THE LIVING WORLD
Unit two. The Living Cell
4.2. The Plasma Membrane
Encasing all living cells is a delicate sheet of molecules called the plasma membrane. It would take more than 10,000 of these molecular sheets, which are about 5 nanometers thick, piled on top of one another to equal the thickness of this sheet of paper. However, the sheets are not simple in structure, like a soap bubble’s skin. Rather, they are made up of a diverse collection of proteins floating within a lipid framework like small boats bobbing on the surface of a pond. Regardless of the kind of cell they enclose, all plasma membranes have the same basic structure of proteins embedded in a sheet of lipids, called the fluid mosaic model.
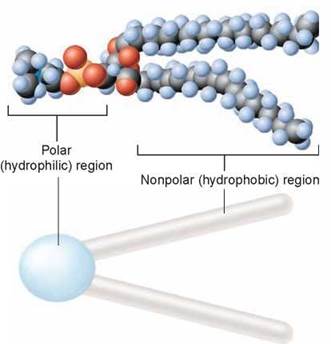
The lipid layer that forms the foundation of a plasma membrane is composed of modified fat molecules called phospholipids. A phospholipid molecule can be thought of as a polar head with two nonpolar tails attached to it, as shown above. The head of a phospholipid molecule has a phosphate chemical group linked to it, making it extremely polar (and thus water-soluble). The other end of the phospholipid molecule is composed of two long fatty acid chains. Recall from chapter 3 that fatty acids are long chains of carbon atoms with attached hydrogen atoms. The carbon atoms are the gray spheres you see above. The fatty acid tails are strongly nonpolar and thus water-insoluble. The phospholipid is often depicted diagrammatically as a ball with two tails.
Imagine what happens when a collection of phospholipid molecules is placed in water. A structure called a lipid bilayer forms spontaneously. How can this happen? The long nonpolar tails of the phospholipid molecules are pushed away by the water molecules that surround them, shouldered aside as the water molecules seek partners that can form hydrogen bonds. After much shoving and jostling, every phospholipid molecule ends up with its polar head facing water and its nonpolar tail facing away from water. The phospholipid molecules form a double layer, called a bilayer. As you can see in the figure above, the watery environments inside and outside the plasma membrane push the nonpolar tails to the interior of the bilayer. Because there are two layers with the tails facing each other, no tails are ever in contact with water. Thus, the interior of a lipid bilayer is completely nonpolar, and it repels any water-soluble molecules that attempt to pass through it, just as a layer of oil stops the passage of a drop of water (that’s why ducks do not get wet).
Cholesterol, another nonpolar lipid molecule, resides in the interior portion of the bilayer. Cholesterol is a multiringed molecule that affects the fluid nature of the membrane. Although cholesterol is important in maintaining the integrity of the plasma membrane, it can accumulate in blood vessels, forming plaques that lead to cardiovascular disease.
Proteins Within the Membrane
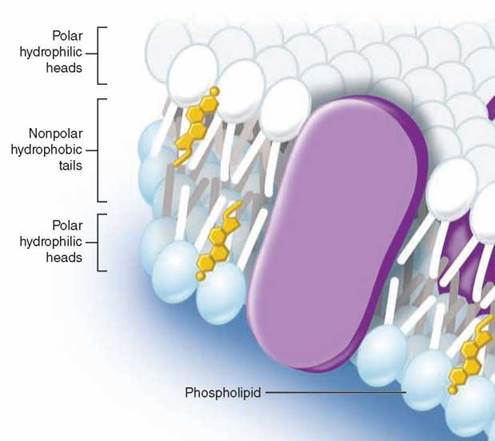
The second major component of every biological membrane is a collection of membrane proteins that float within the lipid bilayer. Membrane proteins function as channels, receptors, and cell surface markers. As you can see here in the figure, some proteins pass through the lipid bilayer, providing channels through which molecules and information pass. While some membrane proteins are fixed into position, others move about freely.
Many membrane proteins project up from the surface of the plasma membrane like buoys, often with carbohydrate chains or lipids attached to their tips like flags. These cell surface proteins act as markers to identify particular types of cells, or as beacons to bind specific hormones or proteins to the cell.
Protein channels that extend all the way across the bilayer provide passageways for ions and polar molecules like water so they can pass into and out of the cell. How do these transmembrane proteins manage to span the membrane, rather than just floating on the surface in the way that a drop of water floats on oil? The part of the protein that actually traverses the lipid bilayer is a specially constructed spiral helix of nonpolar amino acids—the red coiled areas of the transmembrane protein you see in the inset. Water responds to these nonpolar amino acids much as it does to non-polar lipid chains, and as a result the helical spiral is held within the lipid interior of the bilayer, anchored there by the strong tendency of water to avoid contact with these nonpolar amino acids.

