freckles, each labeled with a recessive allele, f. In a similar manner, rows are established across the columns and labeled to represent all the possible gametes formed by the other parent, in this case, the mother with freckles. There would be two rows in this Punnett square, each labeled F. Each square in the resulting table is then filled in by combining the labels of the corresponding rows and columns. The squares represent the possible offspring of these parents. In this first case, all the children would have freckles and would be heterozygous for the trait (Ff).
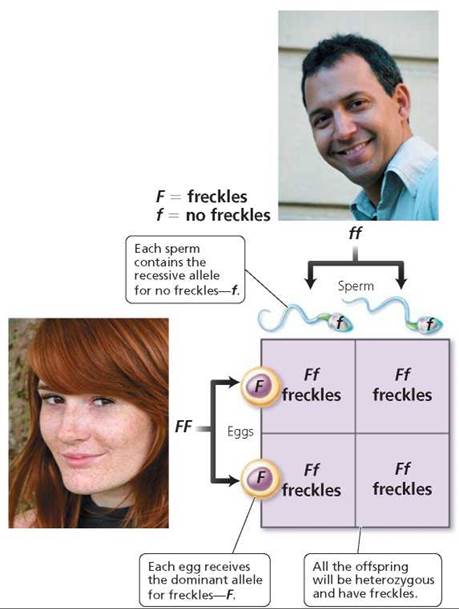
FIGURE 20.4. This Punnett square illustrates the probable offspring from a cross between a homozygous dominant female with freckles (FF) and a homozygous recessive male without freckles (ff). The columns are labeled with the possible gametes the male could produce (one gamete per column). The rows are labeled with the possible gametes the female could produce (one gamete per row). Combining the labels on the corresponding rows and columns yields the genotype of possible offspring.
Now let's consider why it is possible for parents who are both heterozygous for the freckle trait (Ff) to have a child without freckles. The F and f alleles segregate during meiosis. So half the gametes bear the F allele, and half bear the f allele (Figure 20.5a). This time the two rows in the Punnett square are labeled with F and f, and so are the two columns (Figure 20.5b). After filling the boxes according to the labels on rows and columns, we see that the probable genotypes of the children would be homozygous dominant (FF), heterozygous (Ff), and homozygous recessive (ff), in a genotypic ratio of 1 FF : 2 Ff : 1 ff. The ratio of phenotypes of children with freckles (FF and Ff) compared with children without freckles (ff) would be 3 : 1. This ratio means that each child born to this couple will have a 75% (3/4) chance of having freckles. But there is a 1 in 4, or 25%, chance that each child will not have freckles. A cross in which both parents are heterozygous for one trait of interest is called a monohybrid cross.
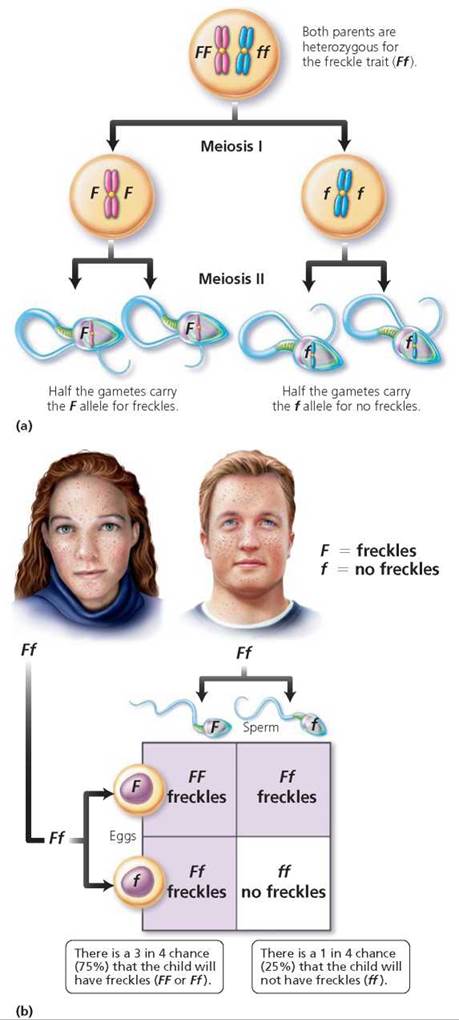
FIGURE 20.5. (a) Gamete formation by a person who is heterozygous for the freckle trait (Ff). (b) A Punnett square showing the probable outcome of a mating between two people who are heterozygous for the freckle trait (Ff).
Stop and think
At the beginning of the chapter, a question was raised about why only one sibling, your hypothetical brother, inherited your mother's freckles. Implicit in the way the question was phrased was that there is at least one other sibling (you), who, like your father, does not have freckles. Use a Punnett square to explain why the mother in this example must be heterozygous for the freckle trait.
Two-trait crosses. We use the same steps to predict the probable outcome for inheritance of two traits of interest that we used for inheritance of a single trait—as long as the genes for the two traits are on different chromosomes.
1. Identify the possible gametes that each parent can produce. Like freckles, a widow's peak is controlled by a dominant allele. What would the children look like if the parents were a woman who is homozygous for both freckles and a widow's peak (FFWW) and a man with no freckles and a straight hairline (ffww)? To answer that question, we begin by determining the possible gametes each mate can produce. Since they are homozygous for both traits, the woman can produce only gametes bearing dominant alleles (FW), and the man can produce only gametes with the recessive alleles (fW).
2. Use a Punnett square to determine the probable outcome of the genetic cross. In this case, each parent can produce only one type of gamete, so all the children will have the same genotype and the same phenotype. All the children will be heterozygous for each trait (FfWw) and will have both freckles and a widow's peak.
Suppose one of these children mates with someone who is also heterozygous for freckles and a widow's peak. This would be a dihybrid cross, a mating of individuals who are both heterozygous for two traits of interest. Would it be possible for this couple to have a child who had neither freckles nor a widow's peak? It would be possible, but the chances are only 1 in 16. Let's see why.
First, determine the possible gametes that each parent could produce (Figure 20.6). Keep in mind that alleles for a gene segregate and that genes for different traits that are located on different chromosomes will segregate independently of one another. There are, therefore, four possible allele combinations in gametes produced by a person who is heterozygous for these two different genes. The gametes are FW, Fw, fW, and fw. In this instance, we need to construct a Punnett square with four columns, because this time there are four possible male gametes, and four rows labeled with the possible female gametes. Each type of gamete has an equal chance of joining with any other type at fertilization. The squares are filled by combining the labels of the corresponding columns and rows, giving us the possible genotypes of the offspring. Notice in Figure 20.7 on page 423 that the relative numbers of phenotypes possible for the next generation are 9 freckled, widow's peak; 3 freckled, straight hairline; 3 no freckles, widow's peak; 1 no freckles, straight hairline. We see, then, that the expected phenotypic ratio resulting from a dihybrid cross is always 9 : 3 : 3 : 1.
