THE LIVING WORLD
Unit two. The Living Cell
7. How Cells Harvest Energy from Food
7.4. Using the Electrons to Make ATP
Moving Electrons Through the Electron Transport Chain
In eukaryotes, aerobic respiration takes place within the mitochondria present in virtually all cells. The internal compartment, or matrix, of a mitochondrion contains the enzymes that carry out the reactions of the Krebs cycle. As described earlier, the electrons harvested by oxidative respiration are passed along the electron transport chain, and the energy they release transports protons out of the matrix and into the intermembrane space.
The NADH and FADH2 molecules formed during the first stages of aerobic respiration each contain electrons and hydrogens that were gained when NAD+ and FAD were reduced (refer back to figure 7.3). The NADH and FADH2 molecules carry their electrons to the inner mitochondrial membrane (an enlarged area of the membrane is shown below), where they transfer the electrons to a series of membrane-associated molecules collectively called the electron transport chain. The electron transport chain works much as does the electron transport system you encountered in studying photosynthesis.
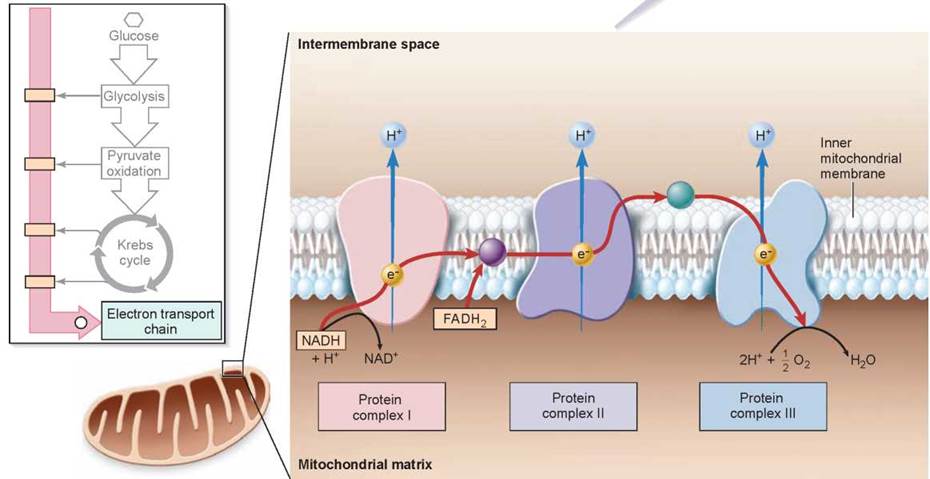
A protein complex (the pink structure above) receives the electrons and, using a mobile carrier, passes these electrons to a second protein complex (the purple structure). This protein complex, along with others in the chain, operates as a proton pump, using the energy of the electron to drive a proton out across the membrane into the intermembrane space. The arrows indicate the transport of the protons into the top half of the figure, which represents the intermembrane space.
The electron is then carried by another carrier to a third protein complex (the light blue structure). This complex uses electrons such as this one to link oxygen atoms with hydrogen ions to form molecules of water.
It is the availability of a plentiful supply of electron acceptor molecules like oxygen that makes oxidative respiration possible. The electron transport chain used in aerobic respiration is similar to, and may well have evolved from, the electron transport system employed in photosynthesis. Photosynthesis is thought to have preceded cellular respiration in the evolution of biochemical pathways, generating the oxygen that is necessary as the electron acceptor in cellular respiration. Natural selection didn’t start from scratch and design a new biochemical pathway for cellular respiration; instead, it built on the photosynthetic pathway that already existed, and uses many of the same reactions.
Producing ATP: Chemiosmosis
As the proton concentration in the intermembrane space rises above that in the matrix, the concentration gradient induces the protons to reenter the matrix by diffusion through a special proton channel called ATP synthase. ATP synthase channels are embedded in the inner mitochondrial membrane, as shown in the figure to the left. As the protons pass through, these channels synthesize ATP from ADP and Pi within the matrix. The ATP is then transported by facilitated diffusion out of the mitochondrion and into the cell’s cytoplasm. This ATP synthesizing process is the same chemiosmosis process that you encountered in studying photosynthesis in chapter 6.
