THE LIVING WORLD
Unit Three. The Continuity of Life
13.9. Gene Therapy
The third major advance in cell technology involves introducing “healthy” genes into cells that lack them. For decades scientists have sought to cure often-fatal genetic disorders like cystic fibrosis, muscular dystrophy, and multiple sclerosis by replacing the defective gene with a functional one.
Early Success
A successful gene transfer therapy procedure was first demonstrated in 1990 (see section 13.3). Two girls were cured of a rare blood disorder due to a defective gene for the enzyme adenosine deaminase. Scientists isolated working copies of this gene and introduced them into bone marrow cells taken from the girls. The gene-modified bone marrow cells were allowed to proliferate, then were injected back into the girls. The girls recovered and stayed healthy. For the first time, a genetic disorder was cured by gene therapy.
The Rush to Cure Cystic Fibrosis
Researchers quickly set out to apply the new approach to one of the big killers, cystic fibrosis. The defective gene, labelled cf, had been isolated in 1989. Five years later, in 1994, researchers successfully transferred a healthy cf gene into a mouse that had a defective one—they in effect had cured cystic fibrosis in a mouse. They achieved this remarkable result by adding the cf gene to a virus that infected the lungs of the mouse, carrying the gene with it “piggyback” into the lung cells. The virus chosen as the “vector” was adenovirus (the red viruses in figure 13.21), a virus that causes colds and is very infective of lung cells. To avoid any complications, the lab mice used in the experiment had their immune systems disabled.
Very encouraged by these well-publicized preliminary trials with mice, several labs set out in 1995 to attempt to cure cystic fibrosis by transferring healthy copies of the cf gene into human patients. Confident of success, researchers added the human cf gene to adenovirus then administered the gene-bearing virus into the lungs of cystic fibrosis patients. For eight weeks the gene therapy did seem successful, but then disaster struck. The gene-modified cells in the patients’ lungs came under attack by the patients’ own immune systems. The “healthy” cf genes were lost and with them any chance of a cure.
Problems with the Vector
Other attempts at gene therapy met with similar results, eight weeks of hope followed by failure. In retrospect, although it was not obvious then, the problem with these early attempts seems predictable. Adenovirus causes colds. Do you know anyone who has never had a cold? When you get a cold, your body produces antibodies to fight off the infection, and so all of us have antibodies directed against adenovirus. We were introducing therapeutic genes in a vector our bodies are primed to destroy.
A second serious problem is that when the adenovirus infects a cell, it inserts its DNA into the human chromosome. Unfortunately, it does so at a random location. This means that the insertion events could cause mutations—if the viral DNA inserts into the middle of a gene, it could inactivate that gene. Because the spot where the adenovirus inserts is random, some of the mutations that result can be expected to cause cancer, certainly an unacceptable consequence.
A More Promising Vector
Researchers are now investigating a much more promising vector, a tiny virus called adeno-associated virus (AAV— the smaller bluish-green viruses in figure 13.21) that has only two genes. To create a vector for gene transfer, researchers remove both of the AAV genes. The shell that remains is still quite infective and can carry human genes into patients. AAV does not elicit a strong immune response— cells infected with AAV are not eliminated by a patient’s immune system. Importantly, AAV enters human DNA far less frequently than adenovirus, and so is less likely to produce cancer-causing mutations.
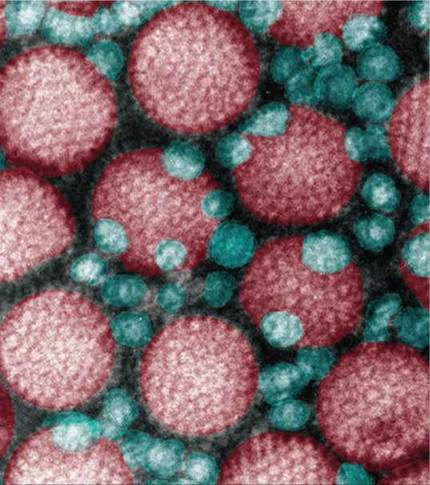
Figure 13.21. Adenovirus and AAV vectors (x200,000).
Adenovirus, the red virus particles above, has been used to carry healthy genes in clinical trials of gene therapy. Its use as a vector is problematic, however. AAV, the much smaller bluish-green virus particles seen in association with adenovirus here, lacks the many problems of adenovirus and is a much more promising gene transfer vector.
Success with New Vectors
In 1999, AAV successfully cured anemia in rhesus monkeys. In monkeys, humans, and other mammals, red blood cell production is stimulated by a protein called erythropoietin (EPO). People with a type of anemia caused by low red blood cell counts, like dialysis patients, get regular injections of EPO. Using AAV to carry a souped-up EPO gene into the monkeys, scientists were able to greatly elevate their red blood cell counts, curing the monkeys of anemia—and they stayed cured.