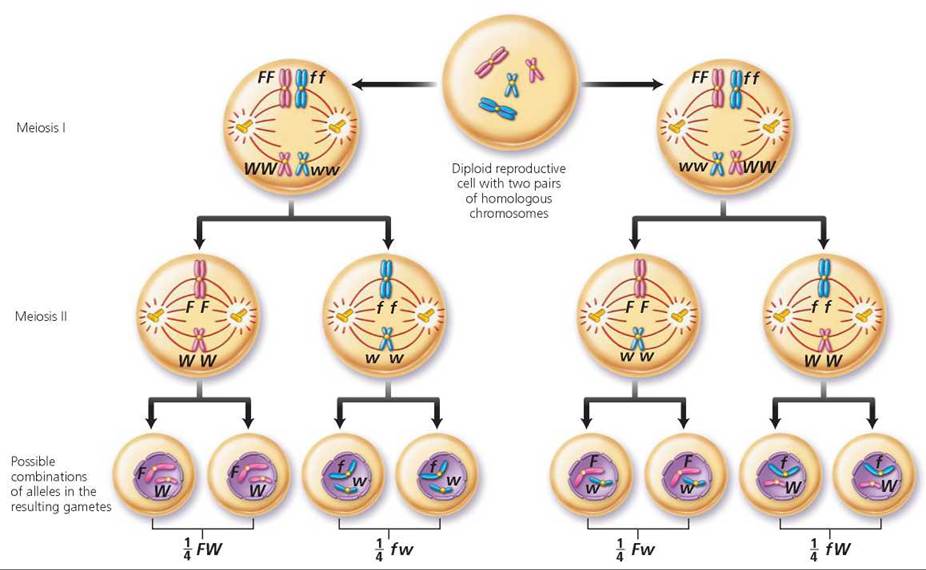
FIGURE 20.6. A person who is heterozygous for two genes that are located on different chromosomes can produce four different types of gametes.
If a parent were homozygous recessive for freckles and heterozygous for widow's peak, how many types of gametes could form?
Two types: fW and fw.
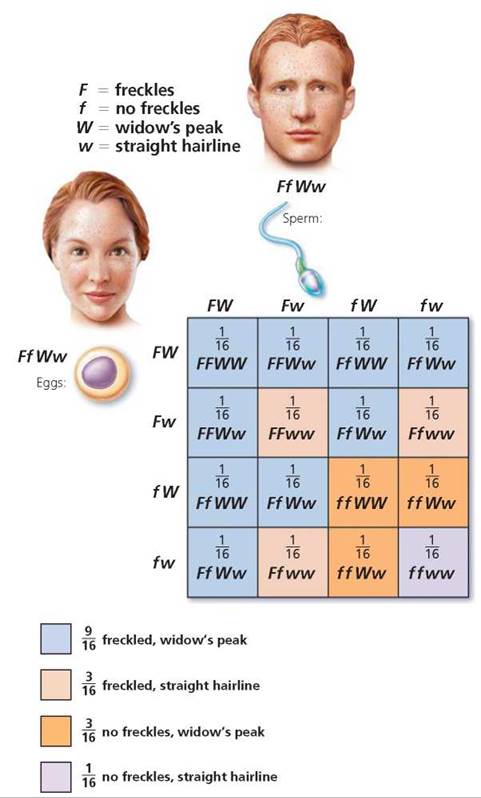
FIGURE 20.7. A dihybrid cross is a mating of individuals heterozygous for two traits, each governed by a gene on a different chromosome. Analyzed on a Punnett square, this cross illustrates the law of independent assortment—that is, each allele pair is inherited independently of others found on different chromosomes. The phenotypes of the offspring of a dihybrid cross would be expected to occur in a 9 : 3 : 3 :1 ratio.
Pedigrees
It is sometimes important to find out the genotype of a human with a dominant phenotype for a particular trait. Since there are two possible genotypes for a dominant trait (homozygous dominant and heterozygous dominant), we cannot know genotype based on phenotype alone. However, we can often deduce the unknown genotype by looking at the expression of the trait in the person's ancestors or descendants. A chart showing the genetic connections between individuals in a family is called a pedigree. Family or medical records are used to fill in the pattern of expression of the trait in question for as many family members as possible. Pedigrees are useful not just in determining an unknown genotype but also in predicting the chances that one's offspring will display the trait. Pedigrees for the inheritance of genetic disorders caused by a dominant allele on one of the non-sex chromosomes (called autosomal dominant) and of genetic disorders that are caused by recessive alleles on a pair of nonsex chromosomes (called autosomal recessive) are shown in Figure 20.8. We will discuss genes on sex chromosomes later in the chapter.
Marfan syndrome is an autosomal dominant disorder. Marfan syndrome is a connective tissue disorder in which a dominant allele for the production of an elastic connective tissue protein, fibrillin, produces a nonfunctional protein. Because connective tissue is widespread in the body, so are the symptoms of Marfan syndrome. Connective tissue is an important component in blood vessel walls, heart valves, tendons, ligaments, and cartilage. A serious problem caused by Marfan syndrome is weak walls of large blood vessels, because they can tear or burst.
Stop and think
If the pedigree in Figure 20.8a were depicting the inheritance of Marfan syndrome, what is the genotype of the males in the third generation who have Marfan syndrome? How do you know?
Genetic disorders are often caused by recessive alleles, so knowing whether the parents carry the allele will help predict both the possibility and the likelihood of that child being homozygous for the trait and therefore born with the condition (Figure 20.8b). For example, cystic fibrosis (CF), a disorder in which abnormally thick mucus is produced, is controlled by a recessive allele. Approximately 1 in every 2000 infants in the United States is born with CF. It is a leading cause of childhood death; for children with the disorder, average length of life is 24 years. The three primary signs of CF are abnormally salty sweat, digestive problems, and respiratory problems. Many children with CF suffer from malnutrition because mucus clogs the pancreatic ducts, preventing pancreatic digestive enzymes from reaching the small intestine, where they would otherwise function in digestion. Thick mucus also plugs the respiratory passageways, making breathing difficult and increasing susceptibility to lung infections such as pneumonia (Figure 20.9).
Because CF is inherited as a recessive allele, children with CF are usually born to normal, healthy parents who had no idea they carried the trait. A carrier is someone who displays the dominant phenotype but is heterozygous for a trait and can, therefore, pass the recessive allele to descendants. Approximately 1 in 22 Caucasians in the United States is a carrier for CF. (CF is less common among Asian Americans and rare among African Americans.)
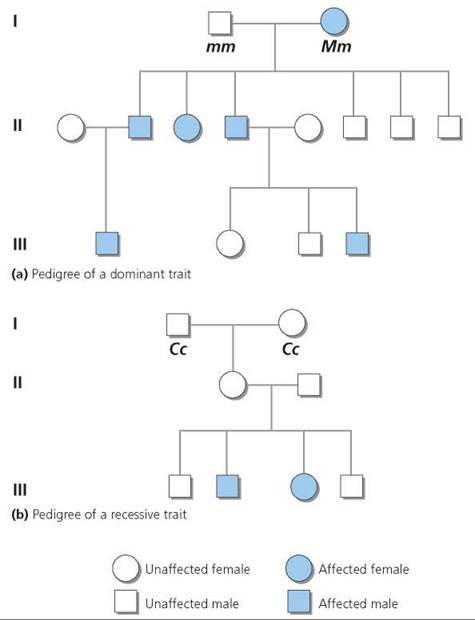
FIGURE 20.8. Pedigrees showing the inheritance of (a) a dominant autosomal trait and (b) a recessive autosomal trait. A pedigree is constructed so that each generation occupies a different horizontal line, numbered from top to bottom, with the most ancestral at the top. Males are indicated as squares, and females as circles. A horizontal line connects mating partners. An affected individual is indicated with a colored symbol.

