THE LIVING WORLD
Unit Four. The Evolution and Diversity of Life
16. Prokaryotes: The First Single-Celled Creatures

These two children wait in May 1995 outside the hospital in the Congo (formerly Zaire) town of Kikwit, where their parents and others infected with Ebola virus are being isolated. Seventy-eight percent of the infected died. Although viruses are not organisms—just fragments of DNA or RNA encased in protein—they can have a deadly impact on living organisms. Even the simplest living creatures, prokaryotes, are subject to viral infection; multiplying within infected cells, the viruses eventually burst forth, killing the cell. A person infected with Ebola virus suffers a similar fate, the virus invading connective tissue and fatally rupturing blood vessels. It used to be popular to talk of viruses as somehow transitional between living and nonliving, but biologists no longer hold this view. Rather, viruses are viewed as renegade segments of genomes, bits of DNA or RNA that have broken away from chromosomes but, using a host cell’s machinery, are still able to produce copies of themselves. In this chapter, we will examine the simplest cellular organisms, the prokaryotes, and the viruses that infect them. We begin with a discussion on the origins of life and an examination of bacteria and archaea. We finish by taking a closer look at the viruses that infect animals and plants. Many of them have a major impact on human health; for example, influenza has been responsible for millions of human deaths.
16.1. Origin of Life
All living organisms are constructed of the same four kinds of macromolecules, discussed in chapter 3, the bricks and mortar of cells. Where the first macromolecules came from and how they came to be assembled together into cells are among the least understood questions in biology—questions that address the very origin of life itself.
No one knows for sure where the first organisms (thought to be like today’s bacteria) came from. It is not possible to go back in time and watch how life originated, nor are there any witnesses. Nevertheless, it is difficult to avoid being curious about the origin of life—about what, or who, is responsible for the appearance of the first living organisms on earth. There are, in principle, at least three possibilities:
1. Extraterrestrial origin. Life may not have originated on earth at all but instead may have been carried to it, perhaps as an extraterrestrial infection of spores originating on a planet of a distant star. How life came to exist on that planet is a question we cannot hope to answer soon.
2. Special creation. Life-forms may have been put on earth by supernatural or divine forces. This viewpoint, called creationism or intelligent design, is common to most Western religions. However, almost all scientists reject creationism and intelligent design because to accept its supernatural explanation requires abandoning the scientific approach.
3. Evolution. Life may have evolved from inanimate matter, with associations among molecules becoming more and more complex. In this view, the force leading to life was selection; changes in molecules that increased their stability caused the molecules to persist longer.
In this text, we focus on the third possibility and attempt to understand whether the forces of evolution could have led to the origin of life and, if so, how the process might have occurred. This is not to say that the third possibility, evolution, is definitely the correct one. Any one of the three possibilities might be true. Nor does the third possibility preclude religion: A divine agency might have acted via evolution. Rather, we are limiting the scope of our inquiry to scientific matters. Of the three possibilities, only the third permits testable hypotheses to be constructed and so provides the only scientific explanation—that is, one that could potentially be disproved by experiment.
Forming Life's Building Blocks
How can we learn about the origin of the first cells? One way is to try to reconstruct what the earth was like when life originated 2.5 billion years ago. We know from rocks that there was little or no oxygen in the earth’s atmosphere then and more of the hydrogen-rich gases hydrogen sulfide (SH2), ammonia (NH3), and methane (CH4). Electrons in these gases would have been frequently pushed to higher energy levels by photons crashing into them from the sun or by electrical energy in lightning (figure 16.1). Today, high-energy electrons are quickly soaked up by the oxygen in earth’s atmosphere (air is 21% oxygen, all of it contributed by photosynthesis) because oxygen atoms have a great “thirst” for such electrons. But in the absence of oxygen, high-energy electrons would have been free to help form biological molecules.

Figure 16.1. Lightning provides energy to form molecules.
Before life evolved, the simple molecules in the earth's atmosphere combined to form more complex molecules. The energy that drove these chemical reactions is thought to have come from UV radiation, lightning, and other forms of geothermal energy.
