THE LIVING WORLD
Unit two. The Living Cell
2. The Chemistry of Life
2.3. Molecules
A molecule is a group of atoms held together by energy. The energy acts as “glue,” ensuring that the various atoms stick to one another. The energy or force holding two atoms together is called a chemical bond. Chemical bonds determine the shapes of the large biological molecules that will be discussed in chapter 3. There are three principal kinds of chemical bonds: ionic bonds, where the force is generated by the attraction of oppositely charged ions; covalent bonds, where the force results from the sharing of electrons; and hydrogen bonds, where the force is generated by the attraction of opposite partial electrical charges. Another type of chemical attraction called van der Waals forces will be discussed later, but keep in mind that this type of interaction is not considered a chemical bond.
Ionic Bonds
Chemical bonds called ionic bonds form when atoms are attracted to each other by opposite electrical charges. Just as the positive pole of a magnet is attracted to the negative pole of another, so an atom can form a strong link with another atom if they have opposite electrical charges. Because an atom with an electrical charge is an ion, these bonds are called ionic bonds.
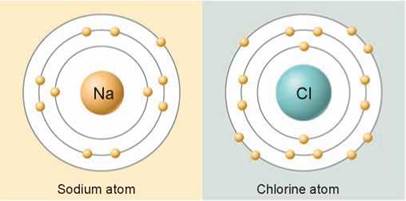
Everyday table salt is built of ionic bonds. The sodium and chlorine atoms of table salt are ions. The sodium you see in the yellow panels gives up the sole electron in its outermost shell (the shell underneath has eight) and chlorine, in the light green panels, gains an electron to complete its outermost shell. Recall from section 2.1 that an atom is more stable when its outermost electron shell is filled (with two electrons in the innermost shell or eight electrons in shells that are farther out from the nucleus).
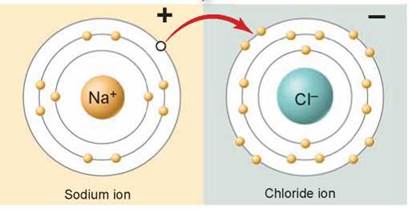
To achieve this stability, an atom will give up or accept electrons from another atom. As a result of this electron hopping, sodium atoms in table salt are positive sodium ions and chlorine atoms are negative chloride ions.
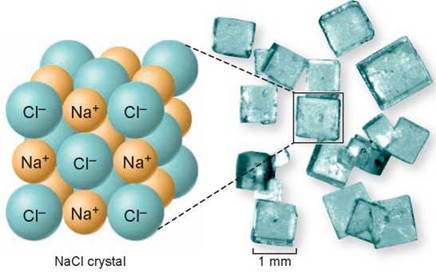
Because each ion is attracted electrically to all surrounding ions of opposite charge, this causes the formation of an elaborate matrix of sodium and chloride ionic bonds—a crystal. The sodium chloride crystal shown above reveals an organized structure of alternating sodium (yellow) and chloride (light green) ions. That is why table salt is composed of tiny crystals and is not a powder.
The two key properties of ionic bonds that make them form crystals are that they are strong (although not as strong as covalent bonds) and that they are not directional. A charged atom is attracted to the electrical field contributed by all nearby atoms of opposite charge. Ionic bonds do not play an important part in most biological molecules because of this lack of directionality. Complex, stable shapes require the more specific associations made possible by directional bonds.
Covalent Bonds
Strong chemical bonds called covalent bonds form between two atoms when they share electrons. Most of the atoms in your body are linked to other atoms by covalent bonds. Why do atoms in molecules share electrons? Remember, all atoms seek to fill up their outermost shell of orbiting electrons, which in all atoms (except tiny hydrogen and helium) takes eight electrons.
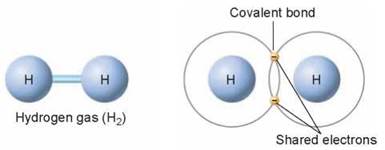
A covalent bond is formed when electrons are shared between atoms. The atoms sharing the electrons may be of the same element or different elements. Some atoms, like hydrogen (H), can form only one covalent bond, because hydrogen needs only one more electron to fill its outermost shell.
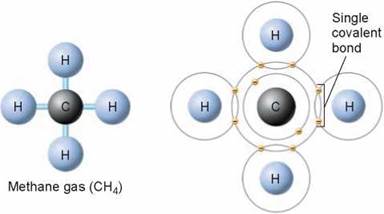
Other atoms, such as carbon (C), nitrogen (N), or oxygen (O), can form more than one covalent bond, depending on the space available in their outermost electron shells. The carbon atom has four electrons in its outermost shell, and carbon can form as many as four covalent bonds in its attempt to fully populate its outermost shell of electrons. Because there are many ways four covalent bonds can form, carbon atoms participate in many different kinds of molecules.
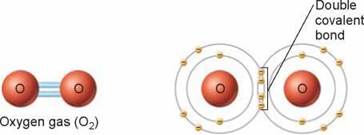
Most covalent bonds are single bonds, which involve the sharing of two electrons, but double bonds (in which four electrons are shared) are also common. Triple bonds (in which six electrons are shared) are much less frequent in nature, but are found in some common compounds, like nitrogen gas (N2).
