Medical Microbiology
Section 4 Clinical manifestation and diagnosis of infections by body system
The clinical manifestations of infection
A system of classification is essential in order to describe the diversity of infectious diseases. In Chapters 18–26, infections are classified according to the body system primarily involved at the clinical level. For example, rhinoviruses specifically cause infection of the upper respiratory tract, and bacillary or amoebic dysentery are gastrointestinal tract infections. Other infections characteristically cause damage predominantly to one part of the body, although other parts may be affected. Therefore, tuberculosis is considered in Chapter 19 (lower respiratory tract infections) and typhoid in Chapter 22 (gastrointestinal tract infections), these being the sites primarily affected, but there may be dissemination to other sites. Finally, some microbes can be grouped together with others acquired in the same way, even though more than one system may be involved. Examples are syphilis and HIV, which are dealt with in Chapter 21 (sexually transmitted diseases) and rubella in Chapter 23 (obstetric and perinatal infections).
The systems approach is useful because it includes infections caused by a wide variety of microbes on the basis of the clinical syndrome produced. As with any system of classification, however, there are grey areas and overlaps. Referral to the Appendix, where definitive accounts of the most important infectious organisms are given, will help clarify any ambiguities.
Chapters 27 and 28 deal with those infections that cannot be readily pigeonholed into systems. These include multisystem infections (i.e. infections that are not obviously localized to any one system of the body). Many multisystem infections are also multihost in that they can be transmitted:
• from person to person by an intermediate vector (usually an arthropod), their distribution depending upon climate, ecology and the presence of adequate numbers of the required arthropod (see Ch. 27)
• directly to humans from other vertebrates, in which case they are known as ‘zoonoses’ (see Ch. 28), with distributions ranging from highly restricted (Rocky Mountain spotted fever, Lassa fever) to widespread (Q fever, leptospirosis).
Finally, there are two further disease groupings, founded on clinical presentation:
• those presenting as ‘fevers of unknown origin’ (see Ch. 29)
• those seen in the compromised host (see Ch. 30).
The latter category has become increasingly important because of the large number of patients whose defences are impaired as a result of disease (cystic fibrosis, diabetes mellitus), infection (HIV/AIDS), immunosuppressive therapy (transplant patients) or other causes (e.g. burns, catheters).
18 Upper respiratory tract infections
Introduction
The mucociliary system and the flushing action of saliva are defences against upper respiratory tract infection
The air we inhale contains millions of suspended particles, including microorganisms, most of which are harmless. However, the air may contain large numbers of pathogenic microorganisms if someone is near an individual with a respiratory tract infection. Efficient cleansing mechanisms (see Chs 9 and 13) are therefore vital components of the body’s defence against infection of both the upper and lower respiratory tract. Infection takes place against the background of these natural defence mechanisms, and it is then appropriate to ask why the defences have failed. For the upper respiratory tract, the flushing action of saliva is important in the oropharynx and the mucociliary system in the nasopharynx traps invaders. As on other surfaces of the body (see Ch. 8), a variety of microorganisms live harmoniously in the upper respiratory tract and oropharynx (Table 18.1); they colonize the nose, mouth, throat and teeth and are well adapted to life in these sites. Normally they are well-behaved guests, not invading tissues and not causing disease. However, as in other parts of the body, resident microorganisms can cause trouble when host resistance is weakened.
Table 18.1 The normal flora of the respiratory tract
|
Type of residenta |
Microorganism |
|
Common residents (> 50% of normal people) |
Oral streptococci |
|
Occasional residents (< 10% of normal people) |
Streptococcus pyogenes |
|
Uncommon residents (< 1% normal people) |
Corynebacterium diphtheria |
|
Residents in latent state in tissues:c |
Pneumocystis jiroveciid |
a All except tissue residents are present in the oronasopharynx or on teeth.
b Present in mouth; also Entamoeba gingivalis, Trichomonas tenax, micrococci, Actinomyces spp.
c All except M. tuberculosis are present in most humans.
d Formerly P. carinii.