FIGURE 20.9. Cystic fibrosis, controlled by a recessive gene, is a condition in which abnormally thick mucus is produced, causing serious digestive and respiratory problems. This child with cystic fibrosis is using a therapeutic toy to enhance airflow. When she exhales with enough force, the ribbons wave.
Stop and think
If the pedigree in Figure 20.8b were depicting the inheritance of CF, what is the genotype of the male in the third generation who has CF? How do you know? Which individuals are carriers for the trait? How do you know?
The U.S. Surgeon General urges all Americans to create a pedigree of their family health history. The Surgeon General's Health Initiative website (www.hhs.gov/familyhistory) provides a computerized tool for creating a pedigree, but it can also be done without a computer. To create your own pedigree, collect information from your relatives about their own and their ancestors' health at your next family gathering. On the pedigree, indicate which individuals have experienced high blood pressure, a heart attack or stroke, cancer, diabetes, or any other disease common in your family. This health history may help predict your risk of developing certain diseases and encourage you to take preventive action before problems develop.
Dominant and Recessive Alleles
You may wonder what makes an allele dominant or recessive. In many cases, the dominant allele produces a normal, functional protein; but the recessive allele produces the protein in an altered form that does not function properly, or it does not produce any protein. Consider the inheritance of the most common form of albinism, the inability to produce the brown pigment melanin that normally gives color to the eyes, hair, and skin. Due to the lack of melanin, a person with albinism has pale skin and white hair (Figure 20.10). A child with the trait has pink eyes, but the eye color darkens to blue in an adult. Because there is no melanin in the skin to protect against sunlight's ultraviolet rays, a person with albinism is quite vulnerable to sunburn and skin cancer.

FIGURE 20.10. A person with albinism lacks the brown pigment melanin in the skin, hair, and irises of the eyes.
The ability to produce melanin depends on the enzyme tyrosinase. The dominant allele that results in normal skin pigmentation produces a functional form of tyrosinase. A single copy of the dominant allele can produce all of this enzyme that is needed. The recessive allele that causes albinism produces a nonfunctional form of tyrosinase. If there are two copies of the recessive allele, melanin cannot be formed, and albinism results.
Codominant Alleles
The examples we have described so far represent complete dominance, a situation in which a heterozygous individual exhibits the trait associated with the dominant allele but not that of the recessive allele. In other words, the dominant allele produces a functional protein, and the protein's effects are apparent, but the recessive allele produces a less functional protein or none at all, and its effects are not apparent. However, complete dominance is not the only possibility for a heterozygous genotype. In some cases, both alleles produce functional proteins. In this situation, the effects of both alleles are apparent in the heterozygous phenotype.
The inheritance of type AB blood is an example of codominance. Two alleles, IA and F, result in the production of two different polysaccharides on the surface of red blood cells. In persons with type AB blood, both alleles are expressed, and their red blood cells have both A and B polysaccharides on their surface. (We return to the example of blood groups shortly.)
Incomplete Dominance
In incomplete dominance, the expression of a trait in a heterozygous individual is somewhere between the expression of the trait in a homozygous dominant individual and the expression of the trait in a homozygous recessive individual. The dominant allele produces a functional protein product. The recessive allele does not produce that product. As a result, a heterozygous person has only one "dose" of the protein product— half the amount in a homozygous dominant person.
Sickle-cell hemoglobin provides an example of incomplete dominance (Figure 20.11). Recall from Chapter 11 that hemoglobin is the pigment in red blood cells that carries oxygen. A red blood cell filled with normal hemoglobin (HbA) is a biconcave disk. The allele for sickling hemoglobin (Hb) produces an abnormal form of hemoglobin that is less efficient in binding oxygen. In the homozygous sickling condition (HbSHbS), called sickle-cell anemia, the red blood cells contain only the abnormal form of hemoglobin. When the oxygen content of the blood drops below a certain level, as might occur during excessive exercise or respiratory difficulty, these red blood cells with abnormal hemoglobin become sickle-shaped and tend to clump together. The clumped cells can break open and clog capillaries, causing great pain. Vital organs may be damaged by lack of oxygen. People who are homozygous for sickle-cell anemia usually die at a young age.
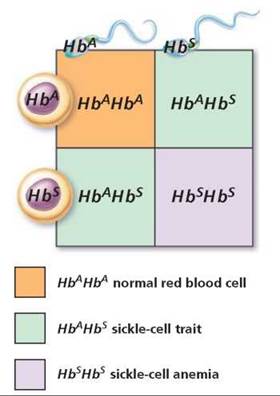
FIGURE 20.11. The inheritance of sickle-cell trait (HbAHbS) is an example of incomplete dominance.
The normal allele for the sickle-cell gene shows incomplete dominance, so people who are heterozygous HbAHbS have the sickle-cell trait. They have only one "dose" of normal hemoglobin (HbA) instead of the normal two doses needed for healthy red blood cells. Thus, people with sickle-cell trait are generally healthy, but sickling and clumping of red cells may occur if there is a prolonged drop in the oxygen content of the blood, as might happen during travel at high elevations. (Sickle-cell anemia is discussed in more detail in Chapter 11.)
Stop and think
Straight hair shows incomplete dominance over curly hair. A homozygous dominant person has straight hair; a heterozygous person has wavy hair; and a homozygous recessive person has curly hair. What is the probability that a curly-haired person and a wavy-haired person would have a child with wavy hair?
Pleiotropy
Besides providing an example of incomplete dominance, sickle-cell anemia is an example of pleiotropy: one gene leading to many effects. As you can see in Figure 20.12, the sickling of red blood cells caused by the abnormal hemoglobin has effects throughout the body. The sickled cells can break down, clog blood vessels, and accumulate in the spleen. These effects can affect the heart, brain, lungs, kidneys, and muscles and joints.