Key Learning Outcome 4.2. All cells are encased within a delicate lipid bilayer sheet, the plasma membrane, within which are embedded a variety of proteins that act as markers or channels through the membrane.
A Closer Look
How Water Crosses the Plasma Membrane
One of the enduring mysteries of cellular biology has been the free movement of water into and out of cells. As early as the middle of the nineteenth century, biologists understood that there must be a way for water to pass across the plasma membrane. With the understanding in the middle of the 1950s that the plasma membrane was composed of a lipid bilayer, the problem posed by the free movement of water became even more puzzling. How could very polar water molecules traverse the very nonpolar environment found in the lipid core of the bilayer?
While some proposed that water leaked into cells through tiny imperfections in the bilayer, or through gaps that open up in the bilayer when the hydrocarbon tails flex and bend, these hypotheses were soon rejected, as they failed to explain how cell membranes managed to prevent the diffusion of protons (hydrogen ions), which are smaller than water. This ability is crucial to the life of a cell, as the difference in proton concentration between the inside and outside of cell organelles is the basis of energy metabolism, as described in chapters 6 and 7.
Clearly the answer to this puzzle lay with the proteins associated with the lipid bilayer. For 30 years researchers searched for the protein machinery that prevented ions from passing across the membrane while allowing water molecules to pass freely. With the acceptance of the fluid mosaic model of membrane structure proposed in 1972, the search focused on proteins that bridge the bilayer. In the mid-1980s, a Johns Hopkins University researcher named Peter Agre, studying red blood cell proteins, identified a previously unknown protein. "No one had seen it before, but we found that it was the fifth most abundant protein in the cell,” said Agre. "That's like coming across a big town that's not on the map. It gets your attention.”
Agre determined the amino acid sequence of the mysterious protein, and saw that it had long nonpolar segments that would allow it to repeatedly traverse the lipid bilayer, just as a cellular water channel would have to do. Perhaps this was the protein so many had sought.
To test this hypothesis, Agre carried out a simple experiment. He compared normal red blood cells that contained the protein with mutant red blood cells that lacked it. When he placed the cells in distilled lab water, cells with the protein in their membrane absorbed water and began to swell, while cells lacking the protein did nothing—they failed to absorb water and did not swell.
To be sure that some other undiscovered membrane protein could not be the cause of the water movement across the red blood cell plasma membrane, Agre repeated the experiment with liposomes, which are artificial cells made of pure lipid bilayer with no proteins—in essence, soap bubbles. As you might expect, water could not cross into liposomes, and he found they did not swell when immersed in distilled lab water. However, the liposomes did become permeable to water if Agre planted the protein in their bilayer.
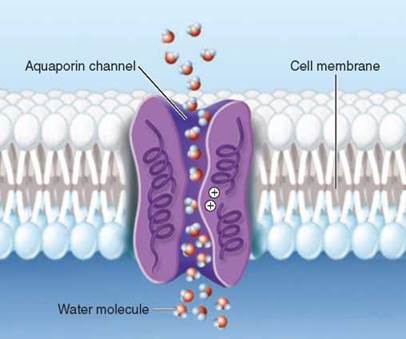
As a final test, Agre knew that mercury ions poison cells by keeping them from taking up and releasing water, and he showed that water transport through liposomes with his protein in their membranes was prevented by mercury too. Agre concluded that the protein he had discovered was indeed the water channel, and named the protein aquaporin, "water pore.” Peter Agre was awarded the Nobel Prize in Chemistry in 2003 for his discovery.
Working with other research teams on this exciting discovery, Agre reported in 2000 the results of X-ray diffraction studies that revealed the three-dimensional structure of aquaporin in atomic detail. Now it was possible to see in detail how the water channel functions.
The aquaporin channel Agre described is illustrated above. Individual water molecules pass through the narrow open channel single file, snaking their way along by orienting themselves in the local electrical field formed by the atoms of the channel wall. And now we see the key feature that allows the channel to exclude protons while passing larger water molecules. Look in the center of the pore. A cluster of positively-charged amino acids line the pore there. While this has no impact on the passage of water molecules because of their partial negative charges, the positive charges repel protons, which are also positively charged. The positive charges in the interior of the aquaporin channel act as a filter to prevent protons leaking through the passage.
In the last 10 years researchers have identified aquaporins in many kinds of bacteria, plants, and animals. There are at least 11 kinds in the human body alone. One kind, called AQP1, acts in your kidney to recover water that would otherwise be lost in your urine. Over 24 hours, AQP1 channels in your kidneys recover about 120 liters of water!