THE LIVING WORLD
Unit two. The Living Cell
4.10. Diffusion
For cells to survive, food particles, water, and other materials must pass into the cell, and waste materials must be eliminated. All of this moving back and forth across the cell’s plasma membrane occurs in one of three ways: (1) water and other substances diffuse through the membrane; (2) proteins in the membrane act as doors that admit certain molecules only; or (3) food particles and sometimes liquids are engulfed by the membrane folding around them. First we will examine diffusion.
Diffusion
Most molecules are in constant motion. How a molecule moves—just where it goes—is totally random, like shaking marbles in a cup. So, if two kinds of molecules are added together, they soon mix. The random motion of molecules always tends to produce uniform mixtures. To see how this works, visualize the simple experiment shown in the Key Biological Process illustration below, in which a lump of sugar is dropped into a beaker of water. The lump slowly breaks apart into individual sugar molecules, which move about randomly until eventually the sugar molecules become evenly distributed throughout the water in the beaker (panel 4 below) as individual molecules take long random journeys. This process of random molecular mixing is called diffusion.
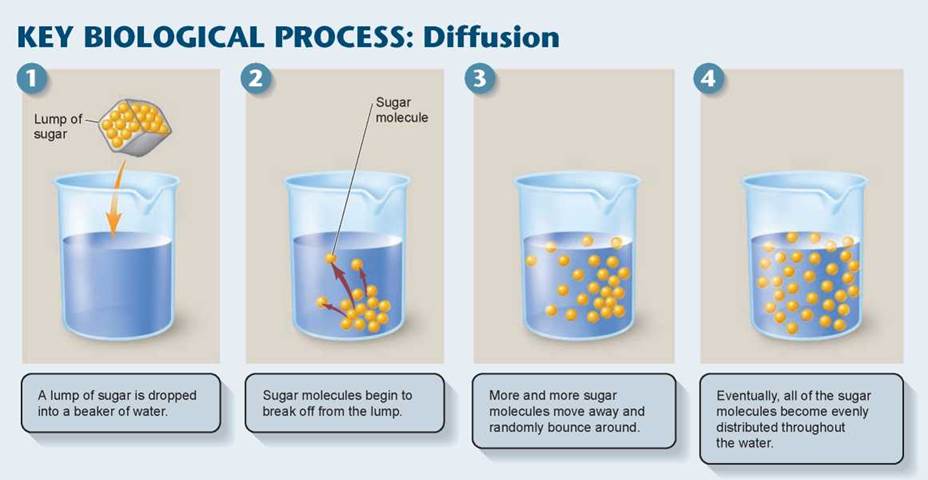
Selective Permeability
Diffusion becomes important to the life of a cell because of the chemical nature of biological membranes. As figure 4.20 illustrates, the nonpolar nature of the lipid bilayer determines which substances can pass and which ones cannot. Oxygen gas and carbon dioxide are not repelled by the bilayer and can freely cross, and so can small nonpolar fats and lipids. However, sugars like glucose cannot, and neither can proteins. In fact, no polar molecule can freely travel across a biological membrane because of the barrier to diffusion imposed by the lipid bilayer. This is true of charged ions like Na+, Cl-, and H+ and is also true of water molecules, which are very polar. It was once thought that water somehow “leaked” across the plasma membrane, slipping through gaps that open up when the hydrocarbon tails of the bilayer flex and bend, but biologists now discount this, as discussed on page 76. Later in this chapter we will further explore the movement of water molecules across plasma membranes.
Because the plasma membrane admits some substances and not others, it is said to be selectively permeable. The selective permeability of a cell’s plasma membrane is arguably its most important property. What a cell is and how it functions are largely determined by which molecules it admits and which it refuses. As you proceed through this chapter, you will encounter a variety of ways in which particular cell types exercise control over their admission policy.
Concentration Gradients
The number of molecules present per unit volume on one side of a membrane is its concentration there. As a direct consequence of a cell membrane’s selective permeability (figure 4.20), polar and charged molecules and ions often have a different concentration on one side of a membrane than on the other. This difference in concentration levels is called a concentration gradient.
