THE LIVING WORLD
Unit two. The Living Cell
2. The Chemistry of Life
2.5. Water Ionizes
The covalent bonds within a water molecule sometimes break spontaneously. When it happens, one of the protons (hydrogen atom nuclei) dissociates from the molecule. Because the dissociated proton lacks the negatively charged electron it was sharing in the covalent bond with oxygen, its own positive charge is no longer counterbalanced, and it becomes a positively charged ion, hydrogen ion (H+). The rest of the dissociated water molecule, which has retained the shared electron from the covalent bond, is negatively charged and forms a hydroxide ion (OH-). This process of spontaneous ion formation is called ionization. It can be represented by a simple chemical equation, in which the chemical formulas for water and the two ions are written down, with an arrow showing the direction of the dissociation:

Because covalent bonds are strong, spontaneous ionization is not common. In a liter of water, only roughly 1 molecule out of each 550 million is ionized at any instant in time, corresponding to 1/10,000,000 (that is, 10-7) of a mole of hydrogen ions. (A mole is a measurement of weight. One mole of any object is the weight of 6.022 X 1023 units of that object.) The concentration of H+ in water can be written more easily by simply counting the number of decimal places after the digit “1” in the denominator:
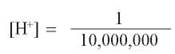
pH
A more convenient way to express the hydrogen ion concentration of a solution is to use the pH scale (figure 2.12). This scale defines pH as the negative logarithm of the hydrogen ion concentration in the solution:
![]()
Since the logarithm of the hydrogen ion concentration is simply the exponent of the molar concentration of H+, the pH equals the exponent times -1. Thus, pure water, with an [H+] of 10-7mole/liter, has a pH of 7. Recall that for every hydrogen ion formed when water dissociates, a hydroxide ion is also formed, meaning that the dissociation of water produces H+ and OH- in equal amounts. Therefore, a pH value of 7 indicates neutrality—a balance between H+ and OH-—on the pH scale.
Note that the pH scale is logarithmic, which means that a difference of 1 on the pH scale represents a 10-fold change in hydrogen ion concentration. This means that a solution with a pH of 4 has 10 times the concentration of H+ present in one with a pH of 5.
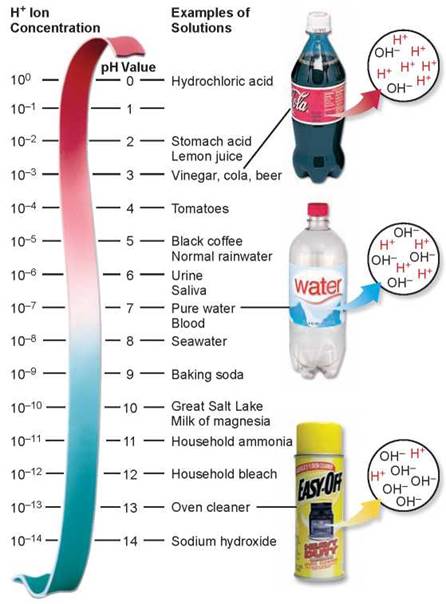
Figure 2.12 The pH scale.
A fluid is assigned a value according to the number of hydrogen ions present in a liter of that fluid. The scale is logarithmic, so that a change of only 1 means a 10-fold change in the concentration of hydrogen ions; thus lemon juice with a pH of 2 is 100 times more acidic than tomatoes with a pH of 4, and seawater is 10 times more basic than pure water.
Acids. Any substance that dissociates in water to increase the concentration of H+ is called an acid. Acidic solutions have pH values below 7. The stronger an acid is, the more H+ it produces and the lower its pH. For example, hydrochloric acid (HCl), which is abundant in your stomach, ionizes completely in water. This means that a dilution of 10-1 mole/liter of HCl will dissociate to form 10-1mole/liter of H+, giving the solution a pH of 1. The pH of champagne, which bubbles because of the carbonic acid dissolved in it, is about 3.
Bases. A substance that combines with H+ when dissolved in water is called a base. By combining with H+, a base lowers the H+ concentration in the solution. Basic (or alkaline) solutions, therefore, have pH values above 7. Very strong bases, such as sodium hydroxide (NaOH), have pH values of 12 or more.
