Medical Microbiology
Section 3 The conflicts
12 Background to the infectious diseases
Introduction
Vertebrates have been continuously exposed to microbial infections throughout their hundreds of millions of years of evolution. Disease or death was the penalty for inadequate defences. Therefore they have developed:
• highly efficient methods for recognizing foreign invaders
• effective inflammatory and immune responses to restrain the growth and spread of foreign invaders and to eliminate them from the body.
The fundamental bases of these defences have been described in Chapters 9 and 10. If these defences were completely effective, microbial infections would be scarce and terminated rapidly, as microorganisms would not be allowed to persist in the body for long periods.
Microbes rapidly evolve characteristics that enable them to overcome the host’s defences
Microorganisms faced with the antimicrobial defences of the host species have evolved and developed a variety of characteristics that enable them to bypass or overcome these defences and carry out their obligatory steps for survival (Table 12.1). Unfortunately, microorganisms evolve with extraordinary speed in comparison with their hosts. This is partly because they multiply much more rapidly, the generation time of an average bacterium being 1 h or less compared with about 20 years for the human host. Rapid evolutionary change is also favoured in bacteria that can hand over genes (carried on plasmids) directly to other bacteria, including unrelated bacteria. Antibiotic resistance genes, for instance, can then be transferred rapidly between species. This rapid rate of evolution ensures that microbes are always many steps ahead of the host’s antimicrobial defences. Indeed, if there are possible ways around the established defences, microorganisms are likely to have discovered and taken