Biology of Humans
21a. Cancer
In the previous few chapters, we learned about cell division, genes, and gene function. In this chapter, we consider how cancer cells escape the normal controls over cell division. We then learn about some causes of cancer and how we can reduce our risk of developing the disease. Finally, we identify some means of diagnosing and treating cancer.
Cancer, the “Big C," is perhaps the disease that people in the industrialized world dread the most—and with good reason. Cancer touches the lives of nearly everyone. One of every three people in the United States will develop cancer at some point in life, and the other two are likely to have a friend or relative with cancer.
Uncontrolled Cell Division
All forms of cancer share one characteristic—uncontrolled cell division. Cancer cells may behave like aliens taking over our bodies, but they are actually traitorous cells from within our own bodies.
Benign or Malignant Tumors
An abnormal growth of cells can form a mass of tissue called a tumor or a neoplasm (meaning "new growth"). However, not all tumors are cancerous: tumors can be either benign or malignant. A benign tumor is an abnormal mass of tissue that is surrounded by a capsule of connective tissue and that usually remains at the site where it forms. Its cells do not invade surrounding tissue or spread to distant locations, although they can and do grow. In most cases, a benign tumor does not threaten life, because it can be removed completely by surgery. Benign tumors can be harmful when they press on nearby tissues enough to interfere with the functioning of those tissues. If a "benign" tumor of that type is also inoperable, as may occur with a tumor in the brain, it can be life threatening. Even so, only malignant tumors, tumors that can invade surrounding tissue and spread to multiple locations throughout the body, are properly called cancerous. The spread of cancer cells from one part of the body to another is called metastasis.
· Exposure to the sun’s ultraviolet light increases one's risk of skin cancer.
Stages of Cancer Development
Cells on their way to becoming cancerous are accumulating genetic damage. As a result, precancerous cells typically look different from normal cells. Dysplasia is the term used to describe the changes in shape, nuclei, and organization within tissues of precancerous cells (Figure 21a.1). Their ragged edges give precancerous cells an abnormal shape. Their nuclei become unusually large and atypically shaped and may contain increased amounts of DNA. We can see the differences by comparing the chromosomes of a normal cell with those from a cancer cell. Notice in Figure 21a.2 that the cancer cell has extra copies of some chromosomes, is missing parts of some chromosomes, and has extra parts of other chromosomes. In a group, precancerous cells form a disorganized clump and, significantly, have an unusually high percentage of cells in the process of dividing.
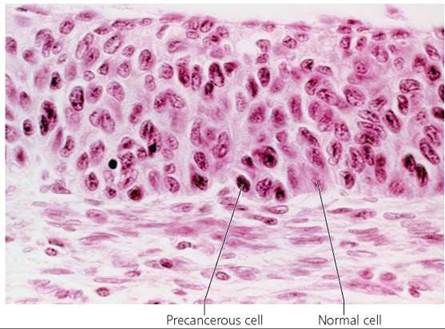
FIGURE 21a.1. Cancer cells have an abnormal appearance. In dysplasia, precancerous cells have large, irregularly shaped nuclei that contain increased amounts of DNA.
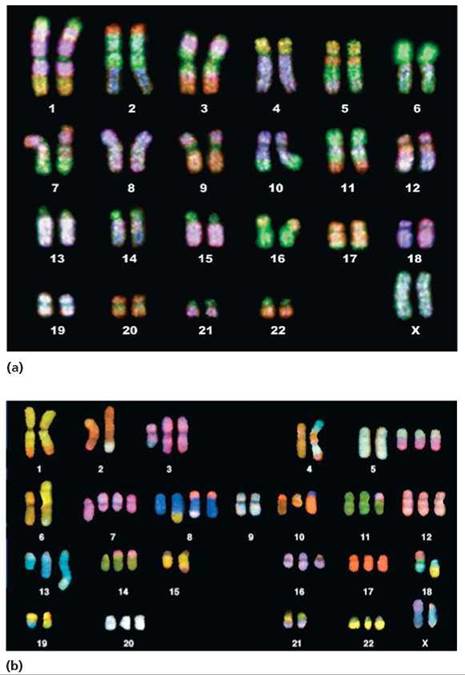
FIGURE 21a.2. A comparison of karyotypes from (a) a normal cell and (b) a cancerous cell shows that cancer cells contain extra chromosomes and chromosomes with extra pieces. (A karyotype is an arrangement of photographed chromosomes in their pairs, identified by physical features.)
Which chromosomes have extra copies in the karyotype of the cancer cell? Which have extra pieces in the karyotype of the cancer cell? Which are missing pieces in the karyotype of the cancer cell?
Chromosomes 3, 5, 7, 8, 10, 11, 12, 13, 14, 16, 17, 20, and 22 have extra copies. Chromosomes 2, 4, 6, and 13 have extra pieces. Chromosomes 3, 4, 5, 6, 7, 8, 10, 13, 14, and 18 are missing pieces.
Eventually, the tumor will reach a critical mass consisting of about a million cells. Although the tumor is still only a millimeter or two in diameter (smaller than a BB), the cells in the interior cannot get a sufficient supply of nutrients, and their own waste is poisoning them. This tiny mass is now called carcinoma in situ, which literally means "cancer in place."
If a tumor this size is to continue growing, it must attract a blood supply. Some of the tumor cells will start to secrete chemicals that cause blood vessels to invade the tumor. This process marks an ominous point of transition, because the tumor cells now have supply lines bringing in nutrients to support continued growth and carry away waste (Figure 21a.3). Of equal importance, the tumor cells have an escape route; they can enter the blood or nearby lymphatic vessels and travel throughout the body. Like cancerous seeds, the cancer cells that spread, or metastasize, can begin to form tumors in their new locations.
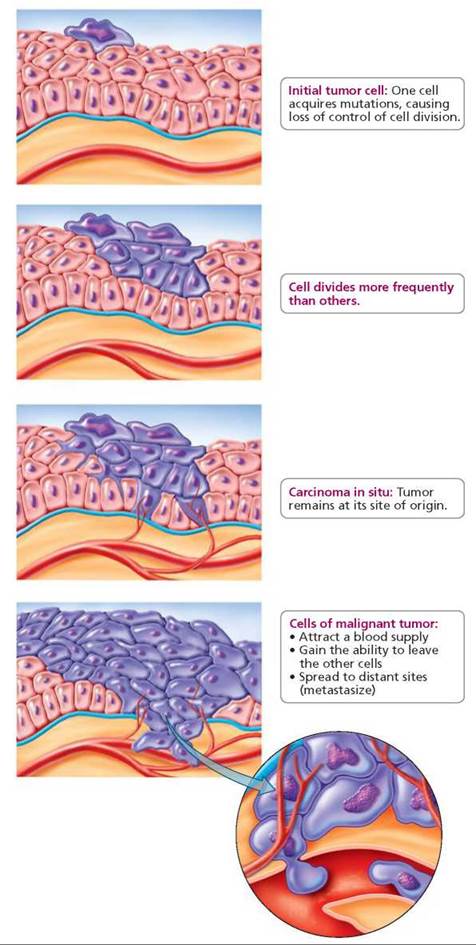
FIGURE 21a.3. The progression of cancer from the initial tumor cell to a malignant tumor
As long as a tumor stays in place, it can grow quite large, and a surgeon would still be able to remove it (depending on the location). However, once cancer cells leave the original tumor, they usually spread to so many locations that a surgeon's scalpel is no longer an effective weapon. At this point, chemotherapy or radiation is generally used to kill the cancer cells wherever they are hiding. (These treatments are discussed later in the chapter.) The original tumor is rarely a cause of death. Instead, the tumors that form in distant sites in the body are responsible for 90% of the deaths of people with cancer.
