THE LIVING WORLD
Unit Six. Animal Life
24.5. How Respiration Works: Gas Exchange
When oxygen has diffused from the air into the moist cells lining the inner surface of the lung, its journey has just begun. Passing from these cells into the bloodstream, the oxygen is carried throughout the body by the circulatory system, described in chapter 23. It has been estimated that it would take a molecule of oxygen three years to diffuse from your lung to your toe if it moved only by diffusion, unassisted by a circulatory system.
Oxygen moves within the circulatory system carried piggyback on the protein hemoglobin. Hemoglobin molecules contain iron, which binds oxygen, as shown in figure 24.6. The oxygen binds in a reversible way, which is necessary so that the oxygen can unload when it reaches the tissues of the body. Hemoglobin is manufactured within red blood cells and gives them their color. Hemoglobin never leaves these cells, which circulate in the bloodstream like ships bearing cargo. Oxygen binds to hemoglobin within these cells as they pass through the capillaries surrounding the alveoli of the lungs. Carried away within red blood cells, the oxygen-bearing hemoglobin molecules later release their oxygen molecules to metabolizing cells at different locations in the body.
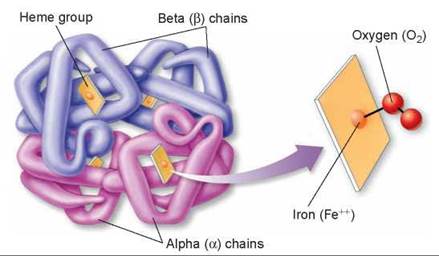
Figure 24.6. The hemoglobin molecule.
The hemoglobin molecule is actually composed of four protein chain subunits: two copies of the "alpha chain" and two copies of the "beta chain." Each chain is associated with a heme group, and each heme group has a central iron atom, which can bind to a molecule of oxygen.
Hemoglobin molecules act like little oxygen (O2) sponges, soaking up oxygen within red blood cells and causing more to diffuse in from the blood plasma. At the high O2 levels that occur in the blood supply of the lung (panel 1 in the Key Biological Process on the facing page), most hemoglobin molecules carry a full load of oxygen atoms. Later, in the tissues, the O2 levels are much lower, so that hemoglobin gives up its bound oxygen (as in panel 3).
In tissue, the presence of carbon dioxide (CO2) causes the hemoglobin molecule to assume a different shape, one that gives up its oxygen more easily. This speeds up the unloading of oxygen from hemoglobin even more. The effect of CO2 on oxygen unloading is of real importance, because CO2 is produced by the tissues at the site of cell metabolism. For this reason, the blood unloads oxygen more readily within those tissues undergoing metabolism and generating CO2.
CO2 Transport
At the same time the red blood cells are unloading oxygen (panel 3), they are also absorbing CO2 from the tissue. About 8% of the CO2 in blood is simply dissolved in plasma. Another 20% is bound to hemoglobin; however, it does not bind to the heme group but rather to another site on the hemoglobin molecule, and so it does not compete with oxygen binding. The remaining 72% of the CO2diffuses into the red blood cells. To maximize the amount of CO2 that diffuses from the tissues into the plasma, it is important to keep the levels of CO2 in the plasma low. The natural tendency is for the CO2 to diffuse out of the red blood cells back to the plasma where CO2 levels are low. To keep this from happening, the enzyme carbonic anhydrase combines CO2 molecules with water molecules in the red blood cells (panel 4) to form carbonic acid (H2CO3). This acid dissociates into bicarbonate (HCO3-) and hydrogen (H+) ions. The H+ binds to hemoglobin. A transporter protein in the membrane of the red blood cell moves the bicarbonate out of the red blood cell into the plasma. This transporter protein exchanges one chloride ion for a bicarbonate, a process called the chloride shift. This reaction keeps the levels of CO2 in the blood plasma low, facilitating the diffusion of more CO2 into it from the surrounding tissue. The facilitation is critical to CO2 removal, because the difference in CO2 concentration between blood and tissue is not large (only 5%). The formation of carbonic acid is also important in maintaining the acid-base balance of the blood, because bicarbonate serves as the major buffer of the blood plasma. You’ll recall from chapter 2 that a buffer is a substance that can adjust the levels of hydrogen ions (H+) in a solution, thereby controlling the pH of a solution.
