THE LIVING WORLD
Unit two. The Living Cell
6. Photosynthesis: Acquiring Energy from the Sun
6.2. How Plants Capture Energy from Sunlight
Where is the energy in light? What is there about sunlight that a plant can use to create chemical bonds? The revolution in physics in the twentieth century taught us that light actually consists of tiny packets of energy called photons, which have properties both of particles and of waves. When light shines on your hand, your skin is being bombarded by a stream of these photons smashing onto its surface.
Sunlight contains photons of many energy levels, only some of which we “see.” We call the full range of these photons the electromagnetic spectrum. As you can see in figure 6.1, some of the photons in sunlight have shorter wavelengths (toward the left side of the spectrum) and carry a great deal of energy—for example, gamma rays and ultraviolet (UV) light. Others such as radio waves carry very little energy and have longer wavelengths (hundreds to thousands of meters long). Our eyes perceive photons carrying intermediate amounts of energy as visible light, because the retinal pigment molecules in our eyes, which are different from chlorophyll, absorb only those photons of intermediate wavelength. Plants are even more picky, absorbing mainly blue and red light and reflecting back what is left of the visible light. To understand why plants are green, look at the green tree in figure 6.2. The full spectrum of visible light shines on the leaves of this tree, and only the green wavelengths of light are not absorbed. They are reflected off the leaf, which is why our eyes perceive leaves as green.
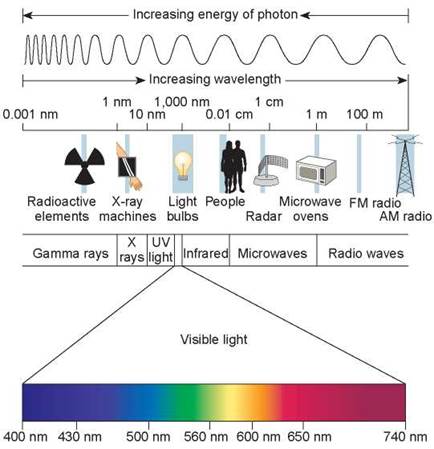
Figure 6.1. Photons of different energy: the electromagnetic spectrum.
Light is composed of packets of energy called photons. Some of the photons in light carry more energy than others. Light, a form of electromagnetic energy, is conveniently thought of as a wave. The shorter the wavelength of light, the greater the energy of its photons. Visible light represents only a small part of the electromagnetic spectrum, that with wavelengths between about 400 and 740 nanometers.
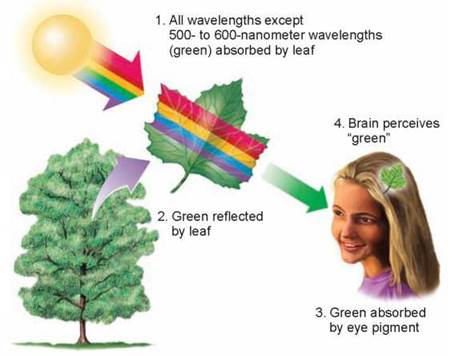
Figure 6.2. Why are plants green?
A leaf containing chlorophyll absorbs a broad range of photons- all the colors in the spectrum except for the photons around 500 to 600 nanometers. The leaf reflects these colors. These reflected wavelengths are absorbed by the visual pigments in our eyes, and our brains perceive the reflected wavelengths as "green."
How can a leaf or a human eye choose which photons to absorb? The answer to this important question has to do with the nature of atoms. Remember that electrons spin in particular orbits around the atomic nucleus, at different energy levels. Atoms absorb light by boosting electrons to higher energy levels, using the energy in the photon to power the move. Boosting the electron requires just the right amount of energy, no more and no less, just as when climbing a ladder you must raise your foot just so far to climb a rung. A particular kind of atom absorbs only certain photons of light, those with the appropriate amount of energy.
Pigments
As mentioned earlier, molecules that absorb light energy are called pigments. When we speak of visible light, we refer to those wavelengths that the pigment within human eyes, called retinal, can absorb—roughly from 380 nanometers (violet) to 750 nanometers (red). Other animals use different pigments for vision and thus “see” a different portion of the electromagnetic spectrum. For example, the pigment in insect eyes absorbs at shorter wavelengths than retinal. That is why bees can see ultraviolet light, which we cannot see, but are blind to red light, which we can see.
As noted, the main pigment in plants that absorbs light is chlorophyll. Its two forms, chlorophyll a and chlorophyll b, are similar in structure, but slight differences in their chemical “side groups” produce slight differences in their absorption spectra. An absorption spectrum is a graph indicating how effectively a pigment absorbs different wavelengths of visible light. For example, chlorophyll molecules will absorb photons at the ends of the visible spectrum, the peaks you see in figure 6.3. While chlorophyll absorbs fewer kinds of photons than our visual pigment retinal, it is much more efficient at capturing them. Chlorophyll molecules capture photons with a metal ion (magnesium) that lies at the center of a complex carbon ring. Photons excite electrons of the magnesium ion, which are then channeled away by the carbon atoms.
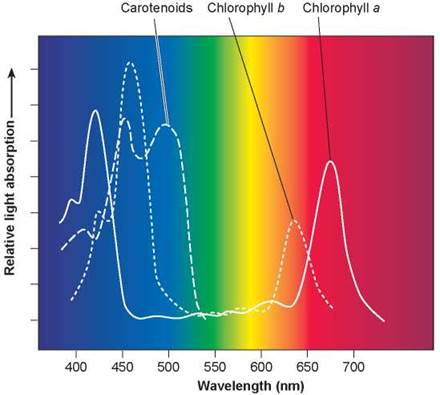
Figure 6.3. Absorption spectra of chlorophylls and carotenoids.
The peaks represent wavelengths of sunlight strongly absorbed by the two common forms of photosynthetic pigment, chlorophyll a and chlorophyll b, and by accessory carotenoid pigments. Chlorophylls absorb predominantly violet-blue and red light, in two narrow bands of the spectrum, while they reflect the green light in the middle of the spectrum. Carotenoids absorb mostly blue and green light and reflect orange and yellow light.
While chlorophyll is the primary pigment involved in photosynthesis, plants also contain other pigments called accessory pigments that absorb light of wavelengths not captured by chlorophyll. Carotenoids are a group of accessory pigments that capture violet to blue-green light. As you can see in figure 6.3, these wavelengths of light are not efficiently absorbed by chlorophyll.
Accessory pigments give color to flowers, fruits, and vegetables but are also present in leaves, their presence usually masked by chlorophyll. During the warm months, when plants are actively producing food through photosynthesis, their cells are filled with chlorophyll-containing chloroplasts that cause the leaves to appear green, like the oak leaves on the left side of figure 6.4. In the fall, the days become shorter and cooler and for many species, leaves stop their food-making processes. Their chlorophyll molecules break down and are not replaced. When this happens, the colors reflected by accessory pigments become visible. The leaves turn colors of yellow, orange, and red, like the oak leaves on the right.

Figure 6.4. Fall colors are produced by pigments such as carotenoids.
During the spring and summer, chlorophyll masks the presence in leaves of other pigments called carotenoids. Cool temperatures in the fall cause leaves of deciduous trees to cease manufacturing chlorophyll. With chlorophyll no longer present to reflect green light, the orange and yellow light reflected by carotenoids gives bright colors to the autumn leaves.
Key Learning Outcome 6.2. Plants use pigments like chlorophyll to capture photons of blue and red light, reflecting photons of green wavelengths.