Once cancer cells have separated from the original tumor, they usually enter the cardiovascular or lymphatic system, which carries the renegade cells to distant sites. Thus, circulatory pathways in the body often explain the patterns of metastasis. For example, cancer cells escaping from tumors in most parts of the body, including the skin, encounter the next capillary bed in the lungs. Consequently, many cancers spread to the lungs. However, blood leaving the intestine travels directly to the liver, so colon cancer typically spreads to the liver.
The simplest explanation of how cancer causes death is that it interferes with the ability of body cells to function normally. For instance, cancer cells are greedy. They deprive normal cells of nutrients and thereby weaken the cells, sometimes to the point of death. Cancer cells can also prevent otherwise healthy cells from performing their usual functions. In addition, tumors can block blood vessels or air passageways in the respiratory system or press on vital nerve pathways in the brain.
Development of Cancer
The 30 trillion to 50 trillion cells in the human body generally work cooperatively, much like the members of any organized society. There are "rules," or controls, that tell a cell when and how often to divide, when to self-destruct, and when to stay in place. However, cancer cells are outlaws. They evade the many controls that would normally maintain order in the body. Let's discuss some of the normal systems of checks and balances that regulate healthy cells and see how cancer cells are able to get around these safeguards to divide indefinitely and spread out of control.
Recall from Chapter 19 that the cell cycle is the life cycle of the cell. During the cell cycle, there are checkpoints at which the cell determines whether conditions are favorable for moving on to the next stage. This is the cell's system of damage control. If a healthy cell detects damage, such as a mutated gene, it stops the cell cycle, assesses the damage, and begins repair (Figure 21a.4). If the repair is successful, the cell cycle resumes. If the damage is recognized as too severe to repair, a program of cell death is initiated, as will be discussed shortly. Unfortunately, if the damage repair is unsuccessful or incomplete, genetic damage accumulates and can lead to cancer.
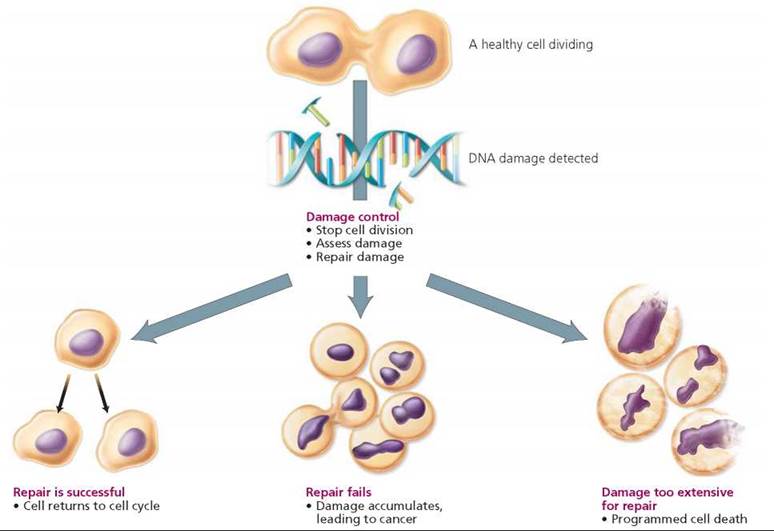
FIGURE 21a.4. Steps in controlling DNA damage during the cell cycle
Tumor-suppressor genes are an important part of the cell's system of damage control. Some tumor-suppressor gene products detect damaged DNA.1 When they do, other tumor-suppressor gene products serve as "managers of the cell's repair shop." These gene products assess the damage and coordinate the activities of other genes, whose products serve as "mechanics" and repair the damage. If the damage turns out to be too extensive, the manager activates still other genes whose products cause cell death. A particularly important tumor-suppressor gene is p53. We consider some of the activities of the p53 protein as we discuss the relationship between genes and cancer.
Lack of Restraint on Cell Division
When genes that regulate cell division are mutated, they usually do not function properly, and the cell loses control over cell division. Cancer is fundamentally a disease in which certain genes mutate and produce proteins that malfunction or produce proteins in abnormal amounts or in inappropriate locations. We now know that gene activity can be turned on or off by changes in how tightly the DNA is coiled without changes in the DNA sequence. Thus, cancer can also result if tumor-suppressor genes are turned off or if oncogenes (cancer-promoting genes) are turned on.
Two types of genes usually regulate cell division: protooncogenes and the tumor-suppressor genes mentioned earlier. Proto-oncogenes stimulate cell division in a variety of ways, including by producing growth factors or affecting their function or by producing proteins that affect the activity of certain genes. In contrast, tumor-suppressor genes inhibit or stop cell division. Thus, tumor-suppressor gene products act like brakes on cell division. The combined activities of these two types of genes allow the body to control cell division to divide and develop normally, repair defective cells, and replace dead cells (Figure 21a.5).
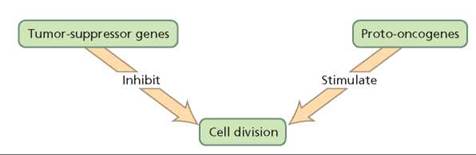
FIGURE 21a.5. Tumor-suppressor genes and proto-oncogenes normally control cell division so that it occurs only when needed. Mutations in these genes lead to loss of control over cell division and cancer.
When mutations affect the functioning of these gene products, the normal system of checks and balances that regulates cell division goes awry, and the disruption can result in the unrestrained cell division that characterizes cancer. A mutation in a tumor-suppressor gene can promote cancer by taking the brakes off cell division. The tumor-suppressor gene p53 produces a protein that regulates another gene whose job it is to produce a protein that keeps cells in a nondividing state. However, when p53 mutates, cell division is no longer curbed. Mutant p53 seems to be an important factor in more than half of all cancers. Mutation in another tumor-suppressor gene, called RB, causes retinoblastoma, which is a rare form of childhood eye cancer. The normal product of RB turns off a protooncogene that stimulates cell division. When RB mutates, the activity of the proto-oncogene is no longer inhibited, and cell division continues because the cell cannot exit the cell cycle. Two tumor-suppressor gene products that play a role in breast cancer—BRCA1 and BRCA2 proteins—initiate DNA repair. When these two genes mutate, damaged DNA is not repaired. Mutations then accumulate, which may set the cell on a course toward cancer.