A similar experiment using AAV cured dogs of a hereditary disorder leading to retinal degeneration and blindness. These dogs had a defective gene that produced a mutant form of a protein associated with the retina of the eye and were blind. Recombinant viral DNA was made using a healthy version of the gene, shown in steps 1 and 2 in figure 13.22. Injection of AAV bearing the needed gene into the fluid-filled compartment behind the retina, step 5, restored sight in the dogs, step 4. This procedure was recently tried on human patients with some success.
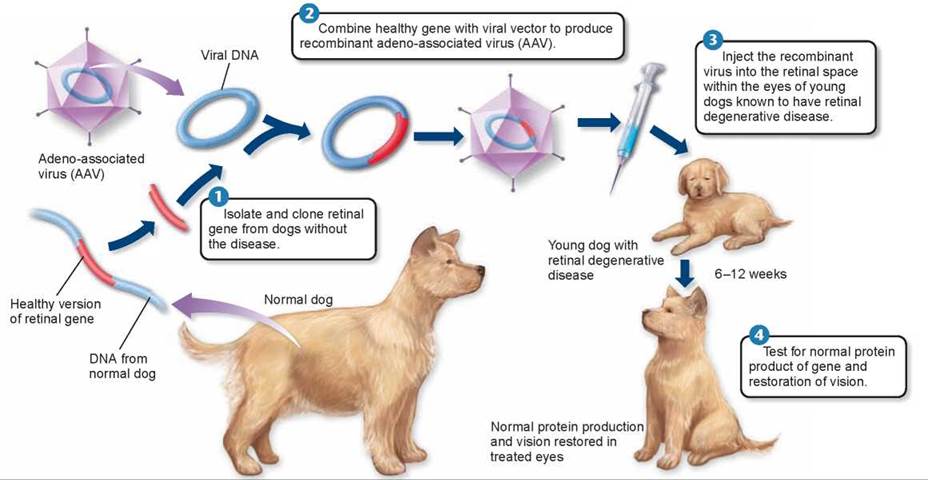
Figure 13.22 Using gene therapy to cure a retinal degenerative disease in dogs.
Researchers were able to use genes from healthy dogs to restore vision in dogs blinded by an inherited retinal degenerative disease. This disease also occurs in human infants and is caused by a defective gene that leads to early vision loss, degeneration of the retinas, and blindness. In the gene therapy experiments, genes from dogs without the disease were inserted into 3-month-old dogs that were known to carry the defective gene and that had been blind since birth. Six weeks after the treatment, the dogs' eyes were producing the normal form of the gene's protein product, and by three months, tests showed that the dogs' vision was restored.
In 2003, gene therapy clinical trials attempting to cure severe combined immune deficiency (SCID) were halted when 5 of the 20 patients in the trial developed leukemia. Apparently the vector had contained a small segment of DNA homologous to a leukemia-causing human gene. When the vector inserted there, the leukemia-causing genes were activated.
Researchers stripped out the leukemia-causing segment of the vector, and launched yet another series of new gene therapy clinical trials. In 2009, a team used an improved vector to successfully treat 12 patients suffering from Leber’s congenital blindness. All patients had some improvement in eyesight. In another study reported in 2009, two patients were treated for a rare, fatal brain disease called adrenoleukodystrophy (ALD), the disease featured in the film “Lorenzo’s Oil.” The treatment stopped the progression of the disease in its tracks. Three years later, the patients remain stable and can attend school.
In 2010, researchers began a new SCID trial with the improved vector, encouraged by the fact that all the patients in the 2003 trial who did not develop leukemia were completely cured of SCID. Trials are also underway for a wide variety of other disorders.
Key Learning Outcome 13.9. In principle, it should be possible to cure hereditary disorders like cystic fibrosis by transferring a healthy gene into the cells of affected tissues. Early attempts using adenovirus vectors were not often successful. New virus vectors avoid the problems of earlier vectors and offer promise of gene transfer therapy cures.
Inquiry & Analysis
Can Modified Genes Escape from GM Crops?
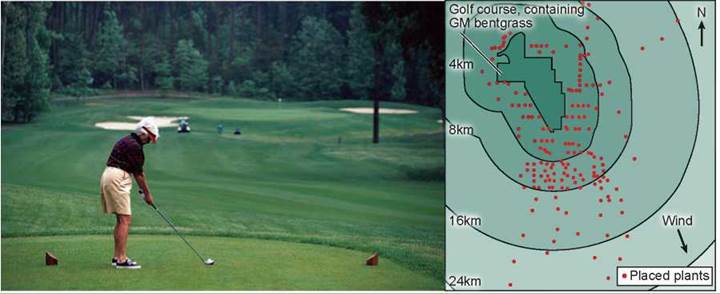
On page 268, the question of whether gene flow of GM crops posed a problem to the environment was discussed. A field experiment conducted in 2004 by the Environmental Protection Agency assessed the possibility that introduced genes could pass from genetically modified golf course grass to other plants. Investigators introduced a gene conferring herbicide resistance (the EPSP synthetase gene for resistance to glyphosate) into golf course bentgrass, Agrostis stolonifera, and then looked to see if the gene passed from the GM grass to other plants of the same species, and also if it passed to other related species.
