THE LIVING WORLD
Unit Six. Animal Life
30. Chemical Signaling Within the Animal Body
The pancreas gland is located behind the stomach and is connected to the front end of the small intestine by a narrow tube. It secretes a variety of digestive enzymes into the digestive tract through this tube, and for a long time it was thought to be solely an exocrine gland. In 1869, however, a German medical student named Paul Langerhans described some unusual clusters of cells scattered throughout the pancreas. In 1893, doctors concluded that these clusters of cells, which came to be called islets of Langerhans, produced a substance that prevented diabetes mellitus. Diabetes mellitus is a serious disorder in which affected individuals’ cells are unable to take up glucose from the blood, even though their levels of blood glucose become very high. Some individuals lose weight and literally starve; others develop poor circulation, sometimes resulting in amputation of limbs with restricted circulation. Diabetes is the leading cause of blindness among adults, and it accounts for one-third of all kidney failures. It is the seventhleading cause of death in the United States.
The substance produced by the islets of Langerhans, which we now know to be the peptide hormone insulin, was not isolated until 1922. Two young doctors working in a Toronto hospital injected an extract purified from beef pancreas glands into a 13-year-old boy, a diabetic whose weight had fallen to 29 kilograms (65 pounds) and who was not expected to survive. The hospital record gives no indication of the historic importance of the trial, only stating, “15 cc of MacLeod’s serum. 7-1/2 cc into each buttock.” With this single injection, the glucose level in the boy’s blood fell 25%—his cells were taking up glucose. A more potent extract soon brought levels down to near normal.
This was the first instance of successful insulin therapy. The islets of Langerhans in the pancreas produce two hormones that interact to govern the levels of glucose in the blood. These hormones are insulin and glucagon. Insulin is a storage hormone, designed to put away nutrients for leaner times. It promotes the accumulation of glycogen in the liver and triglycerides in fat cells. When food is consumed (left side of figure 30.7), beta cells in the islets of Langerhans secrete insulin, causing the cells of the body to take up and store glucose as glycogen and triglycerides to be used later. When body activity causes the level of glucose in the blood to fall as it is used as fuel (right side of figure 30.7), other cells in the islets of Langerhans, called alpha cells, secrete glucagon, which causes liver cells to release stored glucose and fat cells to break down triglycerides for energy use. The two hormones work together to keep glucose levels in the blood within a narrow range.
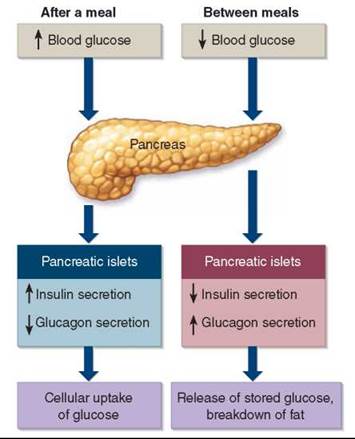
Figure 30.7. Insulin and glucagon secreted by the pancreas regulate blood glucose levels.
After a meal, an increased secretion of insulin by the beta cells of the pancreatic islets of Langerhans promotes the movement of glucose from blood into tissue cells. Between meals, an increased secretion of glucagon by the alpha cells of the pancreatic islets and decreased secretion of insulin cause the release of stored glucose and the breakdown of fat.
Over 23 million people in the United States, and over 246 million people worldwide, have diabetes. There are two kinds of diabetes mellitus. About 5% to 10% of affected individuals suffer from type I diabetes, an autoimmune disease in which the immune system attacks the islets of Langerhans, resulting in abnormally low insulin secretion. Called juvenile-onset diabetes, this type usually develops before age 20. Affected individuals can be treated by daily injections of insulin. Active research on the possibility of transplanting islets of Langerhans holds much promise as a lasting treatment for this form of diabetes.
In type II diabetes, the level of insulin in the blood is often higher than normal, but the cells don’t respond to insulin. This form of diabetes usually develops in people over 40 years of age. It is almost always a consequence of excessive weight; in the United States, 80% of those who develop type II diabetes are obese. The cells of some type II diabetics, overwhelmed with food, adjust their appetite for glucose downward by reducing their sensitivity to insulin. Similar to the way a drug addict’s neurons reduce their number of neurotransmitter receptors after continued exposure to a drug, the obese individual’s cells reduce their number of insulin receptors. To compensate, the pancreas pumps out ever-more insulin, and, in some people, the insulin- producing cells are unable to keep up with the ever-heavier workload and stop functioning. Type II diabetes is usually treatable with diet and exercise, and most affected individuals do not need daily injections of insulin.
Key Learning Outcome 30.4. Clusters of cells within the pancreas secrete the hormones insulin and glucagon. Insulin stimulates the storage of glucose as glycogen, while glucagon stimulates glycogen breakdown to glucose. Working together, these hormones keep glucose levels within a narrow range.