A mutation in a proto-oncogene can also destroy the regulation of cell division. The mutated gene is called an oncogene, and it increases the stimulus for cell division or promotes cell division without a stimulus. An oncogene does to cell division what a stuck accelerator would do to the speed of a car. The proteins produced by many proto-oncogenes are growth factors or receptors for growth factors. When a proto-oncogene mutates and becomes an oncogene, the transformation often causes too much protein to be produced or makes the protein more active than usual. For example, the product of the ras gene normally signals the presence of a growth factor, which stimulates cell division. The ras oncogene protein is hyperactive and stimulates cell division even in the absence of growth factors. The ras oncogene is thought to be important in the development of most pancreatic and colon cancers, as well as some lung cancers. Other oncogenes play a role in leukemia and many of the most deadly forms of breast and ovarian cancer.
When normal cells are grown in tissue culture, they divide until they form a single layer of cells. If healthy cells contact a neighbor, they stop dividing. This phenomenon is called contact inhibition. But cancer cells do not exhibit contact inhibition. Instead, they continue to divide, pile up on one another, and form a tumor.
DNA Damage and Cell Destruction
When the genes that regulate cell division are faulty, backup systems normally swing into play to protect the body from the renegade cell. One such system is programmed cell death, also called apoptosis, in which cells activate a genetic suicide program in response to a biochemical or physiological signal. Activation of the so-called death genes prompts cells to manufacture proteins that then kill the cells. Often, the condemned cells go through a predictable series of physical changes that indicate the cell will die. During apoptosis, the outer membrane of the condemned cell produces bulges, called blebs, that are pinched off the cell (Figure 21a.6). The blebs are an indication that the cell will break down into membrane-enclosed fragments that are engulfed and removed by other cells.
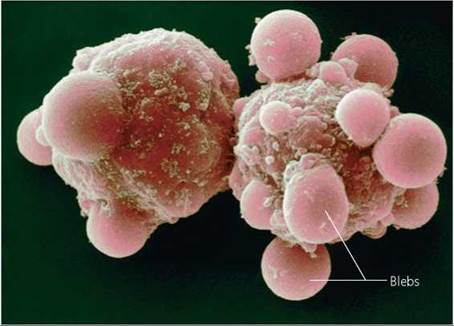
FIGURE 21a.6. Programmed cell death Is a backup system that protects the body from a cell In which the genes regulating cell division have been damaged. DNA damage that is too extensive to repair normally triggers a genetic suicide program that causes the cell to self-destruct, as these cells are doing. As the cell goes through programmed cell death, its plasma membrane forms bulges called blebs. Cancer cells are able to evade this protective mechanism.
Cancer cells often fail to trigger apoptosis. Although it is not the only way that cancer cells evade this safeguard, a faulty p53 tumor-suppressor gene is often at least partly responsible. Besides producing a protein that inhibits cell division, the p53 protein normally prevents the replication of damaged DNA. If damage is detected, the p53 protein halts cell division until the DNA can be mended. If the damage is beyond repair, the p53 protein triggers the events that lead to programmed cell death.
In a cancer cell, however, a faulty p53 protein fails to initiate the events leading to cellular self-destruction, so the cells are free to divide in spite of genetic damage. Tumors containing cells with damaged p53 genes grow aggressively and spread easily and quickly to new locations in the body. Cancer cells containing mutations in p53 are difficult to kill with radiation or chemotherapy, because these techniques are intended to damage the DNA of the cancer cell and trigger programmed self-destruction. Because of mutations in p53, many cancer cells are simply unable to self-destruct in response to DNA damage.
Unlimited Cell Division
Healthy cells have yet another safeguard against unrestrained cell division: a mechanism that limits the number of times a cell can divide during its lifetime. When grown in the laboratory, most human cells divide only about 50 or 60 times before entering a nondividing state called senescence. Like sand running through an hourglass, cell division has a predetermined end.
How does a cell "count" the number of times it has divided? The answer might lie in telomeres—pieces of DNA at the tips of chromosomes that protect the ends of the chromosomes much like the plastic pieces on the ends of shoelaces protect the shoelace—or in telomerase, the enzyme that constructs the telomeres (Figure 21a.7). Soon after an embryo is fully developed, most cells stop producing telomerase, putting an end to the maintenance of telomere length. Each time DNA is copied in preparation for cell division, a tiny piece of every telomere in the cell is shaved off, shortening the chromosomes slightly. When the telomeres are completely gone, the chromosome tips can fuse together, disrupting the genetic message and causing the cell to die. Telomeres, then, may be the cell's way of limiting the number of times division can occur. When the telomeres are gone, time is up for that cell. Thus, telomere length may serve both as a gauge of a cell's age and as an indicator of how long that line of cells will continue to divide.
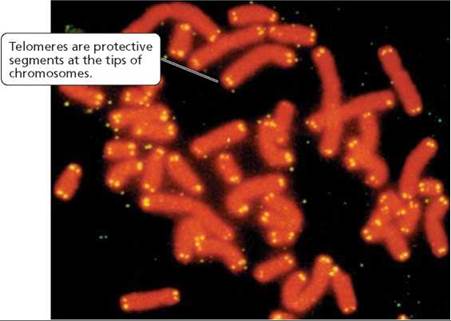
FIGURE 21a.7. Telomeres (shown here in yellow) may serve as molecular counting mechanisms that limit the number of times a cell can divide. Cancer cells retain the ability to construct new telomeres to replace the bits that are shaved off.
The "fountain of youth" that bestows immortality on cancer cells and allows them to divide indefinitely is their unceasing production of telomerase. This enzyme reconstructs the telomeres after each cell division, stabilizing telomere length and protecting the important genes at chromosome tips. Although most types of mature human cells can no longer produce telomerase, the genes for telomerase apparently become turned on in most cancer cells. Telomerase is present in nearly 90% of biopsies of human cancerous tumors.
Stop and think
Why would telomerase activity serve as a tissue marker for cancerous cells?
Blood Supply to Cancer Cells
We have seen that cancer cells have escaped the normal cellular controls on cell division and are unable to issue the orders that would lead to their own death. Instead, they multiply and form a tumor.
As mentioned earlier, when a tumor reaches a critical size of about a million cells, its growth will stop unless it can attract a blood supply to deliver the nutrients it needs to support its growth and to remove waste. Cancer cells release special growth factors that cause capillaries to invade the tumor (Figure 21a.8). These tiny blood vessels become the tumor's lifeline, removing wastes and delivering fresh nutrients and additional growth factors that will spur tumor growth. They also serve as pathways by which the cancer cells are able to leave the tumor and spread to other sites in the body.
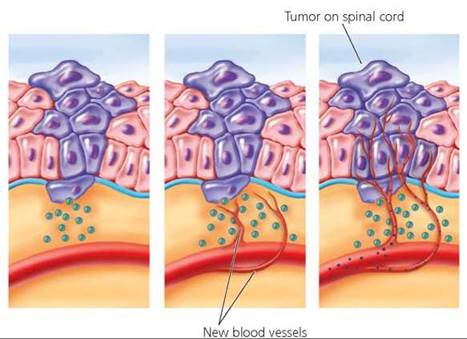
FIGURE 21a.8. Tumor cells release growth factors that cause blood vessels to grow into the tumor mass. These vessels bring nutrients and additional growth factors and remove wastes. They also provide a pathway by which cancer cells can leave the tumor and spread to other locations in the body. Here, blood vessels have formed in a tumor on the spinal cord.