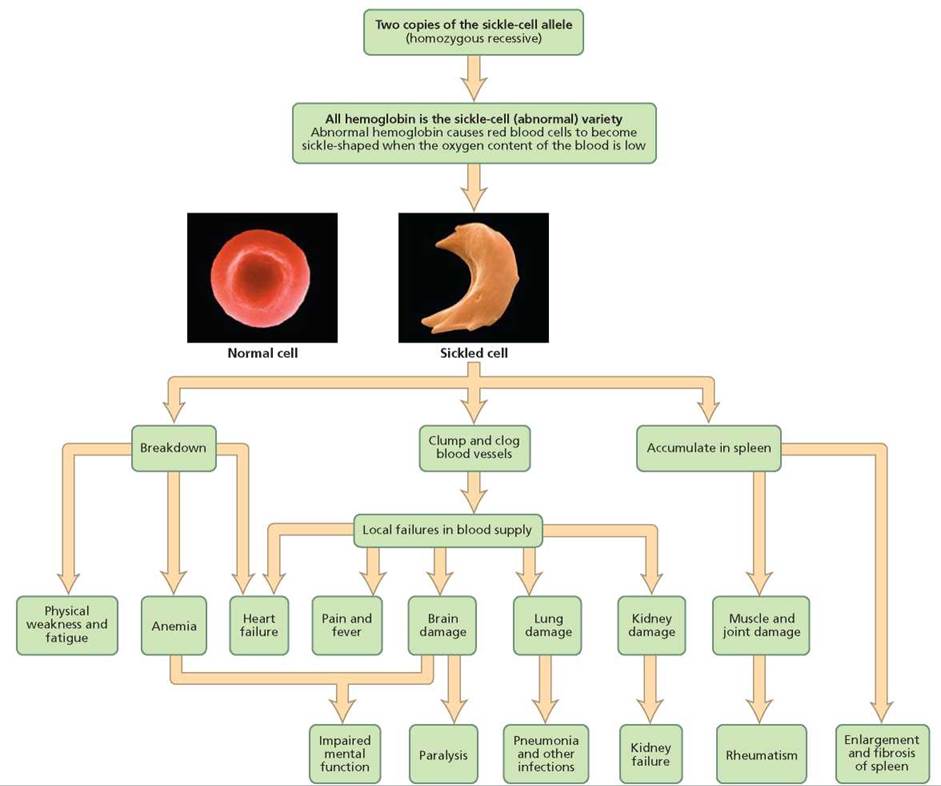
FIGURE 20.12. Sickle-cell anemia is an example of pleiotropy, a condition in which a single gene has many effects.
Multiple Alleles
Many genes have more than two alleles. When three or more forms of a given gene exist, they are referred to as multiple alleles. Keep in mind, however, that one individual has only two alleles for a given gene (one on each homologue), even if multiple alleles exist in the population.
The ABO blood types (discussed in Chapter 11) provide an example of multiple alleles. Blood type is determined by the presence of certain polysaccharides (sugars) on the surface of red blood cells. Type A blood has the A polysaccharide; type B has the B polysaccharide; type AB has both A and B polysaccharides; and type O has neither. The synthesis of each kind of polysaccharide is directed by a specific enzyme: one enzyme produces A; a different enzyme produces B. Each enzyme is specified by a different allele of the gene.
The gene controlling ABO blood types therefore has three alleles, IA, IB, and i. Alleles IA and IB specify the A and B polysaccharides, respectively. When both these alleles are present, both polysaccharides are produced. IA and IB are, therefore, codominant. Allele i, which produces no enzyme, is recessive to both IA and IB. The possible combinations of these alleles and the resulting blood types are shown in Table 20.2.
TABLE 20.2. The Relationship between Genotype and ABO Blood Types
|
Genotype |
Blood Type |
|
IAIA, Ai |
A |
|
IBIB, IBi |
B |
|
IAIB |
AB |
|
ii |
O |
Stop and think
A man who has blood type AB is named as fathering a child with blood type O. The mother has blood type B. Is it possible for the named man to be the father of this child? (Hint: What are the possible genotypes of the man, woman, and child? Given each possible genotype of this man and woman, what gametes could each produce? Use Punnett squares to determine whether any combination of these genotypes could produce a child with the same genotype as this child.)
Polygenic Inheritance
So far, we have discussed traits governed by single genes, although the genes may have multiple alleles. When a single gene controls a trait, the trait is usually either present or it is not, even though incomplete dominance or environmental factors may modify its expression. In the case of multiple alleles, there may be several distinct classes of phenotypes, as in A, B, AB, and O blood types.
The expression of most traits is much more variable, however. Many traits, including height, skin color, and eye color, vary almost continuously from one extreme to another. Environment can play a role in creating such a smooth continuum. For instance, diet and disease influence adult height, and exposure to sunlight darkens skin color. But even when all environmental factors are equal, there is still considerable variation in the expression of certain traits. Such variation results from polygenic inheritance— that is, the involvement of two or more genes, often on different chromosomes, in producing a trait. The more genes involved, the smoother the gradations and the greater the extremes of trait expression.
Although human height is probably controlled by more than three genes, for our purposes we will imagine it is determined by only three, partly to simplify our discussion but also to show how much variation in expression is possible even with as few as three genes—A, B, and C—interacting to determine a trait. Let's assume that the dominant alleles (A, B, C) of each gene add height, and the recessive alleles (a, b, c) do not. How tall would we expect the children to be if both parents were of medium height and heterozygous for all three genes? As you can see in Figure 20.13, there would be seven genetic height classes, ranging from very short to very tall. The probability of the children having either extreme of stature is slim, 1/64. The most likely probability (a 20/64 chance) is that they will be of medium height, like their parents.
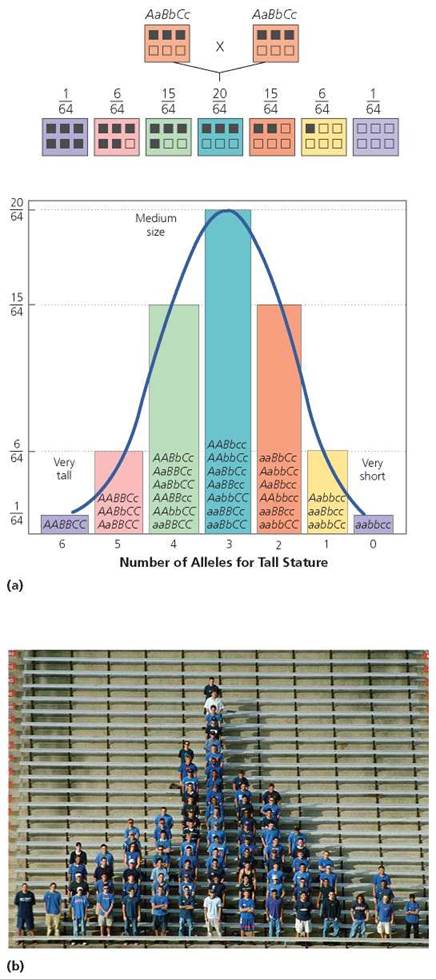
FIGURE 20.13. Human height varies along a continuum. (a) One reason is that height is determined by more than one gene (polygenic inheritance). This figure shows the distribution of alleles for tallness in children of two parents of medium height, assuming that three genes are involved in the determination of height. The top line of boxes shows the parental genotypes, and the second line of boxes indicates the possible genotypes of the offspring. Alleles for tallness are indicated with dark squares, (b) Students organized according to height.
Skin color is also determined by several genes. The allele for the kind of albinism described previously prevents melanin production, so if a person is homozygous recessive for this allele, no melanin can be deposited in the skin. In addition, there are probably at least four other genes involved in determining the amount of melanin deposited in the skin. Two alleles for each of four genes would create nine classes of skin color, ranging from pale to dark.