Biology and Staying Healthy
The Type II Diabetes Epidemic
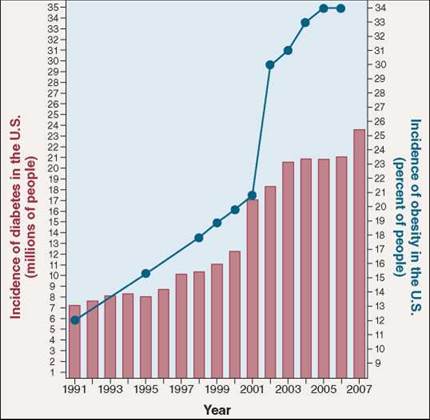
We Americans love to eat, but recently the Centers for Disease Control and Prevention released a report warning we are eating ourselves into a diabetes epidemic. Diabetes affected 7 million Americans in 1991. By the end of 2007, the number was over 23 million, more than 7% of all Americans—an alarming increase in just 16 years!
The same explosion of diabetes is being seen worldwide. Diabetes now affects 246 million people, and kills 3.8 million each year. Every 10 seconds one person dies of diabetes. In the same 10 seconds two more people develop the disease.
Diabetes is a disorder in which the body's cells fail to take up glucose from the blood. Tissues waste away as glucose- starved cells are forced to consume their own proteins.
Diabetes is the leading cause of kidney failure, blindness, and amputation in adults. Almost all the increase in diabetes in the last decade is in the 90% of diabetics who suffer from type II, or "adult-onset,” diabetes. These individuals lack the ability to use the hormone insulin.
Your body manufactures insulin after a meal as a way to alert cells that higher levels of glucose are coming soon. The insulin signal attaches to special receptors on the cell surfaces, which respond by causing the cell to turn on its glucosetransporting machinery. Some individuals who suffer from type II diabetes have normal or even elevated levels of insulin in their blood, and normal insulin receptors, but for some reason the binding of insulin to their cell receptors does not turn on the glucose-transporting machinery like it is supposed to do. For 30 years researchers have been trying to figure out why not.
How does insulin act to turn on a normal cell's glucose transporting machinery? Proteins called IRS proteins (the names refer not to tax collectors, but to insulin receptor substrate) snuggle up against the insulin receptor inside the cell. When insulin attaches to the receptor protein, the receptor responds by adding a phosphate group onto the IRS molecules. Like being touched by a red-hot poker, this galvanizes the IRS molecules into action. Dashing about, they activate a variety of processes, including an enzyme that turns on the glucose-transporting machinery.
When the IRS genes are deliberately taken out of action in so-called "knockout” mice, type II diabetes results. Are defects in the genes for IRS proteins responsible for type II diabetes? Probably not. When researchers look for IRS gene mutations in inherited type II diabetes, they don't find them. The IRS genes are normal.
This suggests that in type II diabetes something is interfering with the action of the IRS proteins. What might it be? An estimated 80% of those who develop type II diabetes are obese, a tantalizing clue. Look at the graph. Over the same 16 years that diabetes has undergone its explosive increase, the obesity rate increased from 12% of the U.S. population to 34%.
What is the link between diabetes and obesity? Recent research suggests an answer to this key question. A team of scientists at the University of Pennsylvania School of Medicine had been investigating why a class of drugs called thiazolidinediones (TZDs) helped combat diabetes. They found that TZDs cause the body's cells to use insulin more effectively, and this suggested to them that the TZD drug might be targeting a hormone.
The researchers then set out to see if they could find such a hormone in mice. In search of a clue, they started by looking to see which mouse genes were activated or deactivated by TZD. Several were. Examining them, they were able to zero in on the hormone they sought. Dubbed resistin, the hormone is produced by fat cells and prompts tissues to resist insulin. The same resistin gene is present in humans too. The researchers speculate that resistin may have evolved to help the body deal with periods of famine.
Mice given resistin by the researchers lost much of their ability to take up blood sugar. When given a drug that lowers resistin levels, these mice recovered the lost glucosetransporting ability.
Researchers don't yet know how resistin acts to lower insulin sensitivity, although blocking the action of IRS proteins seems a likely possibility.
Importantly, dramatically high levels of the hormone were found in mice obese from overeating. Finding this sort of result is like ringing a dinner bell to diabetes researchers.
If obesity is causing high resistin levels in humans, leading to type II diabetes, then resistin-lowering drugs might offer a diabetes cure!
On the scent of something important, resistin researchers are now shifting their efforts from mice to humans. Much needs to be checked, as there are no guarantees that what works in a mouse will do so in the same way in a human. Still, the excitement is tangible.