In a healthy person, blood vessel formation is uncommon and is usually limited to repairing cuts or other wounds. Abnormal invasion of blood vessels into tissues can cause damage. For instance, when blood vessels invade the eye's light- sensitive retina, blindness can result. When vessels invade joints, they can cause arthritis. To avoid such damage, cells produce a protein that prevents new blood vessels from forming in tissues. The gene that normally produces this protein is by now familiar—p53. Mutations in p53 can block the production of the protein that prevents the attraction of blood vessels, allowing blood vessels to invade the tumor.
Adherence to Neighboring Cells
With access to blood vessels, the cancer cells can begin to spread. Their ability to travel through the body is yet another example of their freedom from normal cellular control mechanisms. Normal cells are "glued" in place by special molecules on their surfaces called cellular adhesion molecules (CAMs). When most normal cells become "unglued" from other cells, they stop dividing, and their genetic program for self-destruction is activated.
Cancer cells must become "unglued" from other cells to travel through the body. One way cancer cells break loose is by secreting enzymes that break down the CAMs that hold them and their neighbors in place. In this way, their anchors are broken, and mechanical barriers, such as basement membranes, that would prevent metastasis are breached. Unanchored cancer cells can continue dividing and evade self-destruction because their oncogenes send a false message to the nucleus saying that the cell is properly attached. Table 21a.1 presents a review of the control mechanisms that can fail, resulting in cancer.
TABLE 21a.1. Review of Control Mechanisms That Fail in Cancer
Mechanisms That Protect Cells from Cancer |
Method of Evasion Used by Cancer Cells |
Genetic controls on cell division |
|
Proto-oncogenes stimulate cell division through effects on growth factors and certain other cell-signaling mechanisms |
Oncogenes promote cell division |
Tumor-suppressor genes inhibit cell division |
Mutations in tumor-suppressor genes take the “brakes” off cell division |
Programmed cell death |
|
A genetic program that initiates events that lead to the death of the cell when damaged DNA is detected or another signal is received |
Mutations in tumor-suppressor genes: Mutant gene p53 no longer triggers cell death when damaged DNA is detected |
Limitations on the number of times a cell can divide |
|
Telomeres protect the ends of chromosomes, but a fraction of each is shaved off each time the DNA is copied; when the telomeres are gone, the chromosome tips can stick together, causing the cell to die |
Genes to produce telomerase, the enzyme that reconstructs telomeres, are turned on in cancer cells so telomere length is stabilized |
Controls that prevent the formation of new blood vessels |
|
These controls are normally in effect except in a few instances such as wound healing |
Cancer cells produce growth factors that attract new blood vessels and proteins that counter the normal proteins that inhibit blood vessel formation |
Controls that keep normal cells in place |
|
Cellular adhesion molecules (CAMs) hold cells in place; unanchored cells stop dividing and self-destruct |
Cancer cells' oncogenes send a false message to the nucleus that the cell is properly anchored |
Body Defense Cells
Despite all the safeguards that prevent cells from becoming cancerous, cancer cells develop in our bodies every day. Fortunately, certain body defense cells—natural killer cells and cytotoxic T cells (see Chapter 13)—usually kill those cancer cells. The processes that lead to the creation of cancer cells also produce new and slightly different proteins on cancer cell membranes. The defense cells recognize these proteins as nonself and destroy the cancer cells. But sometimes cancer cells evade destruction. Some types of cancer cells actively inhibit the defense cells, and some simply multiply so quickly that the defense cells cannot destroy them all. In either case, the tumor is able to grow and spread.
Multiple Mutations
Healthy cells have interacting control systems that usually prevent cancer development (Figure 21a.9). Normally, tumor- suppressor genes and proto-oncogenes regulate cell division so that it occurs only for growth and repair. If mutations occur, the cell will normally self-destruct before dividing and passing the genetic damage to its daughter cells. If those safeguards fail, the cell is usually prevented from dividing more than 50 to 60 times, because it lacks telomerase. If the telomerase gene is turned on, the cells will begin to starve due to lack of nutrients when the tumor consists of about a million cells. For a tumor to grow larger than this, the cells must attract a blood supply. To metastasize, cancer cells must break the molecules that anchor them in place.
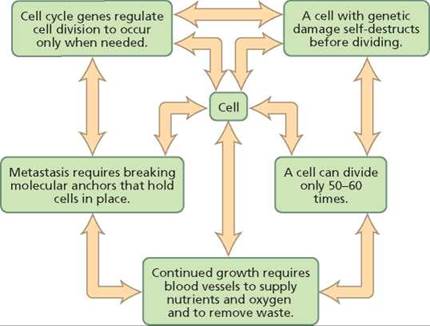
FIGURE 21a.9. Interacting control systems usually provide backup safeguards against cancer development.
With so many controls to circumvent, cancer development is a multistep process involving multiple mutations and changes in gene activity. The first mutation occurs and is passed on to all the descendant cells. Later—usually many years later—a second mutation occurs in one of the descendant cells containing the original mutation. Both mutations are passed to all the descendants of that cell. Many years later, a third mutation might occur in one of the daughter cells that already has two mutations. Each mutation brings the cell closer to becoming cancerous (Figure 21a.10).
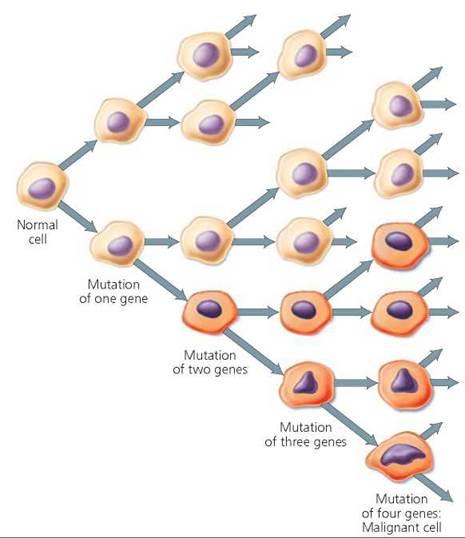
FIGURE 21a.10. Multiple mutations must occur and accumulate in a cell before the cell becomes cancerous.
Damage must occur in at least two genes (and most commonly more than six genes) before cancer occurs. For instance, colon cells must accumulate damage in at least one proto-oncogene and three tumor-suppressor genes before they become cancerous (Figure 21a.11). Furthermore, a tumor may contain different cell lines containing mutations in different sets of genes. In a recent study, researchers found 50,000 mutations in the lung tumor of a heavy smoker. The number of damaged genes needed to produce cancer explains why it is possible to inherit a predisposition to a certain form of cancer. A person who inherits only one mutant gene may be predisposed to cancer. But a second event, a mutation in at least one other gene, is required before uncontrolled cell division is unleashed.