Genes on the Same Chromosome
Scientists estimate that there are about 20,000 to 25,000 human genes distributed among the 23 pairs of chromosomes. Thus, each chromosome bears a great number of genes. Genes on the same chromosome tend to be inherited together because an entire chromosome moves into a gamete as a unit. Genes that tend to be inherited together are described as being linked. We see, then, that linked genes do not usually assort independently. Usually is emphasized here because there is a mechanism that can unlink genes on the same chromosome: crossing over (discussed in Chapter 19).
Sex-Linked Genes
Recall from Chapter 19 that one pair of chromosomes consists of the sex chromosomes. There are two kinds of sex chromosomes, X and Y. They are not truly homologous, because the Y chromosome is much smaller than the X chromosome, and they do not carry all of the same genes.2 The Y chromosome carries very few genes, but it is important in determining gender. If a particular gene found only on the Y chromosome is present, an embryo will develop as a male. Without that gene (whether because there is no Y chromosome or because the gene is missing from the Y chromosome), an embryo will develop as a female. In contrast, most of the genes on an X chromosome have nothing to do with sex determination. For instance, genes for certain blood-clotting factors and for the pigments in cones (the photoreceptors responsible for color vision) are found on the X chromosome but not on the Y chromosome. Furthermore, the X chromosome has about as many genes as a typical autosome does, but the Y chromosome has relatively few. Thus, most genes on the X chromosome have no corresponding alleles on the Y chromosome and are known as X-linked genes.
Because most X-linked genes have no homologous allele on the Y chromosome, they have a different pattern of inheritance than do autosomes. A male is XY and therefore will express virtually all of the alleles on his single X chromosome, even alleles that are recessive. A female, on the other hand, is XX, so she does not always express recessive X alleles. As a result, the recessive phenotype of X-linked genes is much more common in males than in females (Figure 20.14). Furthermore, a son cannot inherit an X-linked recessive allele from his father. To be male, a child must have inherited his father's Y chromosome, not his father's X chromosome. Consequently, a son can inherit an X-linked recessive allele only from his mother. A daughter, however, can inherit an X-linked recessive allele from either parent. She must be homozygous for the recessive allele to show the recessive phenotype. If she is heterozygous for the trait, she will have a normal phenotype but be a carrier for that trait. Among the disorders caused by X-linked recessive alleles are red-green color blindness, two forms of hemophilia, and Duchenne muscular dystrophy. Red-green color blindness, the inability to distinguish red and green, is discussed in Chapter 9. Hemophilia (discussed in Chapter 11) is a bleeding disorder caused by a lack of a blood-clotting factor: Hemophilia A is a lack of blood-clotting factor VIII, and hemophilia B is a lack of clotting factor IX. Duchenne muscular dystrophy is discussed in Chapter 6.
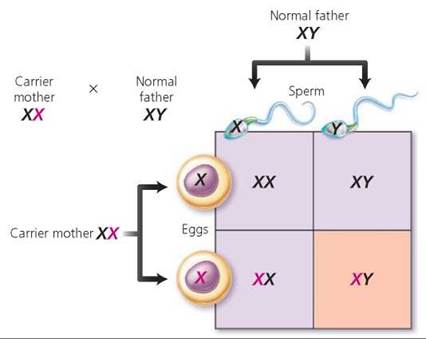
FIGURE 20.14. Genes that are X linked have a different pattern of inheritance than do genes on autosomes, as seen in this cross between a carrier mother and a father who is normal for the trait. The recessive allele is indicated in red. Notice that each son has a 50% chance of displaying the recessive phenotype. All daughters will appear normal, but each daughter has a 50% chance of being a carrier.
Stop and think
Why is Duchenne muscular dystrophy inherited from one's mother but usually expressed only in sons? (Hint: Consider its mode of inheritance.)
Sex-Influenced Genes
The expression of certain autosomal genes, those that are not located on the sex chromosomes, is powerfully influenced by the presence of sex hormones, so their expression differs in males and females. These traits are described as sex-influenced traits.
Male pattern baldness, premature hair loss on the top of the head but not on the sides, is an example of a sex-influenced trait. Male pattern baldness is much more common in men than in women because its expression depends on both the presence of the allele for baldness and the presence of testosterone, the male sex hormone. The allele for baldness, then, acts as a dominant allele in males because of their high level of testosterone and as a recessive allele in females because females have a much lower testosterone level. A male with the allele will develop pattern baldness whether he is homozygous or heterozygous for the trait. However, only women who are homozygous for the trait will develop pattern baldness. When a woman does develop pattern baldness, it usually appears later in life than it does in a man. The allele is expressed in women because the adrenal glands produce a small amount of testosterone. After menopause, when the supply of estrogen declines, adrenal testosterone may cause the expression of the baldness gene. However, in many women, balding may be merely thinning of hair.
Stop and think
Male pattern baldness was passed from father to son through at least four generations of the Adams family. John Adams (1735-1826), the second U.S. president, passed it to his son, John Quincy Adams (1767-1848), the sixth U.S. president. He, in turn, passed the gene to his son, Charles Frances Adams (1807-1886), a diplomat, who passed it to his son, Henry Adams (1838-1918), a historian. Why does father-to-son transmission of the trait rule out X-linked inheritance?
Breaks in Chromosomes
Chromosomes can break, which may lead to other alterations in structure. Breakage can be caused by certain chemicals, radiation, or viruses. Breakage also occurs as an essential part of crossing over. Although it does not happen often, chromosomes can be misaligned when crossing over occurs. Then, when the pieces reattach, one chromatid will have lost a segment, and the other will have gained a segment.
The loss of a piece of chromosome is called a deletion. The most common type of deletion occurs when the tip of a chromosome breaks off and then, during cell division, does not move into the same daughter cell as the rest of the chromosome. Deletion of more than a few genes on an autosome is usually lethal, and the loss of even small regions causes disorders.
In humans, the most common deletion, the loss of a small region near the tip of chromosome 5, causes cri-du-chat syndrome (meaning "cry of the cat"). An infant with this syndrome has a high-pitched cry that sounds like a kitten meowing. The unusual sound of the cry is caused by an improperly developed larynx (voice box). Infants with this syndrome have a round face; wide-set, downward-sloping eyes with a fold of skin at the corner of each eye; and misshapen ears (Figure 20.15). Although the condition is not usually fatal, it does cause severe mental retardation.
The addition of a piece of chromosome is called a duplication. The effects of a duplication depend on its size and position. In general, however, a small duplication is less harmful than a deletion of comparable size. A small region of chromosome 9 is sometimes duplicated, resulting in cells containing three copies of this segment. The result is mental retardation, accompanied by facial characteristics that may include a bulbous nose; wide-set, squinting eyes; and a lopsided grin.
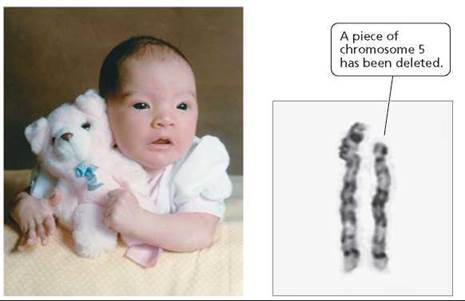
FIGURE 20.15. Occurring in 1 out of every 50,000 live births, cri-du- chat syndrome is the most common genetic deletion found in humans. It is caused by the loss of a small region near the tip of chromosome 5.