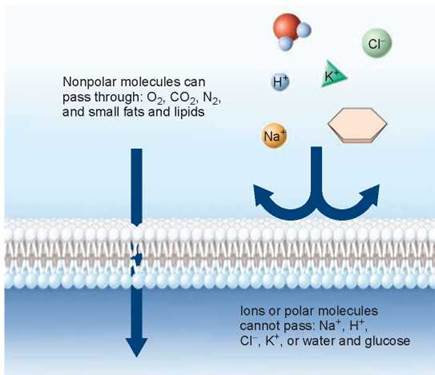
Figure 4.20. Membranes are selectively permeable.
Nonpolar molecules, such as those on the left, can pass through the membrane, but polar molecules and ions (on the right) cannot.
When a substance moves from regions where its concentration is high to regions where its concentration is lower, it is said to move down its concentration gradient. How does a molecule “know” in what direction to move? It doesn’t— molecules don’t “know” anything. A molecule is equally likely to move in any direction and is constantly changing course in random ways. There are simply more molecules able to move from where they are common than from where they are scarce. This mixing process is simply the result of diffusion. In fact, diffusion is formally defined as the net movement of molecules down a concentration gradient toward regions of lower concentration (that is, where there are relatively fewer of them) as a result of random motion.
Importantly, each substance tends to diffuse in the direction established by its own concentration gradient, not the gradients established by other substances present in the same fluid. Thus the rate at which oxygen diffuses across a plant cell’s plasma membrane into the cell is affected by that cell’s oxygen concentration relative to the air, but not by its carbon dioxide concentration.
Molecules in Motion
If you think about it, a concentration gradient is a form of energy. Just as a boulder perched on a hilltop stores the energy it took to lift it there—energy released if the boulder rolls downhill, so a concentration gradient across a membrane can drive the movement of molecules across the membrane in the direction of lower concentration.
How fast molecules diffuse across a membrane (what physiologists call the rate of diffusion) is determined by two characteristics of the cell, and also by the physical characteristics of the environment in which the cell finds itself.
The Steepness of the Concentration Gradient. Diffusion is most rapid when gradients are steepest. You can see why this must be so: Many more molecules randomly move away from a region of high concentration than into it from a region of low concentration. As this process continues, the number of molecules in the region of high concentration continually decreases, and the rate of diffusion slows down as fewer molecules leave and more arrive. Eventually, the same number is arriving as leaving. At this point, diffusion slows to a halt. Molecules are still moving, but the relative concentrations of the two regions no longer change—what a physiologist calls a state of dynamic equilibrium.
The Area of the Membrane Available for Diffusion. Some gases like oxygen and a variety of small lipid-soluble molecules diffuse readily across the lipid bilayer of biological membranes, and the rate of their diffusion into or out of a cell is most rapid when the proportion of membrane surface occupied by the lipid bilayer is greatest. Membranes with larger portions of embedded proteins will exhibit lower rates of diffusion of gases and lipids moving through the bilayer, as the area available for their diffusion is less. Similarly, the rate of diffusion of charged ions like Na+ and polar molecules like sugars and amino acids is greatest in membranes with the greatest number of protein channels with passages through which these molecules can pass. Because these channels are often quite specific, allowing only a particular ion or molecule to pass, the rate of diffusion of a substance is only affected by the number of channels actually available to it, rather than by the overall proportion of the membrane surface taken up by protein.
Physical Characteristics of the Cell Environment. Temperature has a strong influence on the rate of diffusion, for the simple reason that higher temperatures cause molecules to move faster. In general, the rate of diffusion is greater for cells of organisms living at higher temperatures. High pressure also encourages faster diffusion, as molecules collide more often. This effect becomes quite important for organisms living in the deep ocean, where pressures are much higher than on the earth’s surface. A third physical characteristic that influences the rate of diffusion across some cells in an important way is the electrical field in which a cell finds itself. An electric gradient across a nerve cell membrane can have an important influence on the rate at which ions diffuse in and out.
Key Learning Outcome 4.10. Random movements of molecules cause them to mix uniformly in solution, a process called diffusion. Molecules tend to diffuse down their concentration gradients, faster when the gradient is steeper.