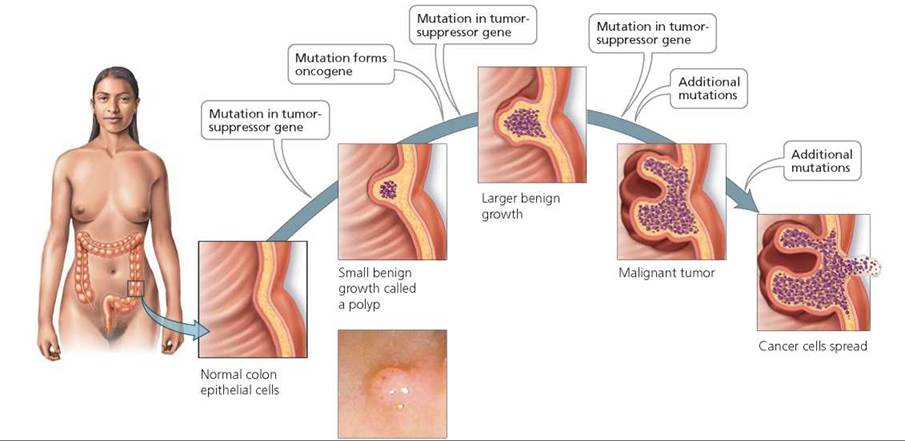
FIGURE 21a.11. Multiple mutations must occur in a single cell before it becomes cancerous. At least one proto-oncogene and three tumor-suppressor genes must mutate in colon cells before they become cancerous.
Cancer Stem Cell Hypothesis
All the cells of a cancerous tumor are genetically identical because they are descendants of a single cell that accumulated the mutations necessary for it to lose control over cell division. Do all the cells of the tumor have the ability to divide without restraint, metastasize, and form new tumors? Perhaps, but an increasing number of scientists are suggesting that only some cells within the tumor are capable of continually dividing. According to the cancer stem cell hypothesis, only a subpopulation of cells within the tumor—cancer stem cells—have the ability of unlimited self-renewal (replenishment) and give rise to the tumor.
Recall from Chapter 19a that stem cells are able to divide continually and to differentiate (specialize) into different types of cells. Embryonic stem cells taken from an early embryo can develop into all the cell types found in the body. In an adult, stem cells are sprinkled throughout tissues to give rise to new cells as needed for growth and repair. When an adult stem cell divides, one of the descendants remains a stem cell. The other daughter cell, sometimes called a progenitor cell, divides rapidly about 5 to 8 times, giving rise to a population of cells that will then specialize to form a particular type of cell.
We do know that not all cells in a tumor have the same capacity for unlimited self-renewal. Those cells with the capacity for unlimited self-renewal—cancer stem cells—have different protein markers on their surface than do other cells in the tumor. Populations of cancer stem cells have been isolated from bone marrow in the case of acute myeloid leukemia and from solid tumors in the breast or brain. Studies involving the injection of cancerous cells in mice have shown that far fewer cells are needed to initiate a tumor in a mouse when only cancer stem cells are used than if randomly selected cancerous cells are used. Some researchers suggest that the reason for the difference in the number of cells needed is that only cancer stem cells initiate tumor growth. Random samples of cells from a tumor may contain some cancer stem cells, but these represent only a fraction of the cells in a tumor. According to the cancer stem cell hypothesis, then, metastasis occurs when a cancer stem cell leaves the initial tumor and settles in a new location.
If the cancer stem cell hypothesis is correct, in which cells do the mutations occur? We don't know. Since stem cells are already able to divide continually, the simplest explanation is that the mutations leading to cancer occur in adult stem cells. However, it is also possible that cancer mutations occur in the progenitor cells that multiply several times before specializing. In this case, the mutations would cause the progenitor cells to become the cancer stem cells, which would give rise to most of the cells of the tumor.
The cancer stem cell hypothesis might also explain why traditional methods of treating cancer—radiation and chemotherapy (discussed shortly)—may shrink tumors, although the tumors often return. Such treatments are aimed at killing rapidly dividing cells such as progenitor cells. Death of progenitor cells would shrink the tumor. However, lingering cancer stem cells could replenish the population. Researchers are looking for ways to cure cancer by killing the cancer stem cells.
Known Causes of Cancer
We have seen that many of the tactics a cancer cell uses to evade normal cellular safeguards are consequences of changes in genes. Those genetic changes are often brought about by viruses or by mutations caused by exposure to certain chemicals or to radiation.
Viruses
It is estimated that viruses cause about 5% of the cancers in the United States (Table 21a.2). Some of the viruses that cause cancer have oncogenes among their genes. Once inside the host cell, the viral oncogene behaves as a host oncogene would, taking the cell one step closer to becoming cancerous. A viral oncogene is partly why the human papilloma virus that causes genital warts can cause cervical and penile cancer. If the viral genetic information is in the form of RNA, the enzyme reverse transcriptase uses viral RNA to synthesize viral DNA, which is then inserted into a host cell chromosome. Proteins produced by the viral DNA may then drive the cellular proto-oncogene to be expressed in abnormal levels or in the wrong place or time. RNA viruses can also pick up a proto-oncogene from one host cell and introduce it into a new host cell, thus promoting cell division. In other cases, a virus causes cancer because viral DNA becomes inserted into the host DNA in a location that disrupts the functioning of a gene that influences cell division. The viral DNA could, for example, be inserted into a regulatory gene that controls a proto-oncogene, breaking the switch that turns off the gene. Some viruses interfere with the function of the immune system, lessening its ability to find and destroy cancer cells as they arise.
TABLE 21a.2. Some Viruses Linked to Human Cancer
Virus |
Types of Cancer |
Human papillomaviruses (HPVs) |
Cervical, penile, and other anogenital cancers in men and women |
Hepatitis B and C viruses |
Liver cancer |
Epstein-Barr virus |
B cell lymphomas, especially Burkitt's lymphoma; nasopharyngeal carcinoma |
Human T cell leukemia virus (HTLV-1) |
Adult T cell leukemia |
Cytomegalovirus (CMV) |
Lymphomas and leukemias |
Kaposi sarcoma-associated virus |
Kaposi's sarcoma |
Chemicals
A carcinogen is an environmental agent that fosters the development of cancer. Some chemicals, especially certain organic chemicals, cause cancer by causing mutations. As we saw in Chapter 21, a mutation in as few as one nucleotide in a DNA sequence can alter a gene's message. Thus, even a small alteration in DNA can wreak havoc with a cell's regulatory mechanisms and lead to cancer.
Chemical carcinogens are around us most of the time—in air, food, water, and other substances in our surroundings. We can attempt to avoid contact with some of them. For instance, tobacco smoke contains a host of chemical carcinogens. Among the carcinogens in tobacco smoke is one that specifically mutates the tumor-suppressor gene p53 and another that specifically mutates one of the ras proto-oncogenes. Tobacco smoke is responsible for 30% of all cancer deaths and may contribute to as many as 60% of all cancer deaths in the United States. Excessive alcohol consumption is another cancer risk factor that can be avoided. Other chemical carcinogens are more difficult to avoid. These include benzene, formaldehyde, hydrocarbons, certain pesticides, and chemicals in some dyes and preservatives.