The map below displays the setup of this elaborate field study. A total of 178 A. stolonifera plants were placed outside the golf course, many of them downwind.
An additional 69 bentgrass plants were found to be already growing downwind, most of them the related species A. gigantea. Seeds were collected from each of these plants, and the DNA of resulting seedlings tested for the presence of the gene introduced into the GM golf course grass.
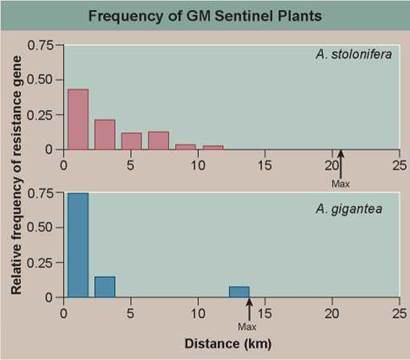
In the graph, the upper red histogram (a histogram is a "bar graph” that sorts data into a series of discontinuous categories, the value of each bar representing the number of individuals in a category, or, as in this case, the average value of entries in that category) presents the relative frequency with which the gene was found in A. stolonifera plants located at various distances from the golf course. The lower blue histogram does the same for A. gigantea plants.
1. Applying Concepts
a. Reading a Histogram. Does the gene conferring resistance to herbicide pass to other plants of this species, A. stolonifera? To individuals of the related species A. gigantea?
b. What is the maximal distance over which the herbicide resistance gene is transferred to other plants of this species? Of the related species? What are these distances, expressed in miles?
2. Interpreting Data
a. What general statement can be made about the effect of distance on the likelihood that the herbicide resistance gene will pass to another plant?
b. Are there any significant differences in the gene flow to individuals of A. stolonifera and to individuals of the related species A. gigantea?
3. Making Inferences. What mechanism do you propose to account for this gene flow?
4. Drawing Conclusions. Is it fair to conclude that genetically modified traits can pass from crops to other plants? What qualifications would you place on your conclusion?
Test Your Understanding
1. The total amount of DNA in an organism, including all of its genes and other DNA, is its
a. heredity.
b. genetics.
c. genome.
d. genomics.
2. A possible reason why humans have such a small number of genes as opposed to what was anticipated by scientists is that
a. humans don’t need more than 25,000 genes to function.
b. the exons used to make a specific mRNA can be rearranged to form different proteins.
c. the sample size used to sequence the human genome was not big enough, so the number of genes estimated could be low.
d. the number of genes will increase as scientists find out what all of the noncoding DNA actually does.
3. A protein that can cut DNA at specific DNA base sequences is called a
a. DNase.
b. DNA ligase.
c. restriction enzyme.
d. DNA polymerase.
4. Complementary DNA or cDNA is produced by
a. inserting a gene into a bacterial cell.
b. exposing the mRNA of the desired eukaryotic gene to reverse transcriptase.
c. exposing the source DNA to restriction enzymes.
d. exposing the source DNA to a probe.
5. Which of the following statements is correct?
a. DNA fingerprinting is not admissible in court.
b. DNA fingerprinting can prove with 100% certainty that two samples of DNA are from the same person.
c. DNA fingerprinting becomes more and more reliable as more probes are used.
d. No two people will ever have the same restriction pattern.
6. Using drugs produced by genetically engineered bacteria allows
a. the drug to be produced in far larger amounts than in the past.
b. humans to permanently correct the effect of a missing gene from their own systems.
c. humans to cure cystic fibrosis.
d. All of the above.
7. Some of the advantages to using genetically modified organisms in agriculture include
a. increased yield.
b. maintaining current nutritive value.
c. mass-producing proteins.
d. curing genetic diseases.
8. Which of the following is not a concern about the use of genetically modified crops?
a. possible danger to humans after consumption
b. insecticide resistance developing in pest species
c. gene flow into natural relatives of GM crops
d. harm to the crop itself from mutations
9. One of the main biological problems with replacing damaged tissue through the use of embryonic stem cells is
a. immunological rejection of the tissue by the patient.
b. that stem cells may not target appropriate tissue.
c. the time needed to grow sufficient amounts of tissue.
d. that genetic mutation of chosen stem cells may cause future problems.
10. In gene therapy, healthy genes are placed into animal cells that have defective genes by using
a. a DNA particle gun.
b. micropipettes (needles).
c. viruses.
d. Cells are not modified genetically. Instead, healthy tissue is grown and transplanted into the patient.