THE LIVING WORLD
Unit two. The Living Cell
4.12. Osmotic
Diffusion allows molecules like oxygen, carbon dioxide, and nonpolar lipids to cross the plasma membrane. The movement of water molecules is not blocked because there are many small channels, called aquaporins, that allow water to pass freely through the membrane (see page 76).
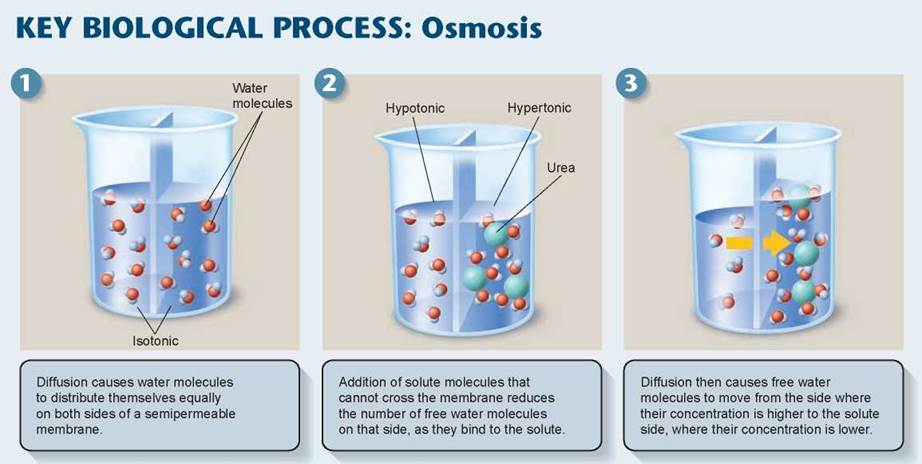
Because water is so important, biologically, the diffusion of water molecules from an area of high concentration to an area of lower concentration is given a specific name, called osmosis. However, the number of water molecules that are free to diffuse across the membrane is dependent upon the concentration of other substances in solution. To understand how water moves into and out of a cell, let’s focus on the water molecules already present inside a cell. What are they doing? Many of them are interacting with the sugars, proteins, and other polar molecules inside. Remember, water is very polar itself and readily interacts with other polar molecules. These “social” water molecules are not randomly moving about as they were outside; instead, they remain clustered around the polar molecules they are interacting with. As a result, while water molecules keep coming into the cell by random motion, they don’t randomly come out again. Because more water molecules come in than go out, there is a net movement of water into the cell. The experiment presented in the Key Biological Process illustration below shows what happens. The right side of the beaker represents the inside of a cell and the left side is a watery environment. When the polar molecule urea is present in the cell, as in panel 2 below, water molecules cluster around each urea molecule and are no longer able to pass through the membrane to the “outside.” In effect, the polar solute has reduced the number of free water molecules. Because the “outside” of the cell (on the left) has more unbound water molecules, water moves by diffusion into the cell (from the left to the right).
The concentration of all particles dissolved in a solution (called solutes) is called the osmotic concentration of the solution. If two solutions have unequal osmotic concentrations, the solution with the higher solute concentration, like the right side of the beaker below, is said to be hypertonic (Greek hyper, more than), and the solution with the lower concentration, like the left side of the beaker, is hypotonic (Greek hypo, less than). If the osmotic concentrations of the two solutions are equal, the solutions are isotonic (Greek iso, the same).
In cells, the plasma membrane separates two aqueous solutions, one inside the cell (the cytoplasm) and one outside (the extracellular fluid). The direction of the net diffusion of water across this membrane is determined by the osmotic concentrations of the solutions on either side. For example, if the cytoplasm of a cell was hypotonic to the extracellular fluid, water would diffuse out of the cell, toward the solution with the higher concentration of solutes (and, therefore, the lower concentration of unbound water molecules). This loss of water from the cytoplasm would cause the cell to shrink until the osmotic concentrations of the cytoplasm and the extracellular fluid become equal.