Some chemicals contribute to the development of cancer by stimulating cell division, which increases the chance of additional mutations arising. If a cancerous cell has already formed, this stimulation will cause the cancer to progress. Certain hormones can promote cancer in this way. For example, the female hormone estrogen stimulates cell division in the tissues of the breast and in the lining of the uterus (the endometrium). Sustained high levels of estrogen are linked with breast cancer,2 and the incidence of endometrial cancer is elevated in postmenopausal women whose hormone replacement therapy consists entirely of estrogen. Estrogen does not seem to promote endometrial cancer when taken in combination with progesterone. However, since 2002, several studies have shown that estrogen taken in combination with progesterone does slightly increase a woman's risk of developing breast cancer. Furthermore, breast tumors in women taking combined hormone pills were larger and more likely to have spread than were tumors in women not taking hormone pills.
Radiation
Radiation, too, can lead to cancer by causing mutations in DNA. It is impossible to avoid exposure to radiation from natural sources, such as the ultraviolet light from the sun, cosmic rays, radon, and uranium. However, we can take reasonable precautions to minimize our risks. For example, sunlight's ultraviolet rays cause skin cancer. Although we probably would not choose to spend our lives entirely indoors to reduce the risk of skin cancer, it is wise to avoid sunbathing, as well as tanning lamps and tanning parlors. It is also a good idea to use a sunscreen whenever exposure to the sun is unavoidable.
Stop and think
A cancer cluster is a greater number of cancer cases in an area than would be expected in a similar area over a given length of time. It usually involves the same type of cancer and is caused by common exposure. If you were asked to investigate a cancer cluster, which of the cancer risks would you look for?
Reducing the Risk of Cancer
Although we tend to think of cancer as one disease, it is in fact a family of more than 200 diseases, usually named for the organ in which the tumor arises. Cancers of the epithelial tissues are carcinomas. Leukemias are cancers of the bone marrow. Sarcomas are cancers of the muscle, bone, cartilage, or connective tissues. Lymphomas are cancers of the lymphatic tissues. Adenocarcinomas are cancers of the glandular epithelia. Figure 21a.12 shows the estimated number of cases of cancer and cancer deaths for various types of cancer, and Table 21a.3 indicates where in this textbook certain types of cancer are discussed.
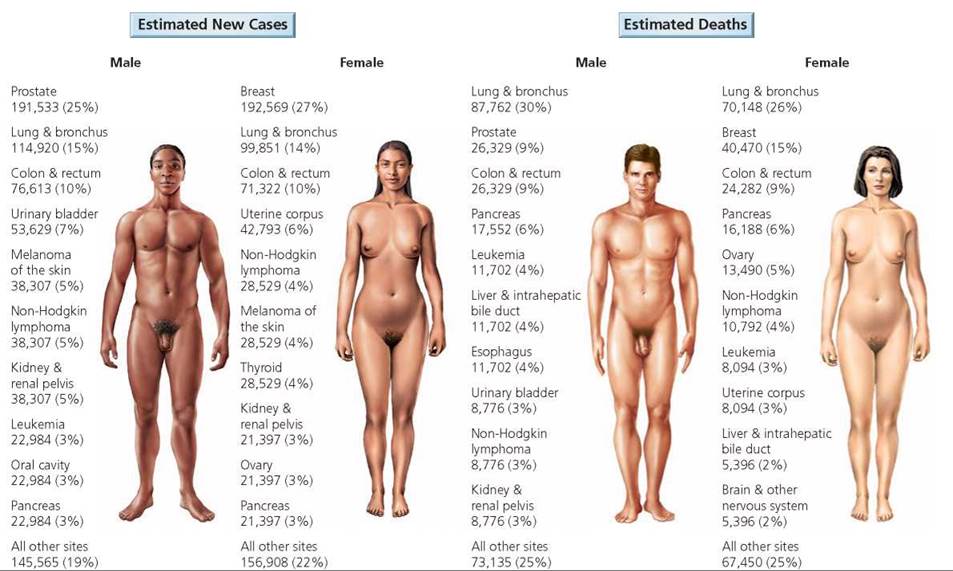
FIGURE 21a.12. The American Cancer Society's 2009 estimates for the leading types of cancer in terms of new cases and deaths. The figures do not include basal and squamous cell skin cancer or in situ carcinomas other than those of the urinary bladder. The percentages may not total 100% because of rounding.
Source: American Cancer Society, Cancer Facts and Figures 2009 (Atlanta: American Cancer Society, 2009).
TABLE 21a.3. Some Discussions of Cancer in This Book
Cancer |
Chapter |
Skin |
Chapter 4 (p. 81) |
Leukemia |
Chapter 11 (p. 207) |
Lung |
Chapter 14 (pp. 281-282) |
Colon, stomach, esophagus |
Chapter 15 (pp. 291-298) |
Testis, prostate |
Chapter 17 (pp. 333-335) |
Breast |
Chapter 17 (pp. 344-345) |
Cervical |
Chapter 17a (p. 357) |
Cancer is the second leading cause of death in industrialized countries, but the good news is that some lifestyle changes can greatly decrease your risk of developing cancer (Table 21a.4). Tobacco use and unhealthy diet are responsible for two-thirds of all cancer deaths in the United States. Tobacco smoke is the leading carcinogen, and it is obvious how to modify that risk.
A few simple diet changes may reduce your risk of developing cancer. The best rules are to eat a well-balanced diet and to eat all foods in moderation. For instance, a high-fat diet is linked to colon and breast cancers. Most people in the United States consume far too much fat. Thus it is wise to reduce fat intake, especially saturated fat, which comes from animal sources such as red meat. Consuming large quantities of smoked, salt-cured, and nitrite-cured foods, such as ham, bologna, and salami, increases the risk of cancers of the esophagus and stomach.
TABLE 21a.4. Tips for Reducing Your Cancer Risk
1. Do not use tobacco. If you do, quit. Avoid exposure to secondhand smoke.
2. Reduce the amount of saturated fat in your diet, especially the fat from red meat.
3. Minimize your consumption of salt-cured, pickled, and smoked foods.
4. Eat at least five servings of fruit and vegetables every day.
5. Avoid excessive alcohol intake. If you consume alcohol, one or two drinks a day should be the maximum.
6. Watch your caloric intake, and maintain a healthy body weight.
7. Avoid excessive exposure to sunlight. Wear protective clothing. Use sunscreen.
8. Avoid unnecessary medical x-rays.
9. Have the appropriate screening exams on a regular basis. Women should have PAP tests and mammograms. Men should have prostate tests. All adults should have tests for colorectal cancer.