Although we have discussed electron transport and chemiosmosis as separate processes, in a cell they are integrated, as shown below, left. The electron transport chain uses two electrons harvested in glycolysis, two harvested in pyruvate oxidation, and eight harvested in aerobic respiration (red arrows) to pump a large number of protons out across the inner mitochondrial membrane (shown in the upper right). Their subsequent reentry back into the mitochondrial matrix drives the synthesis of 34 ATP molecules by chemiosmosis (shown in the lower right). Two additional ATPs were harvested by a coupled reaction in glycolysis, and two more in the Krebs cycle. As two ATPs must be expended to transport NADH into the mitochondria by active transport, the grand total of ATPs harvested is thus 36 molecules.
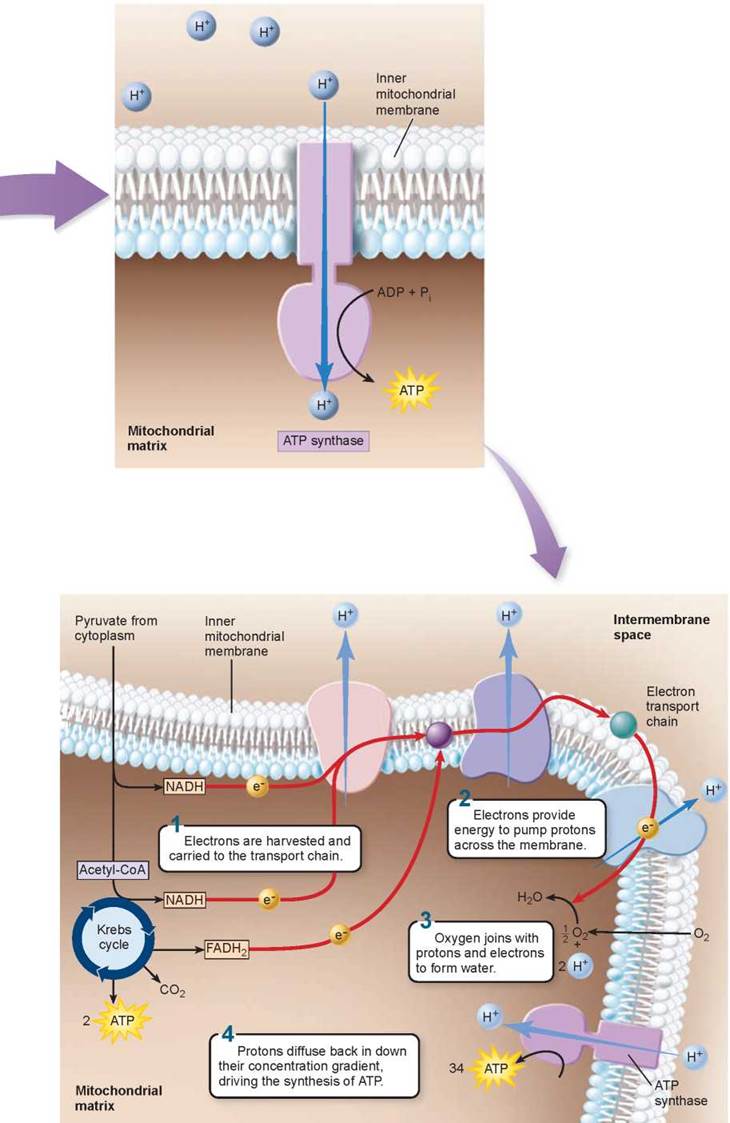
Key Learning Outcome 7.4. The electrons harvested by oxidizing food molecules are used to power proton pumps that chemiosmotically drive the production of ATP.