THE LIVING WORLD
Unit two. The Living Cell
5. Energy and Life

All of life is driven by energy. It took energy for these mice to climb up the wheat stalks. Their mousely activities while perched on the stalks—looking for danger, generating body heat, wiggling their whiskers—take energy. The energy comes from the wheat kernels and other foods these mice eat. By breaking the chemical bonds of carbohydrates and other molecules in the wheat kernels, and transferring the energy of these bonds to those of a “molecular currency” called ATP, the mice are able to capture the chemical energy in their food and put it to work. The cells of the mice perform this feat with the aid of enzymes, which are macromolecules with highly specialized shapes. Each enzyme’s shape has a surface cavity called an active site, into which some specific chemical in the cell fits precisely, like a foot fits into a shoe of the proper size. When the chemical nudges in, the enzyme responds by bending, stressing particular covalent bonds in the chemical and so triggering a specific chemical reaction. The chemistry of life is enzyme chemistry.
5.1. The Flow of Energy in Living Things
We are about to begin our discussion of energy and cellular chemistry. Although these subjects may seem difficult at first, remember that all life is driven by energy. The concepts and processes discussed in the next three chapters are key to life. We are chemical machines, powered by chemical energy, and for the same reason that a successful race car driver must learn how the engine of a car works, we must look at cell chemistry. Indeed, if we are to understand ourselves, we must “look under the hood” at the chemical machinery of our cells and see how it operates.
As described in chapter 2, energy is defined as the ability to do work. It can be considered to exist in two states: kinetic energy and potential energy. Kinetic energy is the energy of motion. Objects that are not in the process of moving but have the capacity to do so are said to possess potential energy, or stored energy. The difference in the two states of energy is being experienced by the young man in figure 5.1. A boulder perched on a hilltop (figure 5.1a) has potential energy; after the man pushes the boulder and it begins to roll downhill (figure 5.1b), some of the boulder’s potential energy is converted into kinetic energy. All of the work carried out by living organisms also involves the transformation of potential energy to kinetic energy.
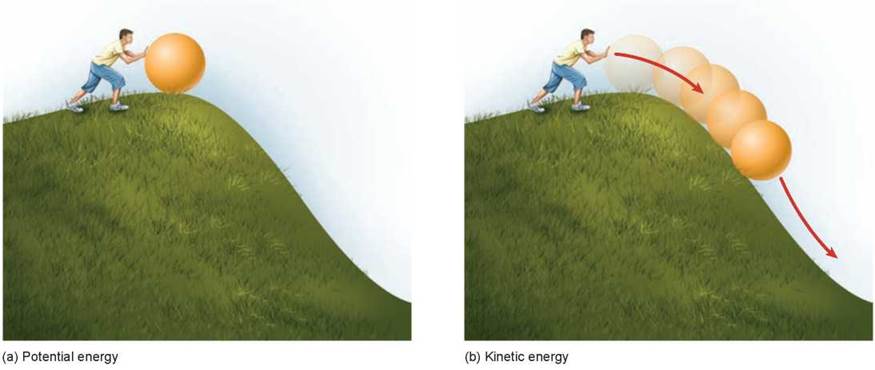
Figure 5.1. Potential and kinetic energy.
Objects that have the capacity to move but are not moving have potential energy, while objects that are in motion have kinetic energy. (a) The energy required to move the ball up the hill is stored as potential energy. (b) This stored energy is released as kinetic energy as the ball rolls down the hill.
Energy exists in many forms: mechanical energy, heat, sound, electric current, light, or radioactive radiation. Because it can exist in so many forms, there are many ways to measure energy. The most convenient is in terms of heat, because all other forms of energy can be converted to heat. Thus the study of energy is called thermodynamics, meaning “heat changes.” Energy flows into the biological world from the sun, which shines a constant beam of light on the earth. It is estimated that the sun provides the earth with more than 13 x 1023 calories per year, or 40 million billion calories per second! Plants, algae, and certain kinds of bacteria capture a fraction of this energy through photosynthesis. In photosynthesis, energy garnered from sunlight is used to combine small molecules (water and carbon dioxide) into more complex molecules (sugars). These complex sugar molecules have potential energy due to the arrangement of their atoms. This potential energy, in the form of chemical energy, does the work in cells. Recall from chapter 2 that an atom consists of a central nucleus surrounded by one or more orbiting electrons, and a covalent bond forms when two atomic nuclei share electrons. Breaking such a bond requires energy to pull the nuclei apart. Indeed, the strength of a covalent bond is measured by the amount of energy required to break it. For example, it takes 98.8 kcal to break 1 mole (6.023 x 1023) of carbon-hydrogen (C-H) bonds.
All the chemical activities within cells can be viewed as a series of chemical reactions between molecules. A chemical reaction is the making or breaking of chemical bonds—gluing atoms together to form new molecules or tearing molecules apart and sometimes sticking the pieces onto other molecules.
Key Learning Outcome 5.1. Energy is the capacity to do work, either actively (kinetic energy) or stored for later use (potential energy). Chemical reactions occur when the covalent bonds linking atoms together are formed or broken.