advantage of them. Infectious microorganisms therefore owe their success to this ability to adapt and evolve, exploiting weak points in the host’s defences, as outlined in Table 12.2 and Figures 12.1, 12.2. The host, in turn, has had to respond to such strategies by slowly improving defences, adding extra features, and having multiple defence mechanisms with overlap and a good deal of duplication.
Table 12.1 Successful infectious microorganisms must take certain obligatory steps
|
Obligatory steps for infectious microorganisms |
||
|
Step |
Requirement |
Phenomenon |
|
Attachment ± entry into body |
Evade natural protective and cleansing mechanisms |
Entry (infection) |
|
Local or general spread in the body |
Evade immediate local defences |
Spread |
|
Multiplication |
Increase numbers (many will die in the host, or en route to new hosts) |
Multiplication |
|
Evasion of host defences |
Evade immune and other defences long enough for the full cycle in the host to be completed |
Microbial answer to host defences |
|
Shedding from body (exit) |
Leave body at a site and on a scale that ensures spread to fresh hosts |
Transmission |
|
Cause damage in host |
Not strictly necessary but often occursa |
Pathology, disease |
a The last step, causing damage in the host, is not strictly necessary, but a certain amount of damage may be essential for shedding. The outpouring of infectious fluids in the common cold or diarrhea, for instance, or the trickle from vesicular or pustular lesions, is required for transmission to fresh hosts.
Table 12.2 Host defences and microbial evasion strategies: mechanical and other barriers
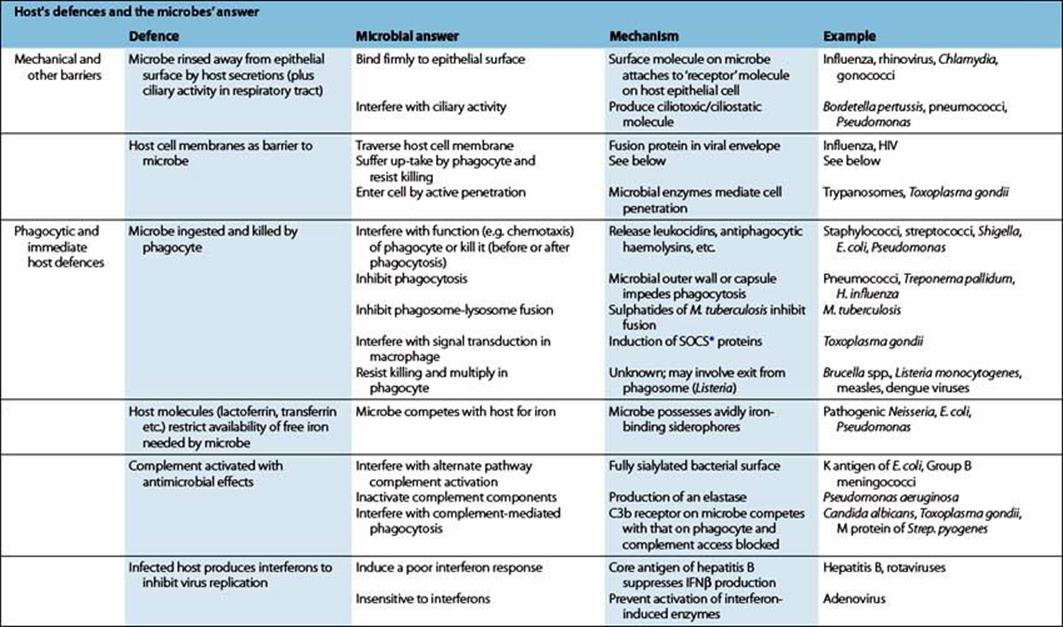
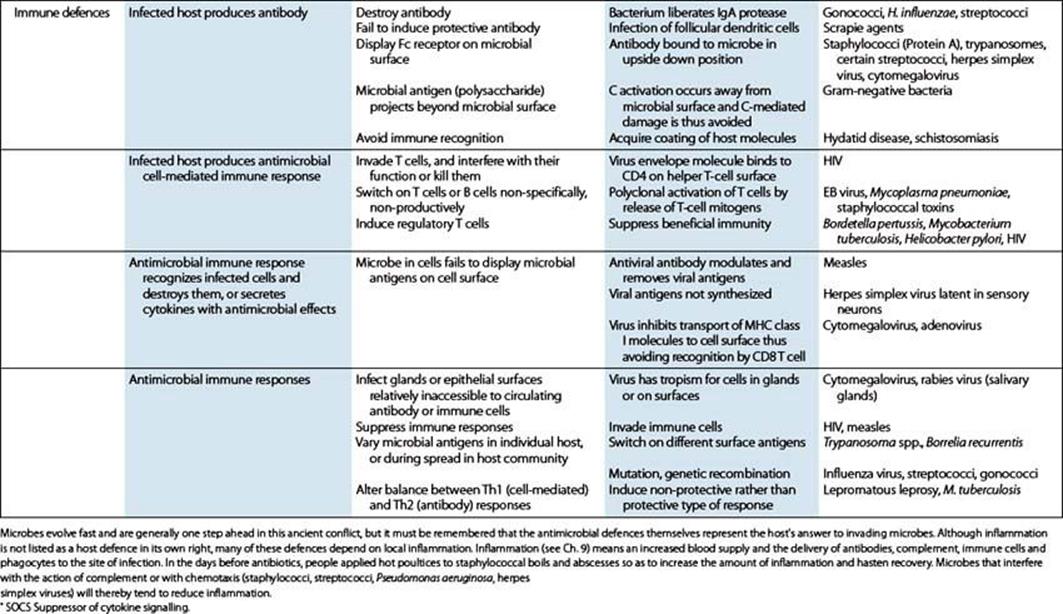
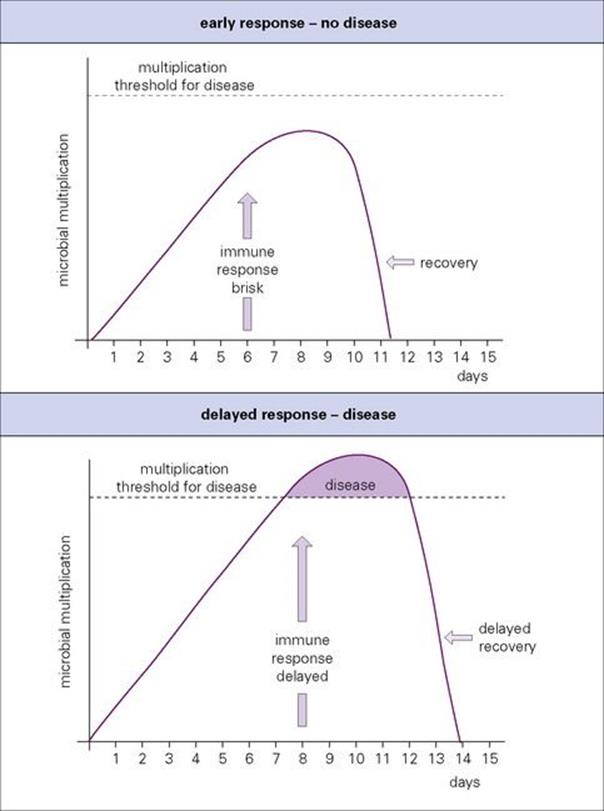
Figure 12.1 Every infection is a race. Delays in mobilizing host adaptive defences can lead to disease or death.
