THE LIVING WORLD
Unit two. The Living Cell
7. How Cells Harvest Energy from Food

Animals such as this chipmunk depend on the energy stored in the chemical bonds of the food they eat to power their life processes. Their lives are driven by energy. All the activities this chipmunk carries out—climbing trees, chewing on acorns, seeing and smelling and hearing its surroundings, thinking the thoughts that chipmunks think—use energy. But unlike the oak tree that produces the nuts on which this chipmunk is dining, no part of the chipmunk is green. It cannot carry out photosynthesis like an oak tree, and so cannot harvest energy from the sun as the tree does. Instead, it must get its energy secondhand, by consuming organic molecules manufactured by plants. The chemical energy that the oak tree invested in making its molecules is harvested by the chipmunk in a process called cellular respiration. The same processes are used by all animals to harvest energy from molecules—and by plants too. There is no sunlight under the soil where the oak tree’s roots penetrate, and like the cells of the chipmunk, these plant root cells obtain the energy to fuel their lives from cellular respiration. In this chapter, we examine cellular respiration up close. As you will see, cellular respiration and photosynthesis have much in common.
7.1. Where Is the Energy in Food?
In both plants and animals, and in fact in almost all organisms, the energy for living is obtained by breaking down the organic molecules originally produced in plants. The ATP energy and reducing power invested in building the organic molecules are retrieved by stripping away the energetic electrons and using them to make ATP. When electrons are stripped away from chemical bonds, the food molecules are being oxidized (remember, oxidation is the loss of electrons). The oxidation of foodstuffs to obtain energy is called cellular respiration. Do not confuse the term cellular respiration with the breathing of oxygen gas that your lungs carry out, which is called simply respiration.
The cells of plants fuel their activities with sugars and other molecules that they produce through photosynthesis and breakdown in cellular respiration. Nonphotosynthetic organisms eat plants, extracting energy from plant tissue in cellular respiration. Other animals, like the lion gnawing with such relish on a giraffe leg in figure 7.1, eat these animals.

Figure 7.1. Lion at lunch.
Energy that this lion extracts from its meal of giraffe will be used to power its roar, fuel its running, and build a bigger lion.
Eukaryotes produce the majority of their ATP by harvesting electrons from chemical bonds of the food molecule glucose. The electrons are transferred along an electron transport chain (similar to the electron transport system in photosynthesis), and eventually donated to oxygen gas. Chemically, there is little difference between this oxidation of carbohydrates in a cell and the burning of wood in a fireplace. In both instances, the reactants are carbohydrates and oxygen, and the products are carbon dioxide, water, and energy:
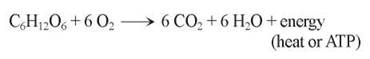
In many of the reactions of photosynthesis and cellular respiration, electrons pass from one atom or molecule to another. When an atom or molecule loses an electron, it is said to be oxidized, and the process by which this occurs is called oxidation. The name reflects the fact that in biological systems, oxygen, which attracts electrons strongly, is the most common electron acceptor. Conversely, when an atom or molecule gains an electron, it is said to be reduced, and the process is called reduction. Oxidation and reduction always take place together, because every electron that is lost by an atom through oxidation is gained by some other atom through reduction. Therefore, chemical reactions of this sort are called oxidation-reduction (redox) reactions. In redox reactions, energy follows the electron, as shown in figure 7.2.
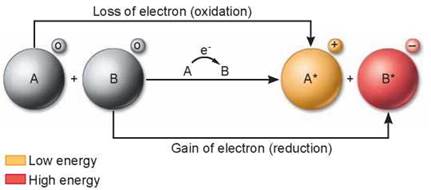
Figure 7.2. Redox reactions.
Oxidation is the loss of an electron; reduction is the gain of an electron. Here the charges of molecules A and B are shown in small circles to the upper right of each molecule. Molecule A loses energy as it loses an electron, while molecule B gains energy as it gains an electron.