Acid Rain
As you study biology, you will learn that hydrogen ions play many roles in the chemistry of life. When conditions become overly acidic—too many hydrogen ions—serious damage to organisms often results. One important example of this is acid precipitation, more informally called acid rain. Acid precipitation is just what it sounds like, the presence of acid in rain or snow. Where does the acid come from? Tall smokestacks from coal-burning power plants send smoke high into the atmosphere through these stacks, each of which is over 65 meters tall. The smoke the stacks belch out contains high concentrations of sulfur dioxide (SO2), because the coal that the plants burn is rich in sulfur. The sulfur-rich smoke is dispersed and diluted by winds and air currents. Since the 1950s, such tall stacks have become popular in the United States and Europe—there are now over 800 of them in the United States alone.

In the 1970s, 20 years after the stacks were introduced, ecologists began to report evidence that the tall stacks were not eliminating the problems associated with the sulfur, just exporting the ill effects elsewhere. The lakes and forests of the Northeast suffered drastic drops in biodiversity, forests dying and lakes becoming devoid of life. It turned out that the SO2 introduced into the upper atmosphere by high smokestacks combines with water vapor to produce sulfuric acid (H2SO4). When this water later falls back to earth as rain or snow, it carries the sulfuric acid with it. When schoolchildren measured the pH of natural rainwater as part of a nationwide project in 1989, rain and snow in the Northeast often had a pH as low as 2 or 3—more acidic than vinegar.
After accumulating in soils for over 50 years, the effects of acid rain are now only too evident. The impact of acid rain on forests first became evident in the Northeast. Some 15% of the lakes in New England have become chronically acidic and are dying biologically as their pH levels fall to below 5.0. Many of the forests of the northeastern United States and Canada have also been seriously damaged. The trees in this photo and on the first page of this chapter show the ill effects of acid precipitation. In the last decades, acid added to forest soils has caused the loss from these soils of over half the essential plant nutrients calcium and magnesium. Researchers blame excess acids for dissolving Ca++ and Mg++ ions into drainage waters much faster than weathering rocks can replenish them. Without them, trees stop growing and die.
Now, some 30 years later, acid rain effects are becoming apparent in the Southeast as well. Researchers suggest the reason for the delay is that southern soils are generally thicker than northern ones and thus able to sponge up far more acid. But now that southern forest soils are becoming saturated, they too are beginning to die. In a third of the southeastern streams studied, fish are declining or already gone.
The solution is straightforward: Capture and remove the emissions instead of releasing them into the atmosphere. Progressively tougher pollution laws over the past three decades have reduced U.S. emissions of sulfur dioxide by about 40% from its 1973 peak of 28.8 metric tons a year. Despite this significant progress, much remains to be done. Unless levels are cut further, researchers predict forests may not recover for centuries.
An informed public will be essential. While textbook treatments have in the past tended to minimize the impact of this issue on students ("the vast majority of North American forests are not suffering substantially from acid precipitation”), it is important that we face the issue squarely and support continued efforts to address this serious problem.
Buffers
The pH inside almost all living cells, and in the fluid surrounding cells in multicellular organisms, is fairly close to 7. The many proteins that govern metabolism are all extremely sensitive to pH, and slight alterations in pH can cause the molecules to take on different shapes that disrupt their activities. For this reason, it is important that a cell maintain a constant pH level. The pH of your blood, for example, is 7.4, and you would survive only a few minutes if it were to fall to 7.0 or rise to 7.8.
Yet the chemical reactions of life constantly produce acids and bases within cells. Furthermore, many animals eat substances that are acidic or basic; Coca-Cola, for example, is acidic, and egg white is basic. What keeps an organism’s pH constant? Cells contain chemical substances called buffers that minimize changes in concentrations of H+ and OH-.
A buffer is a substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution changes. Hydrogen ions are donated to the solution when their concentration falls and taken from the solution when their concentration rises. The graph in figure 2.13 shows how buffers work. The blue line indicates changes in pH. As a base is added to the solution, the H+ concentration falls and the pH should rise sharply, but by contributing H+ to the solution the buffer works to keep the pH within a range, called the buffering range (the darker blue bar). Only when the buffering capacity is exceeded does the pH begin to rise. What sort of substance will act in this way? Within organisms, most buffers consist of pairs of substances, one an acid and the other a base.