When the scientists Stanley Miller and Harold Urey reconstructed the oxygen-free atmosphere of the early earth in their laboratory and subjected it to the lightning and UV radiation it would have experienced then, they found that many of the building blocks of organisms, such as amino acids and nucleotides, formed spontaneously. They concluded that life may have evolved in a “primordial soup” of biological molecules formed in the ancient earth’s oceans.
Recently, concerns have been raised regarding the “primordial soup” hypothesis as the origin of life on earth. If the earth’s atmosphere had no oxygen soon after it was formed, as Miller and Urey assumed (and most evidence supports this assumption), then there would have been no protective layer of ozone to shield the earth’s surface from the sun’s damaging UV radiation. Without an ozone layer, scientists think UV radiation would have destroyed any ammonia and methane present in the atmosphere. When these gases are missing, the Miller-Urey experiment does not produce key biological molecules such as amino acids. If the necessary ammonia and methane were not in the atmosphere, where were they?
In the last two decades, support has grown among scientists for what has been called the bubble model. This model, proposed by geophysicist Louis Lerman in 1986, suggests that the problems with the primordial soup hypothesis disappear if the model is “stirred up” a bit. The bubble model, shown in figure 16.2, proposes that the key chemical processes generating the building blocks of life took place not in a primordial soup but rather within bubbles on the ocean’s surface. Bubbles produced by erupting volcanoes under the sea 1 contain various gases. Because water molecules are polar, water bubbles tend to attract other polar molecules, in effect concentrating them within the bubbles 2. Chemical reactions would proceed much faster in bubbles, where polar reactants would be concentrated. The bubble model solves a key problem with the primordial soup hypothesis. Inside the bubbles, the methane and ammonia required to produce amino acids would have been protected from destruction by UV radiation, with the surface of the bubble reflecting the UV rays. The bubbles pop when they reach the surface 3 and release their chemical contents into the atmosphere 4. Eventually the molecules reenter the oceans packaged in raindrops 5.
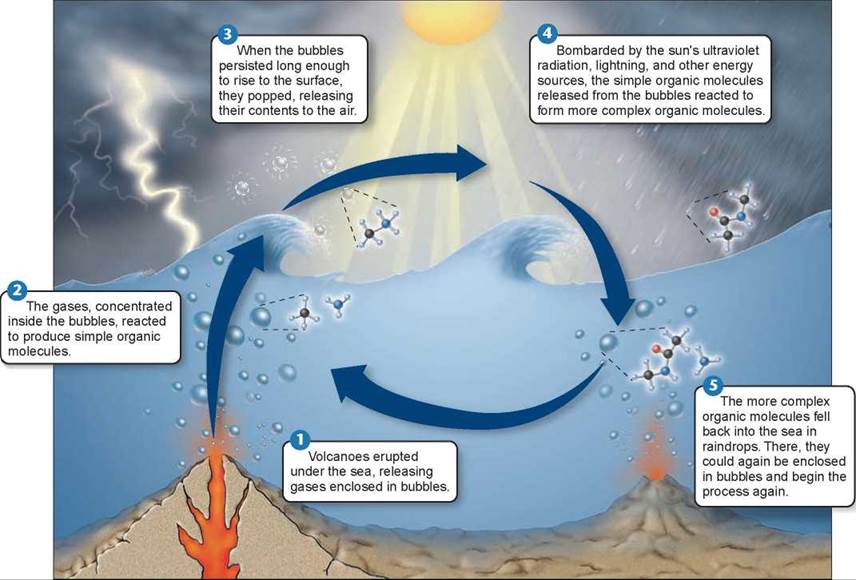
Figure 16.2. A chemical process involving bubbles may have preceded the origin of life.
In 1986, geophysicist Louis Lerman proposed that the chemical processes leading to the evolution of life took place within bubbles on the ocean's surface.
If you have ever watched the ocean surge upon the shore, you may have noticed the foamy froth created by the agitated water. The edges of the primitive oceans were more than likely very frothy places bombarded by ultraviolet and other ionizing radiation, and exposed to an atmosphere that may have contained methane and other simple organic molecules.
Key Learning Outcome 16.1. Life appeared on earth 2.5 billion years ago. It may have arisen spontaneously, although the nature of the process is not clearly understood.