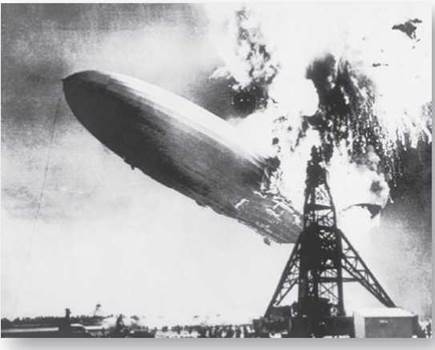
Energy is often released when covalent bonds are broken. The Hindenberg dirigible was filled with hydrogen gas when it exploded and burned in 1937; the energy of the inferno came from the breaking of H2 covalent bonds.
Polar and Nonpolar Covalent Bonds. When a covalent bond forms between two atoms, one nucleus may be much better at attracting the shared electrons than the other, an aspect of the atom called its electronegativity. In water, for example, the shared electrons are much more strongly attracted to the oxygen atom than to the hydrogen atoms; oxygen has a higher electronegativity. When this happens, shared electrons spend more time in the vicinity of the oxygen atom, which as a result becomes somewhat negative in charge; they spend less time in the vicinity of the hydrogens, and these become somewhat positive in charge. The charges are not full electrical charges like in ions but rather tiny partial charges (see next page), signified by the Greek letter delta (S). What you end up with is a sort of molecular magnet, with positive and negative ends, or “poles.” Molecules like this are said to be polar molecules, and the bonds between the atoms are called polar covalent bonds. Molecules that don’t exhibit a large difference in electronegativities of its atoms, like the carbon- hydrogen bonds of methane, are called nonpolar molecules and contain nonpolar covalent bonds.
The two key properties of covalent bonds that make them ideal for their molecule-building role in living systems are that (1) they are strong, involving the sharing of lots of energy; and (2) they are very directional—bonds form between two specific atoms, rather than a generalized attraction of one atom for its neighbors.
Hydrogen Bonds
Polar molecules like water are attracted to one another, a special type of weak chemical bond called a hydrogen bond. Hydrogen bonds occur when the positive end of one polar molecule is attracted to the negative end of another, like two magnets drawn to each other.
In a hydrogen bond, an electropositive hydrogen from one polar molecule is attracted to an electronegative atom, often oxygen (O) or nitrogen (N), from another polar molecule.
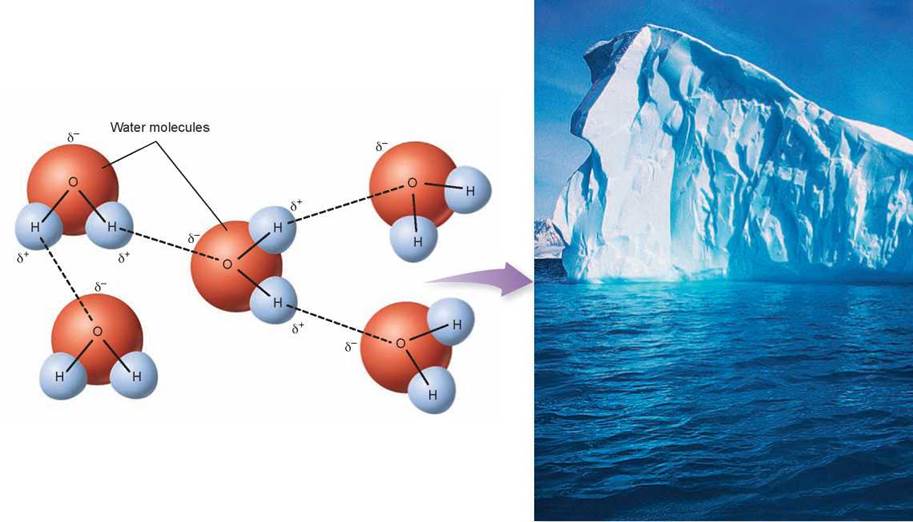
Because the oxygen atoms in water molecules are more electronegative than the hydrogen atoms, water molecules are polar. Water molecules form strong hydrogen bonds with each other, giving liquid water many unique properties. Each oxygen has a partial negative charge (δ-), and each hydrogen has a partial positive charge (δ+). Hydrogen bonds (shown as dashed lines) form between the positive end of one polar molecule and the negative end of another polar molecule. This attraction of partial charges attracts water molecules to one another.
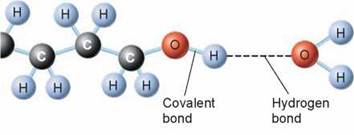
Two key properties of hydrogen bonds cause them to play an important role in the molecules found in organisms. First, they are weak and so are not effective over long distances like more powerful covalent and ionic bonds. Hydrogen bonds are too weak to actually form stable molecules by themselves. Instead, they act like Velcro, forming a tight bond by the additive effects of many weak interactions. Second, hydrogen bonds are highly directional. In chapter 3, we will discuss the role of hydrogen bonding in maintaining the structures of large biological molecules such as proteins and DNA.
Van der Waals Forces
Another important kind of weak chemical attraction is a nondirectional attractive force called van der Waals forces (or van der Waals interactions). These chemical forces come into play only when two atoms are very close to one another. The attraction is very weak, and disappears if the atoms move even a little apart. It becomes significant when numerous atoms in one molecule simultaneously come close to numerous atoms of another molecule—that is, when the shapes of the molecules match precisely. For example, this interaction is important when antibodies in your blood recognize the shape of an invading virus as foreign.
Key Learning Outcome 2.3. Molecules are atoms linked together by chemical bonds. Ionic bonds, covalent bonds, and hydrogen bonds are the three principal types of bonds, and van der Waals forces are weaker interactions.