Genetic disorders also occur when certain sequences of DNA are duplicated multiple times. Fragile X syndrome provides an example. The syndrome is so named because an abnormally long sequence of repeats caused by duplication makes the X chromosome fragile and easily broken. Besides making the chromosome fragile, the repeated DNA can shut down the activity of the entire chromosome. Fragile X syndrome is the most common form of inherited mental retardation, affecting roughly 1 in 1250 males and 1 in 2500 females. It is not known, however, exactly how Fragile X syndrome causes the retardation. Other characteristics may include attention deficit, hyperactivity, autistic symptoms, large ears, long face, and flat feet (Figure 20.16).
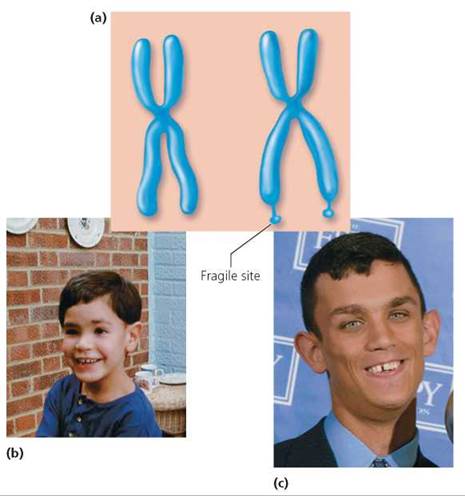
FIGURE 20.16. Fragile X syndrome. (a) Duplication of a region on the X chromosome makes the chromosome fragile and easily broken, (b) A child with Fragile X syndrome appears normal, (c) Characteristics of an adult with Fragile X syndrome include a long face and large ears. In addition, Fragile X syndrome causes mental retardation.
What would you do?
The degree of mental retardation accompanying Fragile X syndrome is variable. Nearly all males who inherit a Fragile X chromosome exhibit retardation. About a quarter of the girls with Fragile X syndrome will develop mental retardation, although others will only have learning problems. Some cities screen children with mental retardation and children with learning difficulties for Fragile X chromosomes. People who endorse this practice argue that the children with Fragile X syndrome are then benefited because specialists such as speech therapists, counselors, and physicians can be called on to help. Others oppose the screening, arguing that there is no special treatment for children with Fragile X syndrome other than that available to all children with learning problems. In your opinion, do the benefits of screening for Fragile X syndrome outweigh the disadvantages?
Detecting Genetic Disorders
More than 4000 disorders are known to have their roots in our genes. Tests are now available to look for predispositions to many of these genetic disorders. Some tests can even confirm the presence of a suspected disease-related allele in a particular person. Knowledge about the presence or absence of a faulty gene can be very helpful to couples who are planning a family. Normal, healthy parents can carry recessive alleles for disorders such as CF and Tay-Sachs disease (a disorder of lipid metabolism that causes death, usually between the ages of 1 and 5; discussed in Chapter 3), which they may pass on to their children. Testing is available for both of these recessive alleles. You can read more about the pros and cons of genetic testing in the Ethical Issue essay, Gene Testing.
When specific tests are unavailable, pedigree analysis can help prospective parents determine whether they might be carriers of recessive alleles. It cannot answer the question with certainty, but it does allow people to weigh the risks of passing a lethal allele to their children versus the possibility of having a child who is not affected.
Prenatal Genetic Testing
Prenatal testing of a fetus is usually recommended when a defective gene runs in the family or when the mother is older than 35 (because age increases the risk of problems due to failure of the homologous chromosomes to separate; see Chapter 19). There are two available procedures for diagnosing genetic problems in the fetus: amniocentesis and chorionic villi sampling (Figure 20.17). Although it is possible to look for more than 100 disorders with these procedures, tests are run only for those disorders that are common as well as any that are of particular concern in that pregnancy. Reassuring results from either form of prenatal testing cannot guarantee a healthy baby, even though these tests are highly accurate in detecting genetic disorders.
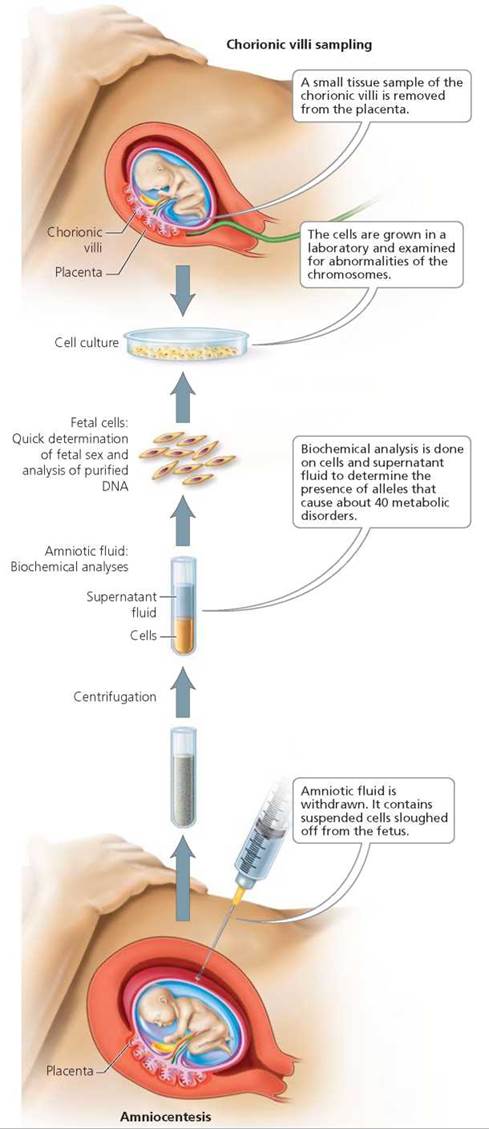
FIGURE 20.17. Amniocentesis and chorionic villi sampling (CVS) are procedures available for prenatal genetic testing.
In amniocentesis, a needle is inserted through the lower abdomen into the uterus, and a small amount of amniotic fluid—10 to 20 ml (about 2 to 4 tsp)—is withdrawn. (Ultrasound is performed first, to find the safest spot for insertion, away from the fetus, umbilical cord, and placenta.) Floating in the amniotic fluid are living cells that were sloughed off the fetus. These cells are grown in the laboratory for a week or two and then examined for abnormalities in the number of chromosomes and for the presence of certain alleles that are likely to cause specific diseases. Biochemical tests are also done on the fluid to look for certain chemicals that indicate problems. For example, a high level of alpha-fetoprotein, a substance produced by the fetus, suggests a problem with the development of the central nervous system (a neural tube defect).
Amniocentesis is usually done between 15 and 20 weeks after the woman's last menstrual period, when there is enough amniotic fluid—about 250 ml (about 1 cup)—to minimize the risk of injuring the fetus. (A few medical centers are now able to perform amniocentesis at 12 weeks of pregnancy.) Amniocentesis is generally safe for both the mother and the fetus, but there is a small risk of triggering a miscarriage or of the needle injuring the mother and causing infection or bleeding.