The blood plasma carries bicarbonate ions back to the lungs. The lower CO2 concentration in the air inside the lungs causes the carbonic anhydrase reaction to proceed in the reverse direction (panel 6), releasing gaseous CO2, which diffuses outward from the blood into the alveoli. With the next exhalation, this CO2 leaves the body. The one-fifth of the CO2 bound to hemoglobin also leaves because hemoglobin has a greater affinity for O2 than for CO2 at low CO2 concentrations. The diffusion of CO2 outward from the red blood cells causes the hemoglobin within these cells to release its bound CO2and take up O2 instead. The red blood cells, with a new load of O2, then start their next respiratory journey.
NO Transport
Hemoglobin also has the ability to hold and release the gas nitric oxide (NO), which is produced in endothelial cells. Nitric oxide, although a noxious gas in the atmosphere, has an important physiological role in the body, acting on many kinds of cells to change their shape and function. For example, in blood vessels the presence of NO causes the blood vessels to expand because it relaxes the surrounding muscle cells. Thus, blood flow and blood pressure are regulated by the amount of NO released into the bloodstream.
Hemoglobin carries NO in a special form called super nitric oxide. In this form, NO has acquired an extra electron and is able to bind to an amino acid, called cysteine, present in hemoglobin. In the lungs, hemoglobin that is dumping CO2 and picking up O2 also picks up NO as super nitric oxide. In tissues, hemoglobin that is releasing its O2 and picking up CO2 may also release super nitric oxide as NO into the blood, making blood vessels expand, thereby increasing blood flow to the tissue. Alternatively, hemoglobin may trap any excesses of NO on its iron atoms left vacant by the release of oxygen, causing blood vessels to constrict, which reduces blood flow to the tissue. When the red blood cells return to the lungs, hemoglobin dumps its CO2 and the NO bound to the iron atoms. It is then ready to pick up O2 and super nitric oxide and continue the cycle.
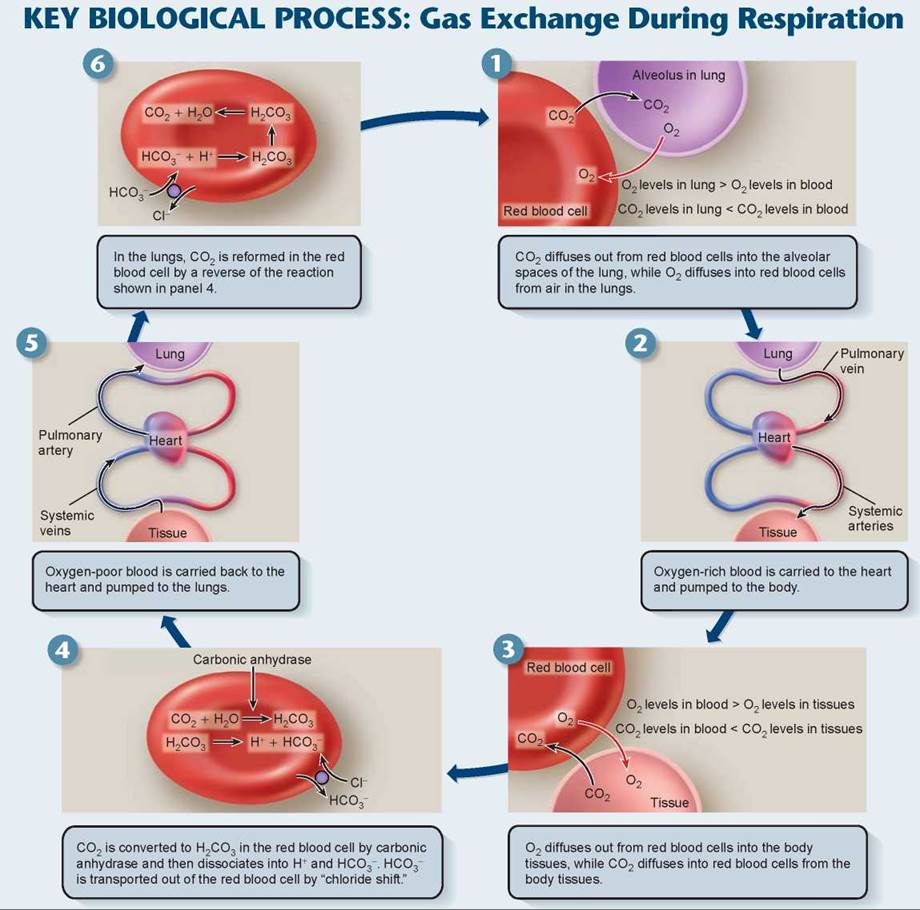
Key Learning Outcome 24.5. Oxygen and NO move through the circulatory system carried by the protein hemoglobin within red blood cells. Most CO2 is transported in the plasma as bicarbonate.