The upper and lower respiratory tracts form a continuum for infectious agents
We distinguish between upper and lower respiratory tract infections, but the respiratory tract from the nose to the alveoli is a continuum as far as infectious agents are concerned (Fig. 18.1). There may, however, be a preferred ‘focus’ of infection (e.g. nasopharynx for coronaviruses and rhinoviruses); but parainfluenza viruses, for instance, can infect the nasopharynx to give rise to a cold, as well as the larynx and trachea resulting in laryngotracheitis (croup), and occasionally the bronchi and bronchioles (bronchitis, bronchiolitis or pneumonia).
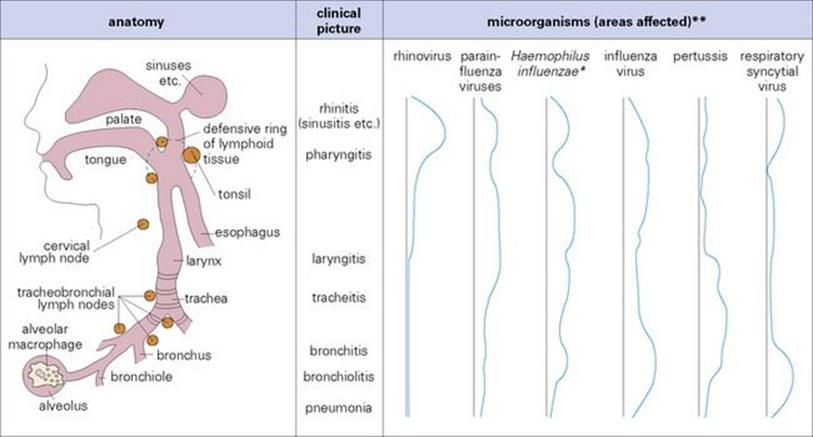
Figure 18.1 The respiratory tract as a continuum. * Asymptomatic nasopharyngeal colonization is common. ** Magnitude of rhinitis, laryngitis, etc. is shown by area between black and blue lines.
Two useful generalizations can be made about upper and lower respiratory tract infections:
1. Although many microorganisms are restricted to the surface epithelium, some spread to other parts of the body before returning to the respiratory tract, oropharynx, salivary glands (Table 18.2).
2. Two groups of microbes can be distinguished: ‘professional’ and ‘secondary’ invaders.
Table 18.2 Two types of respiratory infection
|
Type |
Examples |
Consequences |
|
Restricted to surface |
Common cold viruses |
Local spread |
|
Spread through body |
Measles, mumps, rubella |
Little or no lesion at entry site |
After entry via the respiratory tract, microbes either stay on the surface epithelium or spread through the body.
a Formerly Chlamydia psittaci; CMV, cytomegalovirus; EBV, Epstein–Barr virus.
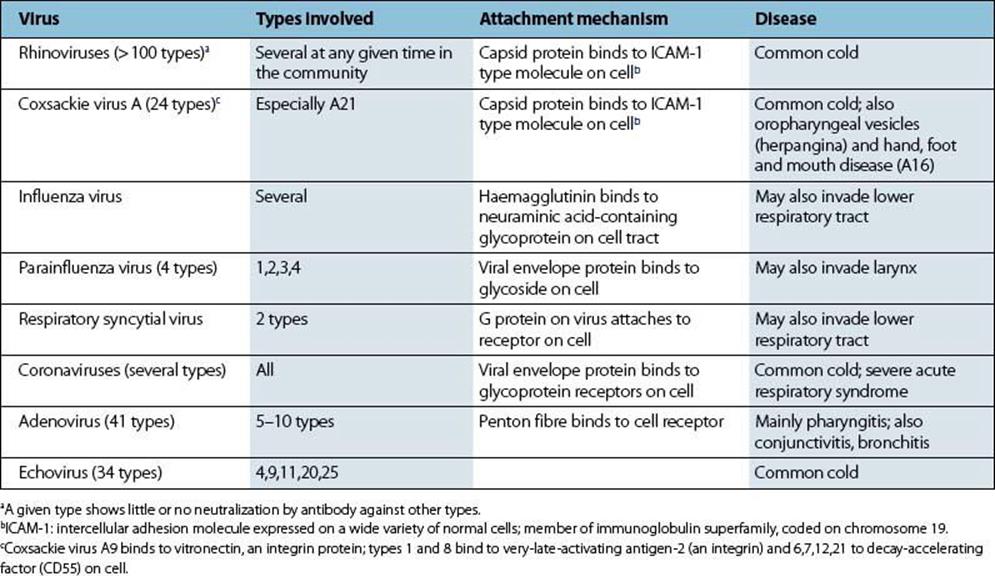
Professional invaders are those that successfully infect the normally healthy respiratory tract (Table 18.3). They generally possess specific properties that enable them to evade local host defences, such as the attachment mechanisms of respiratory viruses (Table 18.4). Secondary invaders only cause disease when host defences are already impaired (Table 18.3).