Osmotic Pressure
What would happen if the cell’s cytoplasm were hypertonic to the extracellular fluid? In this situation, water would diffuse into the cell from the extracellular fluid, causing the cell to swell. The pressure of the cytoplasm pushing out against the cell membrane, called hydrostatic pressure, would increase. On the other hand, the osmotic pressure, defined as the pressure that must be applied to stop the osmotic movement of water across a membrane, would also be at work. If the membrane were strong enough, the cell would reach an equilibrium, at which the osmotic pressure, which tends to drive water into the cell, is exactly counterbalanced by the hydrostatic pressure, which tends to drive water back out of the cell. However, a plasma membrane by itself cannot withstand large internal pressures, and an isolated cell under such conditions would burst like an overinflated balloon. Accordingly, it is important for animal cells to maintain isotonic conditions.
Figure 4.21 illustrates how solutes create osmotic pressure. Look first at the red blood cells at the top of the figure. On the left, in a hypertonic solution like ocean water, there is a net movement of water molecules out of the red blood cell toward the higher concentration of solutes outside, causing the cell to shrivel. In an isotonic solution (in the middle), the concentration of solutes on either side of the red blood cell membrane is the same. Osmosis still occurs, but water diffuses into and out of the cell at the same rate, and the cell doesn’t change size. In a hypotonic solution on the right, the concentration of solutes is higher within the cell than outside, so the net movement of water is into the cell. This is the situation utilized by Peter Agre in the experiment described on page 76 in which he demonstrated that aquaporin was a functioning water channel. A red blood cell is an enclosed structure, so as water entered a cell placed in a hypotonic solution (in his case, pure water), pressure is applied to the cell membrane causing the cell to swell, becoming spherical. This swelling can continue until the cell membrane can stretch no more and ruptures.
Now look at the plant cells at the bottom of figure 4.21. In these cells, unlike animal cells, the hydrostatic pressure generated by osmosis is counterbalanced by osmotic pressure, the force required to stop the flow of water into the cell. Plant cells have strong cell walls that can apply adequate osmotic pressure to keep the cell from rupturing.

Figure 4.21. How solutes create osmotic pressure.
A cell is an enclosed structure, and so as water enters the cell from a hypotonic solution, pressure is applied to the plasma membrane until the cell ruptures. This hydrostatic pressure is counterbalanced by osmotic pressure, the force required to stop the flow of water into the cell. Plant cells have strong cell walls that can apply adequate osmotic pressure to keep the cell from rupturing.
Maintaining Osmotic Balance
Organisms have developed many solutions to the osmotic dilemma posed by being hypertonic to their environment.
Extrusion. Some single-celled eukaryotes like the protist Paramecium use organelles called contractile vacuoles to remove water. Each vacuole collects water from various parts of the cytoplasm and transports it to the central part of the vacuole, near the cell surface. The vacuole possesses a small pore that opens to the outside of the cell. By contracting rhythmically, the vacuole pumps water out through the pore that is continuously seeping into the cell by osmosis.
Isosmotic Solutions. Some organisms that live in the ocean adjust their internal concentration of solutes to match that of the surrounding seawater. Isotonic with respect to their environment, there is no net flow of water into or out of these cells. Many terrestrial animals solve the problem in a similar way, by circulating a fluid through their bodies that bathes cells in an isotonic solution. The blood in your body, for example, contains a high concentration of the protein albumin, which elevates the solute concentration of the blood to match your cells. Sharks maintain a high concentration of urea in their blood and body fluids, keeping their cells isosmotic with respect to the sea water in which they swim.
Turgor. Most plant cells are hypertonic to their immediate environment, containing a high concentration of solutes in their central vacuoles. The resulting internal hydrostatic pressure, known as turgor pressure, presses the plasma membrane firmly against the interior of the cell wall as you saw in figure 4.7, making the cell rigid. Most green plants depend on turgor pressure to maintain their shape, and wilt when they lack sufficient water.
Key Learning Outcome 4.12. Water molecules associated with polar solutes are not free to diffuse, so there is a net movement of water across a membrane toward the side with less "free" water. Osmosis is the diffusion of water, but not solutes, across a membrane. Cells must maintain an osmotic balance to function properly.