THE LIVING WORLD
Unit Four. The Evolution and Diversity of Life
16. Prokaryotes: The First Single-Celled Creatures
16.9. How Animal Viruses Enter Cells
As we just discussed, bacterial viruses punch a hole in the bacterial cell wall and inject their DNA inside. Plant viruses like TMV enter plant cells through tiny rips in the cell wall at points of injury. Animal viruses typically enter their host cells by membrane fusion, or sometimes by endocytosis, a process described in chapter 4, in which the cell’s plasma membrane dimples inward, surrounding and engulfing the virus particle.
A diverse array of viruses occur among animals. A good way to gain a general idea of how they enter cells is to look at one animal virus in detail. Here we will look at the virus responsible for a relatively new and fatal disease, acquired immunodeficiency syndrome (AIDS). AIDS was first reported in the United States in 1981. It was not long before the infectious agent, human immunodeficiency virus (HIV), was identified by laboratories. HIV is shown budding off of a cell in figure 16.12. The cell is the purple and yellow structure at the bottom, and the HIVs are the circular structures suspended above the surface of the cell. HIV’s genes are closely related to those of a chimpanzee virus, suggesting that HIV first entered humans in Africa from chimpanzees.
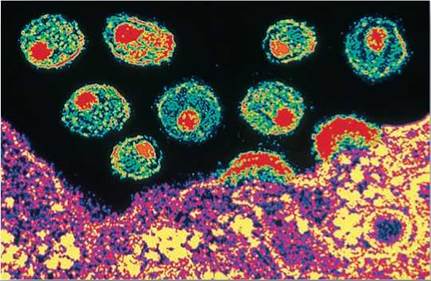
Figure 16.12. The AIDS virus.
HIV particles exit a cell to spread and infect neighboring cells.
One of the cruelest aspects of AIDS is that clinical symptoms typically do not begin to develop until long after infection by the HIV virus, generally 8 to 10 years after the initial exposure to HIV. During this long interval, carriers of HIV have no clinical symptoms but are typically fully infectious, making the spread of HIV very difficult to control.
Attachment
When HIV is introduced into the human bloodstream, the virus particle circulates throughout the entire body but will only infect certain cells, ones called macrophages (Latin, big eaters). Macrophages are the garbage collectors of the body, taking up and recycling fragments of ruptured cells and other bits of organic debris. It is not surprising that HIV specializes in infecting this one kind of cell—many other animal viruses are similarly narrow in their requirements. For example, poliovirus has an affinity for motor nerve cells, and hepatitis virus infects primarily liver cells.
How does a virus such as HIV recognize a specific kind of target cell such as a macrophage? Every kind of cell in the human body has a specific array of cell surface marker proteins, molecules that serve to identify the cells. HIV viruses are able to recognize the macrophage cell surface markers. Studding the surface of each HIV virus are spikes that bang into any cell the virus encounters. Look back to figure 16.9c, a drawing of HIV that shows these spikes (the lollipop-looking structures embedded in the envelope). Each spike is composed of a protein called gp120. Only when gp120 happens onto a cell surface marker that matches its shape does the HIV virus adhere to an animal cell and infect it. It turns out that gp120 precisely fits a cell surface marker called CD4, and that CD4 occurs on the surfaces of macrophages. Panel 1 in figure 16.13 shows the gp120 protein of HIV docking onto the CD4 surface marker on the macrophage.