Chorionic villi sampling (CVS) removes and analyzes a small amount of tissue containing chorionic villi, the small, fingerlike projections of the part of the placenta called the chorion (see Chapter 18). Cells of the chorion have the same genetic material as those of the fetus. With the help of ultrasound, a small tube is inserted through the vagina and cervix to where the villi are located. Gentle suction is then used to remove a small tissue sample, which can be analyzed for genetic abnormalities.
There are pros and cons to CVS. Advantages are that it can be performed several weeks earlier in the pregnancy than amniocentesis can, and the results are available within a few days. More than 95% of the high-risk women who opt for prenatal tests receive good news, and an early diagnosis saves weeks of worry over the health of the fetus. Also, if there is a genetic problem in the fetus and the couple wishes to terminate the pregnancy, the procedure can be performed earlier in the pregnancy, when it is safer for the mother. When abortion is not chosen, early diagnosis allows more time to plan the safest time, location, and method of delivery. A disadvantage of CVS is that it has a slightly greater risk of triggering miscarriage than does amniocentesis.
If detected early enough, certain birth defects can be prevented. Congenital adrenal hyperplasia (an overgrowth of the adrenal glands), for instance, will cause a female fetus to develop abnormal genitalia, unless she is treated with hormones from week 10 to week 16 of gestation. Early diagnosis of this disorder through CVS can tell a physician whether hormone treatment is needed.
Ethical Issue
Gene Testing
Genetic screening is the practice of testing people who have no symptoms to determine whether they carry genes that will influence their chances of developing certain genetic diseases. Genetic screening technologies are advancing rapidly, and their use is gaining popularity. However, genetic screening raises many ethical questions.
Among the advantages of genetic testing is that it enables people who discover they are at risk for a treatable or preventable condition to take steps to reduce their risk. By informing people that they carry a recessive allele they were unaware of or a dominant allele that is not expressed until late in life, it can also help reduce the incidence of serious genetic disorders in future generations. Consider Tay-Sachs disease, an autosomal recessive disorder that causes the death of children, usually by the age of 5. Tay-Sachs disease is especially prevalent in descendants of Jewish people from eastern Europe. As a result of voluntary screening programs, the number of children born with Tay-Sachs disease has decreased tenfold in many communities.
Genetic testing also has a dark side. The psychological consequences of test results can be devastating. Many genetic disorders cannot be prevented or treated. How does a person who may have one of these disorders prepare for the consequences of knowing now what will cause his or her death? Huntington's disease is caused by a dominant allele that provides no hints of its existence until relatively late in life, usually past child-bearing years. About 60% of the people with Huntington's disease are diagnosed between the ages of 35 and 50. The gene causes degeneration of the brain, leading to muscle spasms, personality disorders, and death, usually within 10 to 15 years. Because Huntington's disease is caused by a dominant allele, a bearer has a 50% chance of passing it to his or her children. Thus, a person whose parent died of Huntington's disease might well be tested and receive the good news that the test did not detect the allele. But it is equally likely that the allele will show up in the test. Many persons at risk for Huntington's disease prefer to live without knowing their possible fate.
There is also concern that the results of gene tests will not remain private information but instead be used by employers as well as life and health insurers. If you were an employer who had genetic information about prospective employees, would you choose to invest time and money in training a person who carried an allele that increased the risk of cancer, heart disease, Alzheimer's disease, or alcoholism? As an insurer, would you knowingly cover such a carrier?
The results of gene testing can have both positive and negative consequences for those being tested and for their families. Who, then, should decide whether screening should be done, for which genes, on whom, and in which communities? At first blush, one might be tempted to say, “There oughta be a law!” Should we leave ethical issues to judges and legislators? Should moral matters be decided by society or clergy? Or should they be personal decisions? These are not easy questions to answer, or even to think about—yet if we do not take part in the debate, we will be allowing others to decide these crucial issues for us.
Questions to Consider
• If gene testing is done, should the person being tested be told the results no matter what? If the affected person is an infant, should the parents always be told the results, even if the condition is poorly understood? How do we balance helping such children with the possibility of stigmatizing them?
• We live in a world of limited resources. In addition to deciding who should be tested, we must decide who should pay the bill. Both testing and treatment are expensive. Should testing be done only when treatment or preventive measures are available? How much say should the agent that pays for the procedure have in who is tested and who receives medical treatment?
Newborn Genetic Testing
A simple blood test is now used routinely to screen newborns for phenylketonuria (PKU), an inherited metabolic disorder. People with PKU produce a defective enzyme that prevents them from converting phenylalanine (an amino acid in food) to tyrosine. As a result, they have too much phenylalanine in their bodies and too little tyrosine, an imbalance that somehow causes brain damage. Although nothing can be done to correct the enzyme, diagnosis of the genetic disorder allows doctors and parents to prevent brain damage by keeping the infant on a strict diet that excludes most phenylalanine. Nearly all proteins contain phenylalanine, so it is often necessary to substitute a specially prepared mixture of amino acids for most protein- containing foods, such as meat. It is also necessary to avoid foods containing the artificial sweetener NutraSweet, which contains aspartame, consisting of the amino acids phenylalanine and aspartic acid. When aspartame is digested, phenylalanine is separated from aspartic acid and can reach dangerous levels.
Adult Genetic Testing
Many predictive genetic tests for adults are now available or being developed. These tests identify people who are at risk of getting a disease but do not yet have symptoms. They are simple tests that can usually be done with a small blood sample. When steps can be taken to prevent the disease, predictive gene tests can be lifesaving. For instance, colon cancer will develop in nearly everyone who has the alleles for familial adenomatous polyposis, a condition in which thousands of benign polyps grow in the intestine. If the disorder is diagnosed, a person can be routinely inspected for colon polyps. Any polyps found can be removed, and cancer can be prevented. Genetic counselors help people understand their risks of developing inherited disorders and the medical consequences of such disorders.
Other predictive gene tests look for alleles that might predispose a person to a disorder. A protein that transports cholesterol in the blood, called ApoE, comes in three forms, each specified by a different allele. Having two alleles for ApoE-2, one form of the protein, causes catastrophically high blood cholesterol levels, which can lead to heart attack and stroke. Knowing that a person has this genetic makeup, a physician could prescribe medication to lower blood cholesterol.
What would you do?
Having two copies of another ApoE allele, ApoE-4, increases a person's risk of heart disease by 30% to 50%. It also nearly guarantees that the person will develop Alzheimer's disease by 80 years of age. Alzheimer's disease is an untreatable condition in which brain tissue degenerates (see Chapter 7), gradually robbing the person of memories, of the ability to function normally in society, and eventually of life itself. Suppose a gene test that is performed out of concern for a person's risk of heart disease reveals the presence of two copies of ApoE-4. In your opinion, is a physician with this knowledge morally obligated to tell the patient about the inevitability of Alzheimer's disease? If your genes were tested and found to have two copies of ApoE-4, would you want to be told? Why or why not?
Looking ahead
In this chapter, we learned about the chromosomal basis of human inheritance. In the next chapter, we will look more closely at DNA and the mechanisms by which genes affect cellular activity and development.