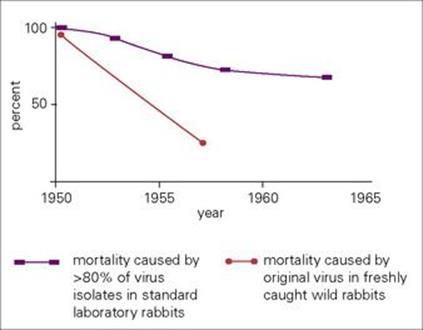
Figure 12.2 Myxomatosis is the best-studied example of the appearance of a highly lethal microbe in a host population that gradually settles down to a state of more balanced pathogenicity. Vibrio cholerae has progressed in this direction, and perhaps HIV is destined to tread the same path.
Host–parasite relationships
The speed with which host adaptive responses can be mobilized is crucial
Every infection is a race between the capacity of the microorganism to multiply, spread and cause disease and the ability of the host to control and finally terminate the infection (Fig. 12.1). For instance, a 24-h delay before an important host response comes into operation can give a decisive advantage to a rapidly growing microorganism. From the host’s point of view, it may allow enough damage to cause disease. More importantly, from the microbe’s point of view, it may give the microbe the opportunity to be shed from the body in larger amounts or for an extra day or two. A microbe that achieves this will be rapidly selected for in evolution.
Adaptation by both host and parasite leads to a more stable balanced relationship
The picture of conflict between host and parasite, usually and appropriately described in military terms, is central to an understanding of the biology of infectious disease. As with military conflicts, adaptation on both sides (Box 12.1) tends to lessen the damage and incidence of death in the host population, leading to a more stable and balanced relationship. The successful parasite gets what it can from the host without causing too much damage, and in general, the more ancient the relationship, the less the damage. Many microbial parasites, not only the normal flora (see Ch. 8), but also polioviruses, meningococci and pneumococci and others, live for the most part in peaceful coexistence with their human host.
![]()
Box 12.1  Lessons in Microbiology
Lessons in Microbiology
Myxomatosis
Myxomatosis provides a well-studied classic example of the evolution of an infectious disease unleashed on a highly susceptible population. Myxomavirus, which is spread mechanically by mosquitoes, normally infects South American rabbits (Sylvilagus brasiliensis), but they remain perfectly well, developing only a virus-rich skin swelling at the site of the mosquito bite. The same virus in the European rabbit (Oryctolagus cuniculus) causes a rapidly fatal disease.
Myxomavirus was successfully introduced into Australia in 1950 as an attempt to control the rapidly increasing rabbit population. Initially, more than 99% of infected rabbits died (Fig. 12.2), but then two fundamental changes occurred:
1. New, less lethal strains of virus appeared and replaced the original strain. This occurred because rabbits infected with these strains survived for longer and their virus was therefore more likely to be transmitted.
2. The rabbit population changed its character, as those that were genetically more susceptible to the infection were eliminated. In other words, the virus selected out the more resistant host, and the less lethal virus strain proved to be a more successful parasite. If the rabbit population had been eliminated, the virus would also have died out, but the host–parasite relationship quite rapidly settled down to reach a state of better balanced pathogenicity, and by the 1970s only about half the rabbits died from infection. Australian rabbits have since faced a new threat, a calicivirus introduced from Europe, which spreads by contact and causes a lethal haemorrhagic disease.
![]()
Some microorganisms remain at body surfaces, perhaps spreading locally, but failing to invade deeper tissues. These include the common cold viruses, wart viruses, mycoplasmas and skin fungi. Often the disease is mild, but severe illness can occur when powerful toxins are produced and act either locally (cholera) or at distant sites (diphtheria).
Infecting microorganisms can gain entry and cause disease in four ways (Fig. 12.3). There are:
• microorganisms with specific mechanisms for attaching to, or penetrating, the body surfaces of normal healthy hosts (most viruses and certain bacteria)
• microorganisms introduced into normal healthy hosts by biting arthropods (e.g. malaria, plague, typhus, yellow fever)
• microorganisms introduced into otherwise normal healthy hosts via skin wounds or animal bites (clostridia, rabies, Pasteurella multocida)
• microorganisms able to infect a normal healthy host only when surface or systemic defences are impaired (see Ch. 30) – as occurs with burns, insertion of foreign bodies (cannulas and catheters), urinary tract infections in men (stones, enlarged prostate, see Ch. 20), bacterial pneumonia following initial viral damage (post-influenza) or depressed immune responses (immunosuppressive drugs or diseases such as AIDS).