Table 18.3 The two types of respiratory invader – professional or secondary
|
Type |
Requirement |
Examples |
|
Professional invaders (infect healthy respiratory tract) |
Adhesion to normal mucosa (in spite of mucociliary system) |
Respiratory viruses (influenza, rhinoviruses) |
|
Ability to interfere with cilia |
Bordetella pertussis, M. pneumoniae, Strep. pneumoniae (pneumolysin) |
|
|
Ability to resist destruction in alveolar macrophage |
Legionella, Mycobacterium tuberculosis |
|
|
Ability to damage local (mucosal, submucosal) tissues |
Corynebacterium diphtheriae (toxin), Strep. pneumoniae (pneumolysin) |
|
|
Secondary invaders (infect when host defences impaired) |
Initial infection and damage by respiratory virus (e.g. influenza virus) |
Staphylococcus aureus; Strep. pneumoniae, pneumonia complicating influenza |
|
Local defences impaired (e.g. cystic fibrosis) |
Staph. aureus, Pseudomonas |
|
|
Chronic bronchitis, local foreign body or tumour |
Haemophilus influenzae, Strep. pneumoniae |
|
|
Depressed immune responses (e.g. AIDS, neoplastic disease) |
Pneumocystis jirovecii, cytomegalovirus, M. tuberculosis |
|
|
Depressed resistance (e.g. elderly, alcoholism, renal or hepatic disease) |
Strep. pneumoniae, Staph. aureus, H. influenzae |
Table 18.4 Common cold viruses and their mechanisms of attachment
Rhinitis
Rhinoviruses and coronaviruses together cause more than 50% of colds
Viruses are the most common invaders of the nasopharynx, and a great variety of types (Table 18.4) are responsible for the symptoms referred to as the common cold. They induce a flow of virus-rich fluid which is called rhinorrhoea from the nasopharynx, and when the sneezing reflex is triggered, large numbers of virus particles are discharged into the air. Transmission is therefore by aerosol and also by virus-contaminated hands (see Ch. 13). Most of these viruses possess surface molecules that bind them firmly to host cells or to cilia or microvilli protruding from these cells. As a result, they are not washed away in secretions and are able to initiate infection in the normally healthy individual. Virus progeny from the first-infected cell then spread to neighbouring cells and via surface secretions to new sites on the mucosal surface. After a few days, damage to epithelial cells and the secretion of fluid containing inflammatory mediators such as bradykinin lead to common cold-type symptoms (Fig. 18.2).
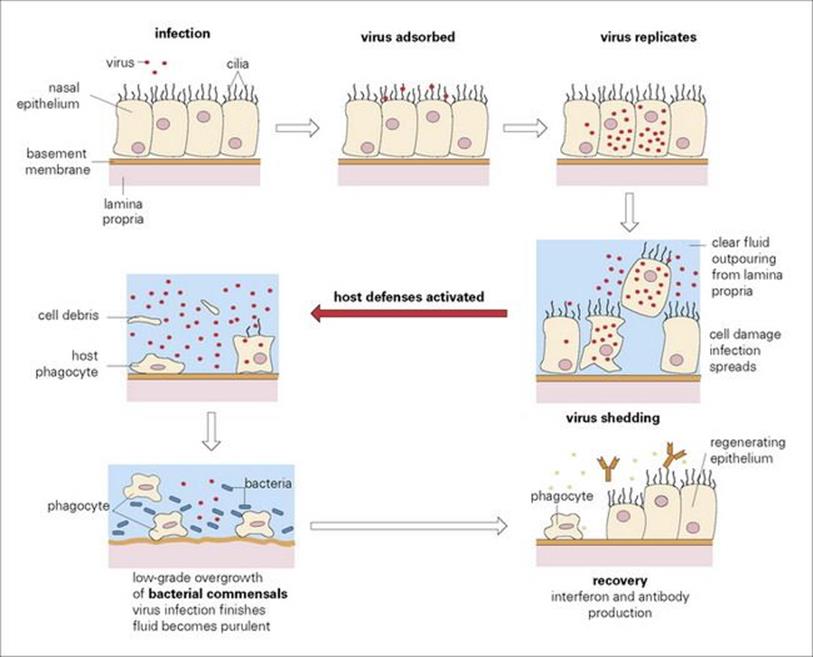
Figure 18.2 The pathogenesis of the common cold. For simplification, the epithelium is represented as one cell thick.