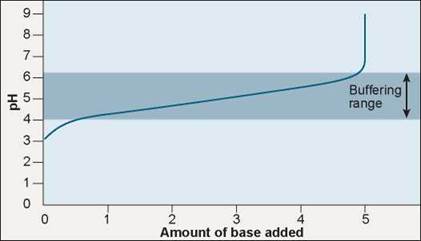
Figure 2.13. Buffers minimize changes in pH.
Adding a base to a solution neutralizes some of the acid present and so raises the pH. Thus, as the curve moves to the right, reflecting more and more base, it also rises to higher pH values. What a buffer does is to make the curve rise or fall very slowly over a portion of the pH scale, called the "buffering range" of that buffer.
The key buffer in human blood is an acid-base pair consisting of carbonic acid (acid) and bicarbonate (base). These two substances interact in a pair of reversible reactions. First, carbon dioxide (CO2) and H2O join to form carbonic acid (H2CO3) (step 2 in figure 2.14), which in a second reaction dissociates to yield bicarbonate ion (HCO3) and H+ (step 3). If some acid or other substance adds H+ to the blood, the HCO3- acts as a base and removes the excess H+ by forming H2CO3. Similarly, if a basic substance removes H+ from the blood, H2CO3 dissociates, releasing more H+ into the blood. The forward and reverse reactions that interconvert H2CO3 and HCO3- thus stabilize the blood’s pH.
For example, when you breathe in, your body takes up oxygen from the air and when you breathe out, your body releases carbon dioxide. When you hold your breath, CO2 accumulates in your blood and drives the chemical reactions in figure 2.14, producing carbonic acid. Can you hold your breath indefinitely? No, but not for the reason you might think. It is not lack of oxygen that forces you to breathe, but too much carbon dioxide. If you try and hold your breath for very long, CO2 accumulates in the blood, as shown in step 1 in figure 2.14, triggering the formation of carbonic acid (step 2) that dissociates into bicarbonate ion and H+ (step 3). This increase in H+ causes the blood to become more acidic. If the pH in the blood drops too low, pH sensors that are located in some of the major blood vessels of the body detect the change (step 4) and send signals to the brain. These signals, along with other sensory processes, stimulate the area of the brain that controls respiration, causing it to increase the rate of breathing. Hyperventilating, breathing very quickly, has the opposite effect, lowering the levels of CO2 in the blood. That is why you are told to breathe into a paper bag when you are hyperventilating, to increase your intake of CO2.
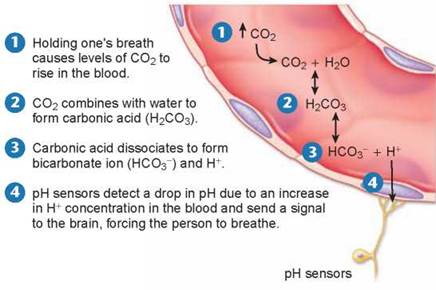
Figure 2.14. Holding your breath.
CO2 accumulates in the blood when a person holds his/her breath. CO2 combines with water, forming carbonic acid. Carbonic acid dissociates into bicarbonate and H+, which acts to lower the pH. The drop in pH is detected by sensors that stimulate the brain, causing the person to breathe.
Key Learning Outcome 2.5. A tiny fraction of water molecules spontaneously ionize at any moment, forming H+ and OH-. The pH of a solution is a measure of its H+ concentration. Low pH values indicate high H+ concentrations (acidic solutions), and high pH values indicate low H+ concentrations (basic solutions).
INQUIRY & ANALYSIS
Using Radioactive Decay to Date the Iceman
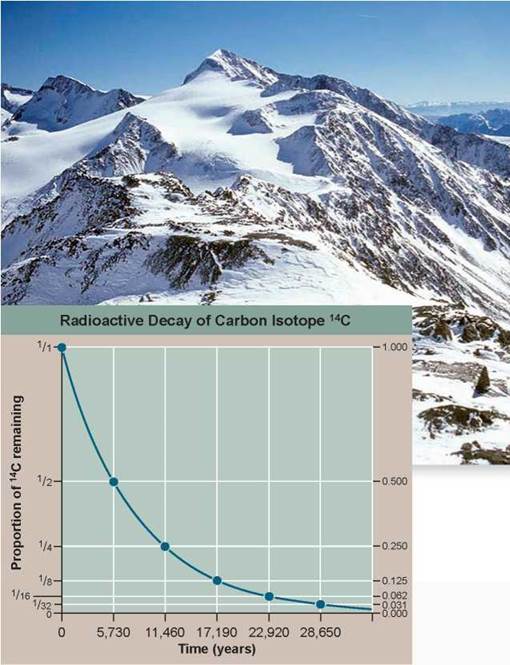
In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two Austrian hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he had sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, whom they named Otzi. They know his age, his health, the shoes and clothing he wore, what he ate, and that he died from an arrow that ripped through his back.
Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died.
How long ago did this Iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14C in Otzi's body. This procedure is discussed earlier in this chapter (see figure 2.8). The graph to the right displays the radioactive decay curve of the carbon isotope carbon-14 (14C); it takes 5,730 years for half of the 14C present in a sample to decay to nitrogen-14 (14N). When Otzi's carbon isotopes were analyzed, researchers determined that the ratio of 14C to 12C (a ratio is the size of one variable relative to another), also written as the fraction14C/12C, in Otzi's body is 0.435 of the fraction found in tissues of a person who has recently died.

1. Applying Concepts
a. Variable. In the graph, what is the dependent variable?
b. Proportion. What proportion (a proportion is the size of a variable relative to the whole) of the 14C present in Otzi's body when he died is still there today?
2. Interpreting Data Plot this proportion on the 14C radioactive decay curve above. How many half-lives does this point represent?
3. Making Inferences If Otzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14C to 12C to be, relative to that in your own body?
4. Drawing Conclusions How old are the remains of the Iceman Otzi?
5. Further Analysis
a. The radioactive iodine isotope 131I decays at a halflife of eight days. Plotted on the graph above, would its radioactive decay curve be above or below that of 14C?
b. Scientists often employ the radioactive decay of isotope potassium-40 (40K) into argon-40 (40Ar) to date old material. 40K has a half-life of 1.3 billion years. Would it be a better or poorer isotope than 14C to use in dating Otzi?
Test Your Understanding
1. The smallest particle into which a substance can be divided and still retain all of its chemical properties is
a. matter.
b. an atom.
c. a molecule.
d. mass.
2. An atom that has gained or lost one or more electrons is
a. an isotope.
b. a neutron.
c. an ion.
d. radioactive.
3. Atoms are held together by a force called a bond. The three types of bonds are
a. positive, negative, and neutral.
b. hydrophobic, hydrophilic, and van der Waals interactions.
c. magnetic, electric, and radioactive.
d. ionic, covalent, and hydrogen.
4. Carbon has four electrons in its outer electron shell, therefore
a. it has a completely filled outer electron shell.
b. it can form four single covalent bonds.
c. it does not react with any other atom.
d. it has a positive charge.
5. The partial separation of charge in the water molecule
a. results from the electrons’ greater attraction to the oxygen atom.
b. means the molecule has a positive end and a negative end.
c. indicates that the water molecule is a polar molecule.
d. All of the above.
6. Water has some very unusual properties. These properties occur because of the
a. hydrogen bonds between the individual water molecules.
b. covalent bonds between the individual water molecules.
c. hydrogen bonds within each individual water molecule.
d. ionic bonds between the individual water molecules.
7. Which of the following properties are somehow related to the need for significant heat energy to break hydrogen bonds?
a. cohesion and adhesion
b. hydrophobic and hydrophilic
c. heat storage and heat of vaporization
d. ice formation and high polarity
8. The attraction of water molecules to other water molecules is called
a. cohesion.
b. capillary action.
c. solubility.
d. adhesion.
9. Water sometimes ionizes, a single molecule breaking apart into a hydrogen ion and a hydroxide ion. Other materials may dissociate in water, resulting in either (1) an increase of hydrogen ions or (2) a decrease of hydrogen ions in the solution. We call the results
a. (1) acids and (2) bases.
b. (1) bases and (2) acids.
c. (1) neutral solutions and (2) neutronic solutions.
d. (1) hydrogen solutions and (2) hydroxide solutions.
10. Which of the following is not true about buffers?
a. A buffer takes up H+ from the solution.
b. A buffer keeps the pH relatively constant.
c. A buffer stops water from ionizing.
d. A buffer releases H+ into the solution.