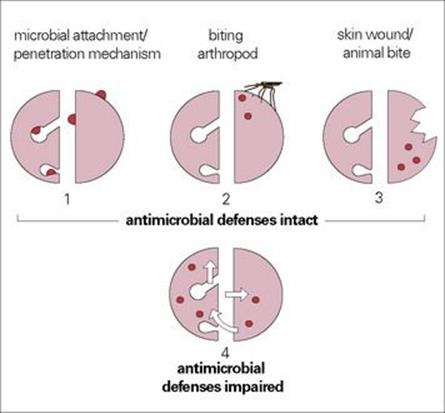
Figure 12.3 Four types of microbial infection can be distinguished. (The diagrams show a schematic representation of the body surfaces of a host, similar to that in Fig. 13.1.) Surface or systemic defences of the host can be impaired in a variety of ways.
Causes of infectious diseases
More than 100 microbes commonly cause infection
Humans are host to many different microorganisms. In addition to the scores of microbes that form the normal flora, there are more than 100 that quite commonly cause infection, some of them remaining in the body for many years afterwards, and several hundred others that are responsible for less common infections. Against this rich background of parasitic activity, how do we prove that a certain microorganism is the culprit in a given disease? In some instances (anthrax, cholera, tetanus), the causative microorganism is identified and incriminated at an early stage, but in the case of glandular fever and viral hepatitis it is not so easy.
Koch’s postulates to identify the microbial causes of specific diseases
In 1890, Robert Koch (Box 12.2) set out as ‘postulates’ the following criteria he felt to be necessary for a microorganism to be accepted as the cause of a given disease:
• The microbe must be present in every case of the disease.
• The microbe must be isolated from the diseased host and grown in pure culture.
• The disease must be reproduced when a pure culture is introduced into a non-diseased-susceptible host.
• The microbe must be recoverable from an experimentally infected host.
![]()
Box 12.2  Lessons in Microbiology
Lessons in Microbiology
Robert Koch (1843–1910)
In 1876, while in general practice in Berlin, Robert Koch (Fig. 12.4) isolated the anthrax bacillus, and became the first to show a specific organism as the cause of a disease. In 1882, he discovered Mycobacterium tuberculosis as the cause of tuberculosis. He then went on to lead the 1883 expedition to Egypt and India, and discovered the cause of cholera: Vibrio cholerae.
Koch was the founder of the ‘germ theory’ of disease, which maintained that certain diseases were caused by a single species of microbe. In 1890, he set out his ‘postulates’ as ground rules. New techniques were necessary to meet the exacting requirements of the postulates, and Koch became the first to grow bacteria in ‘colonies’, initially on potato slices and later, with his pupil Petri, on solid gelatin media.
Koch himself could not reproduce cholera in animals, however, and not all microbes could be cultivated. His neat rules therefore had to be modified. Nevertheless, he brought order and clarity to medicine – until then diseases were attributed to miasmas or mists, to punishments from the Gods or devils, or to unfortunate conjunctions of the stars and planets. However, there was resistance to his ideas. A distinguished Munich physician, Max Von Petternkofer, believed that he had put paid to the new theory when he drank a pure culture of V. cholerae and suffered no more than mild diarrhea!
![]()
In the early days of microbiology, Koch’s postulates brought a welcome clarity. The germ theory of disease causation had only recently been set out following Koch’s classic studies on anthrax (1876) and tuberculosis (1882), and methods for isolating microbes in pure culture and identifying them were only just being developed. However, modifications were needed in order to include certain bacterial diseases and the new world of viral diseases. The microbe could not always be grown in the laboratory (Treponema pallidum, wart viruses), and for certain microbes: hepatitis B, Epstein–Barr virus (EBV), there were (initially) no susceptible animal species. The criteria were modified, therefore, on several occasions to accommodate these problems and finally reformulated by A.S. Evans in 1976.
Conclusions about causation are now reached using enlightened common sense
Nowadays, with our vastly increased technology and understanding of infection, those attempts to make lists and apply rigid criteria may seem old fashioned. Perhaps we can now reach conclusions about causation using common sense. For instance, we recognize that diseases sometimes do not appear until many years after a specific infection (subacute sclerosing panencephalitis, Creutzfeldt–Jakob disease; see Ch. 24). Molecular genetic techniques may now identify previously uncultivable causative organisms. The polymerase chain reaction was used to amplify and sequence small amounts of mRNA from the bowel of patients with Whipple’s disease, a rare multisystem disorder. A unique 16 s mRNA was identified, belonging to a previously uncharacterized, uncultivable bacterium, Tropheryma whippelii. Nevertheless, grey areas remain, especially in diseases of possible or probable microbial aetiology where the microbe does not act alone. Co-factors or genetic and immunologic factors in the host may play a vital part. Examples include:
• the cancers associated with viruses (hepatitis B, genital wart viruses, EBV)
• diseases of possible microbial origin where a number of different microbes may be involved (post-viral fatigue syndrome, exacerbations of multiple sclerosis)
• diseases that might be infectious, but occur in only a very small proportion of genetically predisposed individuals (rheumatoid arthritis, juvenile diabetes mellitus).
