THE LIVING WORLD
Unit Eight. The Living Environment
36.5. Soil Nutrients and Other Chemical Cycles
The Nitrogen Cycle
Organisms contain a lot of nitrogen (a principal component of protein) and so does the atmosphere, which is 78.08% nitrogen gas (N2). However, the chemical connection between these two reservoirs is very delicate because most living organisms are unable to use the N2 so plentifully available in the air surrounding them. The two nitrogen atoms of N2 are bound together by a particularly strong “triple” covalent bond that is very difficult to break. Luckily, a few kinds of bacteria can break the nitrogen triple bond and bind its nitrogen atoms to hydrogen (forming “fixed” nitrogen, ammonia [NH3], which becomes ammonium ion [NH4+]) in a process called nitrogen fixation.
Bacteria evolved the ability to fix nitrogen early in the history of life, before photosynthesis had introduced oxygen gas into the earth’s atmosphere, and that is still the only way the bacteria are able to do it—even a trace of oxygen poisons the process. In today’s world, awash with oxygen, these bacteria live encased within bubbles called cysts that admit no oxygen or within special airtight cells in nodules of tissue on the roots of beans, aspen trees, and a few other plants. Figure 36.9 shows the workings of the nitrogen cycle. Bacteria make needed nitrogen available to other organisms. The nitrogen moves up the food chain as one organism eats another and eventually returns following their deaths or through their excretions. Decomposing bacteria and then ammonifying bacteria return the nitrogen to ammonia and ammonium ion forms. Continuing the cycle, nitrifying bacteria can convert ammonium ion into nitrate (NO3-), and denitrifying bacteria are able to convert nitrate back into atmospheric nitrogen (N2).
The growth of plants in ecosystems is often severely limited by the availability of “fixed” nitrogen in the soil, which is why farmers fertilize fields. This agricultural practice is a very old one, known even to primitive societies—the American Indians instructed the pilgrims to bury fish, a rich source of fixed nitrogen, with their corn seeds. Today most fixed nitrogen added to soils by farmers is not organic but instead is produced in factories by industrial rather than bacterial nitrogen fixation, a process that accounts for a prodigious 30% of the entire nitrogen cycle.

Figure 36.9. The nitrogen cycle.
Relatively few kinds of organisms—all of them bacteria—can convert atmospheric nitrogen into forms that can be used for biological processes.
The Phosphorus Cycle
Phosphorus is an essential element in all living organisms, a key part of both ATP and DNA. Phosphorus is often in very limited supply in the soil of particular ecosystems, and because phosphorus does not form a gas, none is available in the atmosphere. Most phosphorus exists in soil and rock as the mineral calcium phosphate, which, as shown in figure 36.10, dissolves in water to form phosphate ions (Coca-Cola is a sweetened solution of phosphate ions). These phosphate ions are absorbed by the roots of plants and used by them to build organic molecules like ATP and DNA. When the plants and animals die and decay, bacteria in the soil convert the organic phosphorus back into phosphorus ions, completing the cycle.
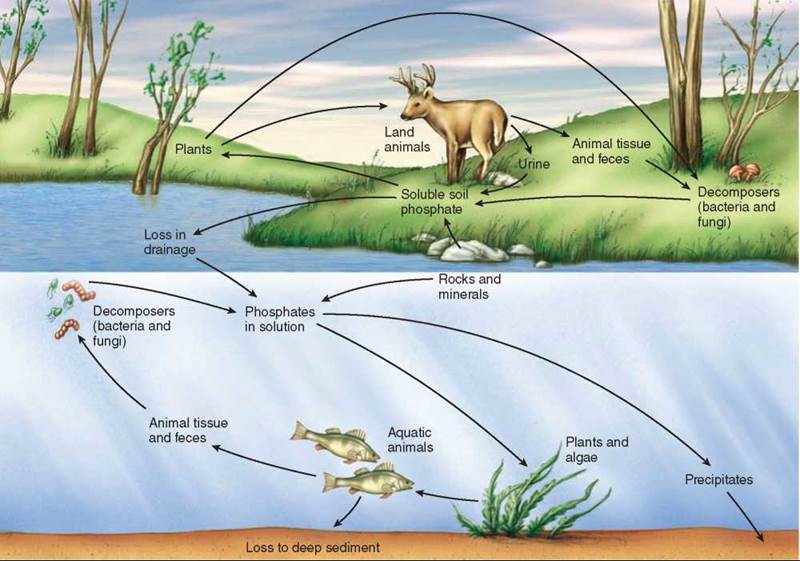
Figure 36.10. The phosphorus cycle.
Phosphorus plays a critical role in plant nutrition; next to nitrogen, phosphorus is the element most likely to be so scarce that it limits plant growth.
The phosphorus level in freshwater lake ecosystems is often quite low, preventing much growth of photosynthetic algae in these systems. Such ecosystems are particularly vulnerable to the inadvertent addition of phosphorus by human activity. For example, agricultural fertilizers and many commercial detergents are rich in phosphorus. Pollution of a lake by the addition of phosphorus to its waters first produces a green scum of algal growth on the surface of the lake, which then, if the pollution continues, proceeds to “kill” the lake. After the initial bloom of rapid algal growth, aging algae die, and bacteria feeding on the dead algae cells use up so much of the lake’s dissolved oxygen that fish and invertebrate animals suffocate. Such rapid, uncontrolled growth caused by excessive nutrients in an aquatic ecosystem is called eutrophication.
The Cycling of Other Chemicals
Many other chemicals cycle through an ecosystem and must be maintained in a balanced state for the ecosystem to be healthy. Proper balance is important. Some chemicals can become harmful when their concentrations exceed normal levels for cycling, as we saw with phosphorus. Other chemicals, when in excess of normal cycling levels, can have similar devastating effects on an ecosystem.
Sulfur, a chemical that cycles through the atmosphere, can harm an ecosystem when large amounts of it are pumped into the atmosphere through coal-burning power plants. The excess sulfur combines with water vapor and oxygen, producing sulfuric acid. This acid then reenters the ecosystem as precipitation. This “acid rain” is discussed further in chapter 38.
Heavy metals, which include mercury, cadmium, and lead, are particularly damaging as they cycle through biological food chains, as they tend to progressively accumulate in organisms of higher trophic levels. This process, called biological magnification, is discussed further in chapter 38.
Key Learning Outcome 36.5. Most of the earth's atmosphere is diatomic nitrogen gas that cannot be used by most organisms. Certain bacteria are able to convert this nitrogen gas into ammonia through nitrogen fixation. These nitrogen atoms then cycle through the earth's ecosystem. Phosphorus, critical to organisms, is available in soil and dissolved in water. It cycles between organisms and the environment and is often the limiting factor in determining what organisms are able to live in an ecosystem.