Common cold virus infections are diagnosed by clinical appearance
In view of the large variety of viruses and because common colds are generally mild and self-limiting with no systemic spread in healthy individuals, determining the aetiology may only be helpful from an epidemiological perspective. Diagnosis becomes important when the lower respiratory tract is involved, as for instance with influenza viruses or in children with respiratory syncytial virus (RSV) infection. The diagnosis can be made using a number of different methods that include detecting viral antigens in exfoliated cells in samples such as nasopharyngeal aspirates or throat swabs using:
• immunofluorescence techniques (see Fig. 19.5)
• or by detecting viral genomic material using molecular methods such as the polymerase chain reaction (PCR) or microarrays
• or by virus isolation in cell culture
Alternatively, collecting an acute and convalescent serum sample and looking for a rise in virus-specific antibodies can confirm the diagnosis retrospectively.
Due to the increased sensitivity in detecting respiratory viruses by PCR, together with automated sample extraction and detection methods, many laboratories use molecular methods for making a diagnosis using combined nose and throat swab samples.
Treatment of the common cold is symptomatic
It is often said that a common cold will resolve in 48 h if vigorous treatment with anticongestants, analgesics and antibiotics is undertaken. There are no vaccines to protect against the common cold viruses as the vaccines would have to be polyvalent to cover this antigenically diverse group of viruses, and treatment is for the most part symptomatic.
Pharyngitis and tonsillitis
About 70% of acute sore throats are caused by viruses
Microorganisms that cause sore throats (acute pharyngitis) are listed in Table 18.5. Those viruses that infect the upper respiratory tract inevitably encounter the submucosal lymphoid tissues that form a defensive ring around the oropharynx (see Fig. 18.1). The throat becomes sore either because the overlying mucosa is infected or because of inflammatory and immune responses in the lymphoid tissues themselves. Adenoviruses are common causes, often infecting the conjunctiva as well as the pharynx to cause pharyngoconjunctival fever. Epstein–Barr virus (EBV) and cytomegalovirus (CMV) multiply locally in the pharynx (Fig. 18.3), and herpes simplex virus (HSV) and certain coxsackie A viruses multiply in the oral mucosa to produce a painful local lesion or ulcer. Certain enteroviruses (e.g. coxsackie A16) can cause additional vesicles on the hands and feet and in the mouth (hand, foot and mouth disease; Fig. 18.4).
Table 18.5 Microorganisms that cause acute pharyngitis
|
Organisms |
Examples |
Comments |
|
Viruses |
Rhinoviruses, coronaviruses |
A mild symptom in the common cold |
|
Adenoviruses (types 3,4,7,14,21) |
Pharyngoconjunctival fever |
|
|
Parainfluenza viruses |
More severe than common cold |
|
|
Influenza viruses, cytomegalovirus |
Not always present |
|
|
Coxsackie A and other enteroviruses |
Small vesicles (herpangina) |
|
|
Epstein–Barr virus |
Occurs in 70–90% of glandular fever patients |
|
|
Herpes simplex virus type 1 |
Can be severe, with palatal vesicles or ulcers |
|
|
Bacteria |
Streptococcus pyogenes |
Causes 10–20% of cases of acute pharyngitis; sudden onset; mostly in 5–10-year-old children |
|
Neisseria gonorrhoeae |
Often asymptomatic; usually via orogenital contact |
|
|
Corynebacterium diphtheriae |
Pharyngitis often mild, but toxic illness can be severe |
|
|
Haemophilus influenzae |
Epiglottis |
|
|
Borrelia vincentii plus fusiform bacilli |
Vincent’s angina; commonest in adolescents and adults |
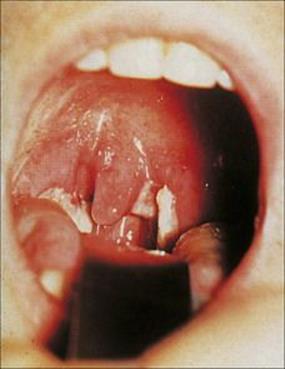
Figure 18.3 Infectious mononucleosis caused by Epstein–Barr virus. The tonsils and uvula are swollen and covered in white exudate. There are petechiae on the soft palate.