Cellular respiration is carried out in two stages, illustrated in figure 7.3. The first stage uses coupled reactions to make ATP. This stage, glycolysis, takes place in the cell’s cytoplasm. Importantly, it is anaerobic (that is, it does not require oxygen). This ancient energy-extracting process is thought to have evolved over 2 billion years ago, when there was no oxygen in the earth’s atmosphere.
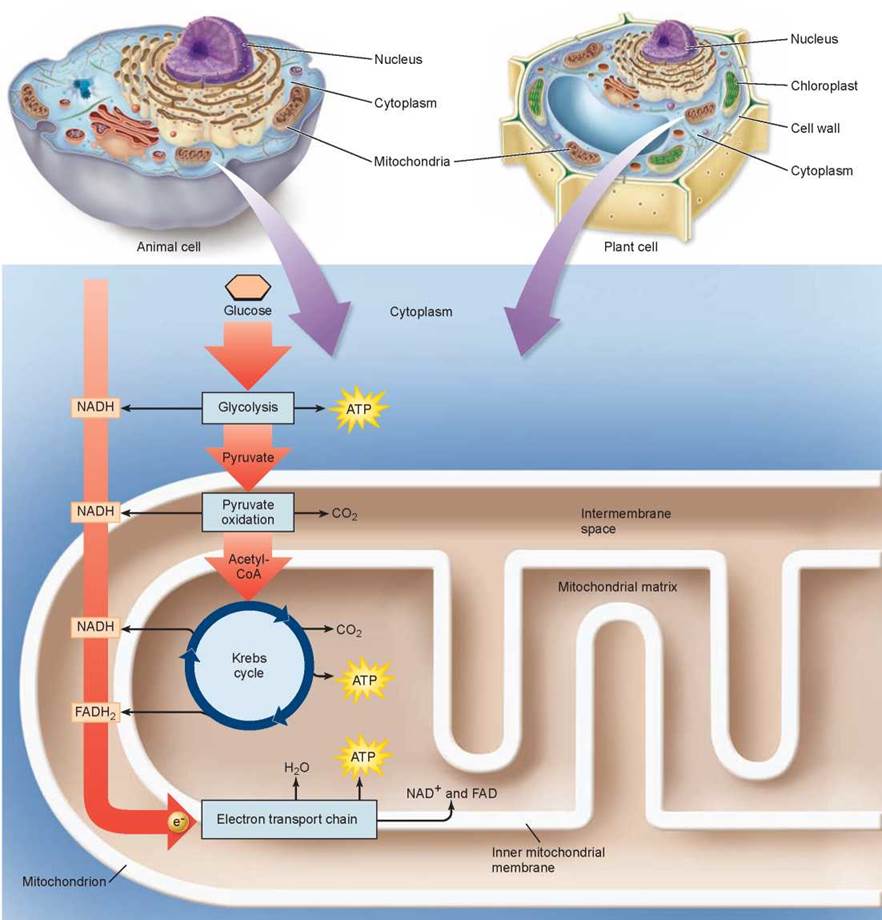
Figure 7.3. An overview of cellular respiration.
The second stage is aerobic (requires oxygen) and takes place within the mitochondrion. The focal point of this stage is the Krebs cycle, a cycle of chemical reactions that harvests electrons from C—H chemical bonds and passes the energy-rich electrons to carrier molecules, NADH and FADH2. These molecules deliver the electrons to the electron transport chain, which uses their energy to power the production of ATP. This harvesting of electrons, a form of oxidation, is far more powerful than glycolysis at recovering energy from food molecules, and is how the bulk of the energy used by eukaryotic cells is extracted from food molecules.
Key Learning Outcome 7.1. Cellular respiration is the dismantling of food molecules to obtain energy. In aerobic respiration, the cell harvests energy from glucose molecules in two stages, glycolysis and oxidation.