Highlighting the Concepts
Principles of Inheritance (pp. 417-428)
• Different forms of a gene are called alleles. An individual who has two of the same alleles is said to be homozygous. An individual with two different alleles for a gene is said to be heterozygous. The allele that is expressed in the heterozygous condition is described as being dominant. The allele that is masked in the heterozygous condition is described as recessive.
• The genotype for one or more traits consists of the specific alleles present in an individual. The phenotype refers to the observable expression of the trait or traits.
• The alleles for each gene separate during gamete formation so that half the gametes receive one allele and the other half receive the other allele.
• Each pair of alleles located on one kind of chromosome separate into gametes independently of the alleles of a gene pair located on a different kind of chromosome.
• If a homozygous dominant individual (AA) mates with a homozygous recessive one (aa), the genotypes of all the offspring would be heterozygous (Aa) and the dominant phenotype. If heterozygous individuals (Aa) mate, the probable genotypes of children will be 1 in 4 (25%) homozygous dominant (AA) : 2 in 4 (50%) heterozygous dominant (Aa) : 1 in 4 (25%) homozygous recessive (aa). Their phenotypes will be 3 dominant : 1 recessive.
• Pedigrees, which are diagrams constructed to show the genetic relationships among individuals in an extended family, are often useful in determining the unknown genotypes of humans showing dominant phenotypes.
• In many cases, the dominant allele produces a functional protein, and the recessive allele produces a nonfunctional protein or no protein at all.
• In complete dominance, the dominant allele completely masks the recessive allele. When two alleles are codominant, both are apparent in the phenotype. In incomplete dominance, the expression of the trait in a heterozygous individual is somewhere in between the expression of the trait in a homozygous dominant individual and the expression of the trait in a homozygous recessive individual.
• Three or more alleles for a particular gene in a population are called multiple alleles. ABO blood types are determined by three alleles: IA, P, and i. Blood type refers to the presence of certain polysaccharides on the surface of red blood cells.
• Many traits, including height, skin pigmentation, and eye color, are determined by more than one gene (polygenic inheritance). Such traits show a wide range of variation. Moreover, when many genes are involved in determining a trait, the variation in expression can be continuous.
• An X-linked gene is one that is located on the X chromosome and that has no corresponding allele on the Y chromosome. A recessive X-linked allele will always be expressed in a male, but it will be expressed only in the homozygous condition in a female.
• The expression of a sex-influenced trait depends on both the presence of the allele and the presence of sex hormones. Therefore, the expression of the allele depends on the person's sex.
Breaks in Chromosomes (pp. 428-429)
• The loss of a piece of a chromosome is called a deletion. The gain of a piece of chromosome is called a duplication. Either chromosome abnormality can cause a genetic disorder.
Detecting Genetic Disorders (pp. 429-432)
• Tests such as amniocentesis and chorionic villi sampling (CVS) are available to determine whether a fetus is likely to develop a genetic disease. In amniocentesis, a sample of amniotic fluid is taken. The fluid and the fetal cells in the fluid are analyzed for genetic problems. CVS samples cells from the chorion of the placenta and analyzes them for genetic disorders.
Reviewing the Concepts
1. What is the ratio of genotypes and phenotypes in the offspring resulting from a cross between a homozygous dominant individual and a homozygous recessive one? p. 420
2. What is a pedigree? What can a family pedigree reveal about the inheritance of a trait? pp. 421-423
3. Explain what is meant by codominance by using an example. p. 424
4. Differentiate between multiple alleles and polygenic inheritance. pp. 425-427
5. What are linked genes? Why are they usually inherited together? What can cause such genes to become unlinked? p. 427
6. Explain why the pattern of inheritance for recessive X-linked genes is different from the pattern for recessive autosomal alleles. pp. 427-428
7. What two procedures are used for prenatal genetic testing? How do they differ? pp. 429-431
8. Which of the following crosses could produce offspring with the recessive phenotype?
a. AA x aa
b. Aa x Aa
c. AA x Aa
d. AA x AA
9. All of the following crosses will have a 50% probability of producing 50% of the offspring with a heterozygous genotype except
a. AA x aa
b. Aa x Aa
c. AA x Aa
d. Aa x aa
10. A trait controlled by many genes is described as being _____.
11. The _____ of an individual is the physical expression of one or more genes of interest, and the _____ is the set of alleles the person possesses for the gene or genes of interest.
12. The genotype of a person with two copies of the same allele is _____, and the genotype of a person with two different alleles for a trait is _____.
13. Genes for different traits that are located on the same chromosome are described as being _____.
Applying the Concepts
1. Klaus has red-green color blindness, which is a sex-linked recessive trait. His wife, Helen, is homozygous for normal color vision. (Normal color vision is dominant to red-green color blindness.) What is the probability of their having a colorblind daughter? What is the probability of their having a color-blind son?
2. George and Sue have an infant, Sammy, who is lethargic, is vomiting, and has liver disease. Sammy is diagnosed with galactosemia, an autosomal recessive disorder in which the affected individuals are unable to metabolize the milk sugar galactose. Sammy is placed on a diet free of lactose and galactose and slowly recovers. George and Sue would like to have a second child. What are the chances that the second child will have galactosemia? (Hint: What are the genotypes of George and Sue? Use a Punnett square to determine the expected results of a cross with those genotypes.)
3. Explain why the offspring of first cousins are more likely to have harmful recessive traits than are offspring of unrelated individuals.
4. A woman with blood type AB names a man with blood type O as the father of her child. The child has blood type AB. Could the man be the father? Why or why not?
5. Straight hair shows incomplete dominance over curly hair. A homozygous dominant person has straight hair; a heterozygote has wavy hair; and a homozygous recessive person has curly hair. The first child of a curly-haired person and a wavy-haired person has wavy hair. What is the probability that a second child would have wavy hair?
Becoming Information Literate
Use at least three reliable sources (books, journals, websites) to answer the following questions. Cite your sources, and explain why you choose them.
1. A "savior sibling" is a child born to save the life of a sibling.
The older sibling's illness is usually a genetic blood disease, such as leukemia or Fanconi's anemia. It is hoped that the savior sibling will be a tissue match for the older sibling, so a bone marrow transplant from the younger sibling may save the older sibling's life. The embryos for a potential savior sibling are often created in a fertility clinic and tested for tissue compatibility before being implanted in the mother's uterus. If you were the parent of a child with leukemia and there were no family members who were tissue matches, would you consider conceiving a savior sibling? Identify the arguments on both sides of the issue, and defend your decision.
2. Identify at least two disorders that are caused by chromosome deletions and two that are caused by chromosome duplications. Which chromosomes are affected? What are the symptoms of the disorders?
____________________________________________________________
1 This is a simplified definition of a gene. As we will see in Chapter 21, some genes contain regulatory regions of DNA within their boundaries. Also, some genes code for RNA molecules that are needed for the production of a protein but are not part of it.
2 X and Y are considered to be a homologous pair because they each have a small region at one end that carries some of the same genes. During meiosis, the tiny homologous region on X and Y will pair in synapsis. As a result, they segregate into gametes in the same way that autosomes do.