Possible problems in assigning disease aetiology
Finally, there are two interesting possibilities that could give problems in assigning disease aetiology, although neither has yet been shown to apply to human disease:
• First, in some infections, the DNA of the causative virus is integrated into the genome of the host, and is transmitted vertically. It therefore behaves as a genetic attribute. This is known to occur, for instance, with mammary tumour virus in mice.
• Second, the causative microbe triggers off the disease process and then disappears completely from the body and is no longer detectable. This is known to be the case in the cerebellar hypoplasia occurring in hamsters and cats after intrauterine infection with parvovirus. There are no known examples in humans.
The biologic response gradient
It is uncommon for a microbe to cause exactly the same disease in all infected individuals
Hence, a physician must be able to make a diagnosis when only some of the possible signs and symptoms are present. The exact clinical picture depends upon many variables such as infecting dose and route, age, sex, presence of other microbes, nutritional status and genetic background. Infections such as measles or cholera give a fairly consistent disease picture, but others such as syphilis cause such a wide spectrum of pathology that Sir William Osler (1849–1919) stated that ‘He who knows syphilis, knows medicine’.
There is great variation not only in the nature, but also in the severity of clinical disease. Many infections are asymptomatic in > 90% individuals, the clinically characterized illness applying to only an occasional unfortunate host (Table 12.3). This illness can be mild or severe. Asymptomatically infected individuals are important because, although they develop immunity and resistance to reinfection, they are not identified, move normally in the community and can infect others. Clearly, there is little point in isolating a clinically infected patient when there is a high frequency of asymptomatically infected individuals in the community. This phenomenon can be represented as an iceberg (Fig. 12.5).
Table 12.3 The likelihood of developing clinical disease often depends upon age and sex
|
Frequency of clinically apparent disease |
|
|
Infection |
Approximate % with clinically apparent diseasea |
|
Pneumocystis jiroveciib |
0 |
|
Poliomyelitis (child) |
0.1–1.0 |
|
Epstein–Barr virus (1–5 year old child) |
1.0c |
|
Influenza (young adult) |
60 |
|
Whooping cough |
|
|
Malaria (1–5 year old child) |
25 |
|
Gonorrhoea (adult male) |
|
|
HIVd |
|
When there is a lengthy incubation period, the proportion with clinical disease may increase with time, from a few percent to (nearer) 100% in the case of HIV.
a On primary infection
b formerly P. carinii
c 30–75% in young adults
d Some individuals infected with HIV can maintain high CD4 counts and very low viral loads for > 5 years, and are called ‘long-term non-progressors’ or ‘controllers’, with a few individuals called ‘elite controllers’ controlling progression to disease for > 20 years.

Figure 12.4 Robert Koch (1843–1910).
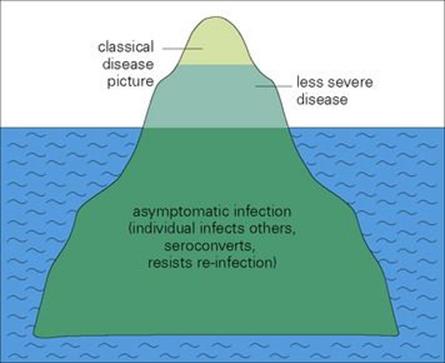
Figure 12.5 The ‘iceberg’ concept of infectious disease.
![]()
 Key Facts
Key Facts
• Faced with host defences (see Chs 9,10), the microbes (see >Chs 1–7) have developed mechanisms to bypass them, and in turn the host defences have had to be modified, although slowly, in response.
• There is a conflict between the microbe and host, and every infectious disease is the result of this ancient battle. Details of the host–microbe conflict are given in Chs 12–17,an outline of diagnostic methods in Ch. 31, and a central account of infectious diseases according to the body systems involved in Chs 18–30.
• Speed matters. Every infection is a race between microbial replication and spread and the mobilization of host responses.
• Microorganisms can infect in four main ways, depending upon whether host defences are intact or impaired.
• It is sometimes difficult to incriminate a specific microbe as the cause of a disease.
• Microbes do not necessarily produce the same disease in all infected individuals. A biologic response gradient causes a spectrum that can range from an asymptomatic to a lethal infection.
![]()