A diet rich in fruits and vegetables can reduce your cancer risk because these foods are high in fiber, which can dilute the contents of the intestines, bind to carcinogens, and reduce the amount of time the carcinogens spend in the intestine by speeding passage of intestinal contents. The so- called colorful vegetables—vegetables having colors other than green—are usually high in antioxidant vitamins, and these vitamins may play a role in protecting against cancer. As their name implies, antioxidants interfere with oxidation, a process that can result in the formation of molecules called free radicals that can damage DNA and thereby lead to cancer. The three major antioxidants are beta-carotene, vitamin E, and vitamin C. The first two are common in red, yellow, and orange fruits and vegetables, and the last abounds in citrus fruits, among other sources.
Diagnosing Cancer
Early detection is critical to cancer survival because treatment is much more likely to be successful if the cancer has not yet spread. You know your own body better than anyone else does. The American Cancer Society suggests that you be alert for cancer's seven warning signs, the first letters of which spell the word CAUTION:
Change in bowel or bladder habit or function
A sore that does not heal
Unusual bleeding or bloody discharge
Thickening or lump in breast or elsewhere
Indigestion or difficulty swallowing
Obvious change in wart or mole
Nagging cough or hoarseness
But self-examination is not enough. There are additional ways to diagnose cancer—some more involved than others:
• Routine screening. Many routine tests can detect cancer in people who do not have symptoms. You can perform some of the tests on yourself; others require a visit to a medical professional (Table 21a.5).
TABLE 21a.5. Recommended Cancer Screening Tests
Guidelines suggested by the American Cancer Society for the early detection of cancer in people without symptoms, age 20 to 40 |
|
Cancer-related checkup every 3 years |
|
Should include the procedures listed below plus health counseling (such as tips on quitting cigarette smoking) and examinations for cancers of the thyroid, testes, prostate, mouth, ovaries, skin, and lymph nodes. Some people are at higher than normal risk for certain cancers and may need to have tests more frequently. |
|
Breast cancer |
• Exam by doctor every 3 years • Self-exam every month • One baseline breast x-ray ages 35 to 40 |
Higher risk for breast cancer: Personal or family history of breast cancer; never had children; had first child after 30 |
|
Uterine cancer |
• Pelvic exam every 3 years |
Cervical cancer |
• Yearly PAP test beginning at age 18 or when sexual activity begins |
Higher risk for cervical cancer: Early age at first intercourse; multiple sex partners |
|
Guidelines suggested by the American Cancer Society for the early detection of cancer in people without symptoms, age 40 and over |
|
Cancer-related checkup every year |
|
Should include the procedures listed below plus health counseling (such as tips on quitting cigarette smoking) and examinations for cancers of the thyroid, testes, prostate, mouth, ovaries, skin, and lymph nodes. Some people are at higher than normal risk for certain cancers and may need to have tests more frequently. |
|
Colon and rectal |
• Fecal occult blood test every year after age 50 |
cancer |
• Flexible sigmoidoscopy beginning at age 50 and every 5 years thereafter |
Higher risk for colorectal cancer: Personal or family history of colon or rectal cancer; personal or family history of polyps in the colon or rectum; ulcerative colitis |
|
Breast cancer |
• Exam by doctor every 3 years • Self-exam every month • Breast x-ray every year after 40 |
Higher risk for breast cancer: Personal or family history of breast cancer; never had children; had first child after 30 |
|
Uterine cancer |
• Pelvic exam every year |
Cervical cancer |
• Yearly PAP test |
Higher risk for cervical cancer: Early age at first intercourse; multiple sex partners |
|
Endometrial cancer |
• Endometrial tissue sample at menopause if at risk |
Higher risk of endometrial cancer: Infertility, obesity, failure of ovulation, abnormal uterine bleeding, estrogen therapy |
|
Prostate cancer |
• Yearly prostate-specific antigen (PSA) blood test and digital rectal exam after age 50 |
• Imaging. Many imaging techniques allow physicians to look inside the body and identify tumors. These include x-rays, computerized tomography (CT) scans, magnetic resonance imaging (MRI), and ultrasound (Figure 21a.13).
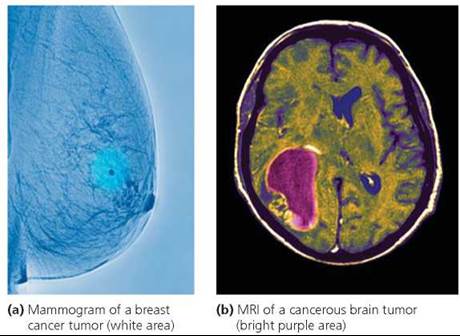
FIGURE 21a.13. Imaging techniques such as x-rays or MRIs can detect tumors.
• Biopsy. Biopsy is the removal and analysis of a small piece of tissue suspected to be cancerous. A biopsy is often done using a needle instead of surgery. In either case, cells are then examined under a microscope to see whether they have the characteristic appearance of cancer cells.
• Tumor marker tests. When cancer is suspected, certain blood tests can be used to look for tumor markers, which are chemicals produced either by the cells of the tumor or by body cells in response to a tumor. Prostate cells, for example, produce prostate-specific antigen (PSA). Men normally have low levels of PSA in their blood; but abnormal proliferation of prostate cells, as would occur if a tumor were developing, can raise those levels. Thus elevated blood PSA levels suggest the presence of prostate cancer. Currently, PSA is the only tumor marker that is useful in the original diagnosis of a cancer, but other tumor markers may reveal whether certain cancers have spread or returned. For example, blood levels of a marker called TA-90 can help determine whether melanoma (a type of skin cancer) has spread. The tumor marker CA 125 can identify ovarian cancer. CA-15-3 indicates a recurrence of breast cancer, and CEA indicates a recurrence of colon cancer.
• Genetic tests. DNA analysis of cells found in certain bodily fluids or excretions can identify gene mutations associated with certain cancers: sputum is examined for signs of lung cancer, urine for signs of bladder cancer, and feces for signs of colon cancer. Other signs of cancer can be detected by still other tests. For instance, as noted earlier, the enzyme telomerase is produced by cancer cells but rarely by normal ones. A test for telomerase appears to be helpful in diagnosing certain cancers, but this test is still in an experimental stage.
Treating Cancer
The conventional cancer treatments—surgery, radiation therapy, and chemotherapy—are still the mainstays of cancer treatment. But many new treatments hold promise.
Surgery
When a cancerous tumor is accessible and can be removed without damaging vital surrounding tissue, surgery is usually performed to eradicate the cancer or remove as much as possible. If every cancer cell is removed, as can be done with early tumors (carcinoma in situ), a complete cure is possible. However, if cancer cells have begun to invade surrounding tissue or have spread to distant locations, surgery alone cannot "cure" the cancer. If the cancer has spread, surrounding tissue and perhaps even nearby lymph nodes may also be removed. Unfortunately, more than half of all tumors have already metastasized by the time of diagnosis, so further treatment is necessary.
Radiation
If cancer has spread from the initial site but is still localized, surgery is usually followed by radiation therapy (Figure 21a.14). In some cases, such as cancer of the larynx (voice box), localized tumors that are difficult to remove surgically without damaging surrounding tissue may be treated with radiation alone.
