THE LIVING WORLD
Unit Eight. The Living Environment
38. Human Influences on the Living World
For 2 billion years, life was trapped in the oceans because radiation from the sun seared the earth’s surface unchecked. Nothing could survive that bath of destructive energy. Living things were able to leave the oceans and colonize the surface of the earth only after a protective shield of ozone had been added to the atmosphere by photosynthesis. Imagine if that shield were taken away. Alarmingly, it appears that we are destroying it ourselves. Starting in 1975, the earth’s ozone shield began to disintegrate. Over the South Pole in September of that year, satellite photos revealed that the ozone concentration was unexpectedly less than elsewhere in the earth’s atmosphere. It was as if some “ozone eater” were chewing it up in the Antarctic sky, leaving a mysterious zone of lower-than- normal ozone concentration, an ozone hole. For many years after that, more of the ozone was depleted, and the hole grew bigger and deeper. The satellite image in figure 38.5 shows lower levels of ozone as light purple (Antarctica is also colored purple, indicating that the ozone hole completely covers it). The graph indicates the size of the ozone hole over a 10-year period, with the largest hole appearing in September of 2000 (the blue line).
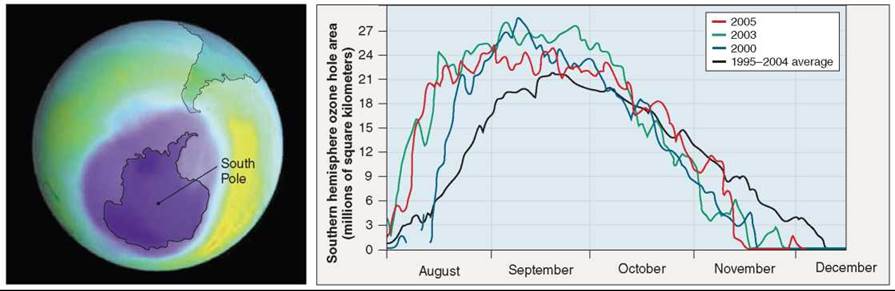
Figure 38.5. The ozone hole over Antarctica.
For decades NASA satellites have tracked the extent of ozone depletion over Antarctica. Every year since 1975 an ozone "hole" has appeared in August when sunlight triggers chemical reactions in cold air trapped over the South Pole during Antarctic winter. The hole intensifies during September before tailing off as temperatures rise in November-December. In 2000, the 28.4-million-square-kilometer hole (purple in the satellite image) covered an area larger than the United States, Canada, and Mexico combined, the largest hole ever recorded. In September 2000, the hole extended over Punta Arenas, a city of about 120,000 people in southern Chile, exposing residents to very high levels of UV radiation.
What is eating the ozone? Scientists soon discovered that the culprit was a class of chemicals that everyone had thought to be harmless: chlorofluorocarbons (CFCs). CFCs were invented in the 1920s, a miracle chemical that was stable, harmless, and a near-ideal heat exchanger. Throughout the world, CFCs are used in large amounts as coolants in refrigerators and air conditioners, as the gas in aerosol dispensers, and as the foaming agent in Styrofoam containers. All of these CFCs eventually escape into the atmosphere, but no one worried about this until recently, both because CFCs were thought to be chemically inert and because everyone tends to think of the atmosphere as limitless. But CFCs are very stable chemicals, and have continually accumulated in the atmosphere.
It turned out that the CFCs were causing mischief the chemists had not imagined. High over the South and North Poles, nearly 50 kilometers up, where it is very, very cold, the CFCs stick to frozen water vapor and act as catalysts of a chemical reaction. Just as an enzyme carries out a reaction in your cells without being changed itself, so the CFCs catalyze the conversion of ozone (O3) into oxygen (O2) without being used up themselves. Very stable, the CFCs in the atmosphere just keep at it—little machines that never stop. They are still there, still doing it, today. The drop in ozone worldwide is now over 3%.
Ultraviolet radiation is a serious human health concern. Every 1% drop in the atmospheric ozone content is estimated to lead to a 6% increase in the incidence of skin cancers. At middle latitudes, the drop of approximately 3% that has occurred worldwide is estimated to have led to an increase of perhaps as much as 20% in lethal melanoma skin cancers.
Experts generally agree that levels of ozone-killing chemicals in the upper atmosphere are leveling off since more than 180 countries in the 1980s signed an international agreement, which phases out the manufacture of most CFCs. The 2005 ozone hole peaked at about 25 million square kilometers (the size of North America), below the 2000 record size of about 28.4 million square kilometers. Current computer models suggest the Antarctic ozone hole should recover by 2065, and the lesser-damaged ozone layer over the Arctic by about 2023.
Key Learning Outcome 38.4. CFCs and other chemicals are catalytically destroying the ozone in the upper atmosphere, exposing the earth's surface to dangerous radiation. International attempts to solve the problem appear to be succeeding.