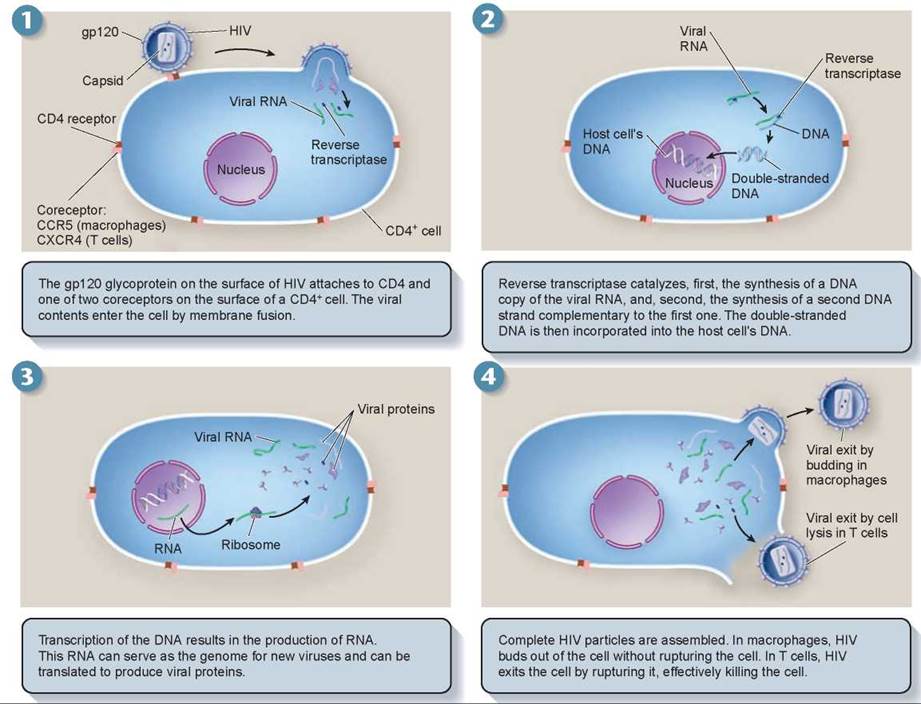
Figure 16.13. The HIV infection cycle.
Entry into Macrophages
Certain cells of the immune system, called T lymphocytes, or T cells, also possess CD4 markers. Why are they not infected right away, as macrophages are? This is the key question underlying the mystery of the long AIDS latent period. When T lymphocytes become infected and killed, AIDS commences. So what holds off T cell infection so long?
Researchers have learned that after docking onto the CD4 receptor of a macrophage, the HIV virus requires a second receptor protein, called CCR5, to pull itself across the plasma membrane. After gp120 binds to CD4, its shape becomes twisted (a chemist would say it goes through a conformational change) into a new form that fits the CCR5 coreceptor molecule. Investigators speculate that after the conformational change, the coreceptor CCR5 passes the gp120-CD4 complex through the plasma membrane by triggering membrane fusion. Macrophages have the CCR5 coreceptor, as shown in panel 1, but T lymphocytes do not.
Replication
Panel 1 also shows that once inside the macrophage cell, the HIV virus particle sheds its protective coat. This leaves the virus nucleic acid (RNA in this case) floating in the cell’s cytoplasm, along with a viral enzyme that was also within the virus shell. This enzyme, called reverse transcriptase, binds to the tip of the virus RNA and slides down it, synthesizing DNA that matches the information contained in the virus RNA, shown in panel 2. Importantly, the HIV reverse transcriptase enzyme doesn’t do its job very accurately. It often makes mistakes in reading the HIV RNA, and so creates many new mutations. The mistake-ridden double-stranded DNA that it produces may integrate itself into the host cell’s DNA, as in panel 2; it can then direct the host cell’s machinery to produce many copies of the virus, shown in panel 3.
In all of this process, no lasting damage is done to the host cell. HIV does not rupture and kill the macrophage cells it infects. Instead, the new viruses are released from the cell by budding (shown in the upper right of panel 4), a process much like exocytosis, in which the new viruses fold out opposite to the way that HIV initially gained entry into the cell at the start of the infection.
This, then, is the basis of the long latency period characteristic of AIDS. The HIV virus cycles through macrophages over a period of years, multiplying powerfully but doing little apparent damage to the body.
Starting AIDS: Entry into T Cells
During this long latent period, HIV is constantly replicating and mutating as it cycles through successive generations of macrophages. Eventually, by chance, HIV alters the gene for gp120 in a way that causes the gp120 protein to change its coreceptor allegiance. This new form of gp120 protein prefers to bind instead to a different coreceptor, CXCR4, a receptor that occurs on the surface of T cells that have the CD4 cell surface marker. Soon the body’s T cells become infected with HIV.
This has deadly consequences, as new viruses exit T cells by bursting through the plasma membrane. This rupturing destroys the T cell’s physical integrity and kills it (as shown at the lower right in panel 4). Therefore, HIV can either bud off the cell as in macrophages or it can cause cell lysis in T cells. In the case of T cells, as the released viruses infect nearby CD4+ T cells, they in turn are ruptured, in a widening circle of cell death. It is this destruction of the body’s T cells, which fight other infections in the body, that blocks the body’s immune response and leads directly to the onset of AIDS. Cancers and opportunistic infections are free to invade the defenseless body.
Key Learning Outcome 16.9. Animal viruses enter cells using specific receptor proteins to cross the plasma membrane.