As we have seen, radiation damages DNA, and extensive DNA damage triggers programmed cell death. The greatest damage caused by radiation is done to cells that are dividing rapidly. The intended targets of radiation, cancer cells, are dividing actively; but so are the cells of several types of tissues, called renewal tissues, whose cells normally continue dividing throughout life. These tissues include cells of the reproductive system, cells that replace layers of skin or the lining of the stomach, cells that give rise to blood cells, or cells that give rise to hair. Unfortunately, radiation cannot distinguish between cancer cells and renewal tissue, so good cells are sacrificed in killing the harmful ones. The destruction of renewal tissues leads to the side effects of radiation such as temporary sterility, nausea, anemia, and hair loss.
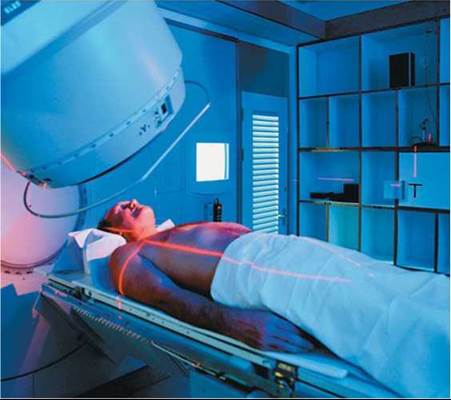
FIGURE 21a.14. Radiation therapy is often used to treat cancer that is localized.
Chemotherapy
When cancer is thought to have spread by the time of diagnosis, chemotherapy is often used. In general, the drugs used in chemotherapy reach all parts of the body and kill all rapidly dividing cells, just as radiation does. Some of the drugs block DNA synthesis, others damage DNA, and a few others prevent cell division by interfering with other cellular processes. The side effects are similar to those that accompany radiation therapy.
As we have seen, the idea underlying radiation and chemotherapy is that the damage they do to DNA in rapidly dividing cells will cause the cells to self-destruct. However, p53, the gene that detects DNA damage and initiates programmed cell death, is mutant in more than half of all cancers. As a result, even though the treatment succeeds in damaging the DNA in cancer cells, the cells do not self-destruct, and treatment fails.
Immunotherapy
Cytotoxic T cells of the immune system continually search the body for abnormal cells, such as cancer cells, and kill any that they find (see Chapter 13). The goal of immunotherapy, then, is to boost the patient's immune system so that it becomes more effective in destroying cancer cells. One form of immunotherapy involves administering factors normally secreted by lymphocytes, including interleukin-2 (which stimulates lymphocytes that attack cancer cells), interferons (which stimulate the immune system and also directly affect the tumor cells), and tumor necrosis factor (which directly affects cancer cells, causing them to self-destruct).
Two types of vaccines can be used in the battle against cancer. One type is a vaccine against a virus that causes cancer. For example, there is a vaccine against four of the human papillomaviruses that cause cervical cancer. Two of these viruses are HPV16 and HPV18, which cause 70% of the cases of cervical cancer. Federal health officials now recommend that all girls 10 to 12 years of age receive this vaccination.
The other type of vaccine is being tested in clinical trials with promising results. It is designed to work by stimulating T cells to attack and kill the cancer cells. Unlike most vaccines, these vaccines cannot prevent disease (cancer); they can only treat it. Cancer vaccines contain dead cancer cells, parts of cells, or proteins from cancer cells. Recall that vaccines cause the immune system to fight cells having the same characteristics as the cells in the vaccine. A vaccine strategy is now available to treat prostate cancer. Vaccines are being tested to treat melanoma (a deadly form of skin cancer) and leukemia, as well as cancers of the kidney, prostate, colon, and lung.
Inhibition of Blood Vessel Formation
In recent years, there has been a major change in the way doctors and scientists think about cancer. Gone are the days when the only aim was to kill the cancer cells. As researchers have learned more about the molecular biology of cancer, new ways of slowing its progression or dealing with it as a genetic disease have been developed.
The formation of blood vessels is a critical step in the life of a tumor, because blood vessels bring nourishment and provide a pathway for cell migration. Researchers are working on ways to cut off these lifelines and starve the tumor. Many drugs have been developed for this purpose, and some have been approved by the Food and Drug Administration. When a certain drug that blocks blood vessel formation is combined with chemotherapy, the survival rate of patients with colorectal cancer improves.
Gene Therapy
There are currently more than 400 clinical trials using gene therapy to treat cancer, but no means of gene therapy has been approved by the Food and Drug Administration (FDA) for cancer treatment. Nearly all these studies are in very early stages. One of the treatment strategies being tested is to insert normal tumor-suppressor genes into the cancerous cells. For instance, you may recall that gene p53 normally triggers programmed cell death when DNA damage is detected but that this gene is often faulty in cancer cells. Researchers hope that inserting a healthy form of p53 into cancer cells will lead to their death, causing the tumor to shrink.
Another strategy is to insert into a cancer cell a piece of DNA that will prevent an oncogene from exerting its effects. The inserted DNA is called antisense DNA because it is complementary to the mRNA produced by the oncogene. The antisense DNA would be expected to bind to the mRNA produced by the oncogene and prevent it from being translated into protein, thereby preventing the oncogene from exerting its effects. Currently, this method is being tried as a means of treating leukemia.
One of the most promising approaches using gene therapy is to insert a gene into tumor cells that makes the cells sensitive to a drug that will kill them. This method is now being evaluated as treatment for brain, ovarian, and prostate cancers. A viral gene for the enzyme thymidine kinase is inserted into the tumor cells, and when the gene is expressed, the resulting enzyme makes the cell sensitive to a drug called ganciclovir. The drug is inactive except inside the cells that produce the enzyme coded for by the inserted gene. Thus, only the tumor cells are killed.
Researchers have used gene therapy to cure two patients (out of 17 patients who were treated) of the aggressive skin cancer melanoma. The researchers used a virus to transfer a gene into body defense cells called T cells (discussed in Chapter 13). The inserted gene codes for a protein called T-cell receptor, or TCR. With this gene, the T cell can find tumor cells and destroy them.
In 2010, researchers delivered a gene-altered virus intravenously to treat colorectal, skin, ovarian, and lung cancers that had spread. The virus was administered in a single dose at 5 dosage levels. The virus reached and replicated within tumors throughout the body. Some patients at every level of dosage saw improvement; patients receiving the highest dosage showed the greatest improvement. Thus, gene therapy for cancer treatment holds promise for the future.
What would you do?
The Relay for Life is an overnight event held by the American Cancer Society to celebrate survivorship and raise money. Teams gather to walk or run on a track. Each team keeps one member on the track at all times. When a Relay for Life comes to your neighborhood, will you participate?
Looking ahead
In this and the previous few chapters we considered genes and inheritance. We have seen that meiosis and mutations increase variability in a population. In the next chapter, we will see that this genetic variability in a population is important for evolution by natural selection.
____________________________________________________
1 Recall that genes exert their effect through the proteins they code for. Thus it is really the proteins that are acting.
2 The link between estrogen and breast cancer is discussed in Chapter 17.