(Courtesy of J.A. Innes.)
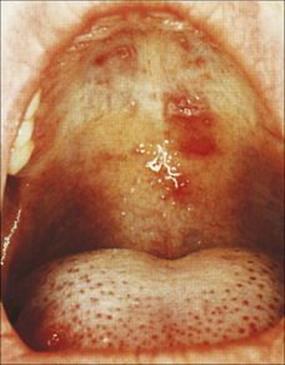
Figure 18.4 Ulcers on the hard palate and tongue in hand, foot and mouth disease due to coxsackie A virus.
(Courtesy of J.A. Innes.)
Cytomegalovirus infection
Cytomegalovirus can be transmitted by saliva, urine, blood, semen and cervical secretions
Cytomegalovirus is the largest human herpesvirus (Fig. 18.5) and is species specific; humans are the natural hosts. Cytomegalovirus refers to the multinucleated cells, which together with the intranuclear inclusions, are characteristic responses to infection with this virus. CMV was originally called ‘salivary gland’ virus and is transmitted by saliva and other secretions. In addition, CMV can be transmitted by sexual contact, as semen and cervical secretions may also contain this virus, and by blood transfusions (although leukodepletion reduces the risk significantly) and organ transplants from CMV antibody-positive donors. The CMV load will be high in the urine from babies with congenital CMV infection and careful hand washing and disposal of nappies will reduce the risk of transmission to susceptible individuals. CMV can be detected in breast milk, but this is of doubtful significance in transmission.
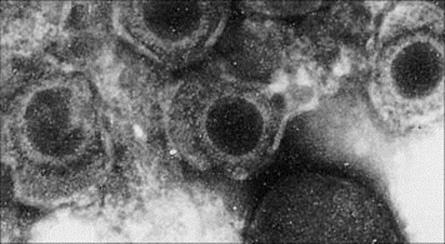
Figure 18.5 Electron micrograph of cytomegalovirus particles. This is the largest human herpes virus, with a diameter of 150–200 nm, and a dense DNA core.
(Courtesy of D.K. Banerjee.)
Cytomegalovirus infection is often asymptomatic, but can reactivate and cause disease when cell-mediated immunity (CMI) defences are impaired
After clinically silent infection in the upper respiratory tract, CMV spreads locally to lymphoid tissues and then systemically in circulating lymphocytes and monocytes to involve lymph nodes and the spleen. The infection then localizes in epithelial cells in salivary glands and kidney tubules, and in cervix, testes and epididymis, from where the virus is shed to the outside world (Table 18.6).
Table 18.6 The effects of cytomegalovirus (CMV) infection
|
Site of infection |
Result |
Comment |
|
Salivary glands |
Salivary transmission |
Via kissing and contaminated hands |
|
Tubular epithelium of kidney |
Virus in urine |
Probable role in transmission by contaminating environment |
|
Cervix, testis/epididymis |
Sexual transmission |
Up to 107 infectious doses/ml of semen in an acutely infected male |
|
Lymphocytes, macrophages |
Virus spread through body via infected cells |
|
|
Placenta, fetus |
Congenital abnormalities |
Greatest damage in fetus after primary maternal infection rather than reactivation |
CMV is a ‘well-behaved parasite’, causing little or no damage to the host unless it infects the fetus or placenta to cause congenital abnormalities or it reactivates following depressed cell-mediated immunity (post-transplant, immunosuppression) to cause viraemia, fever, hepatitis or pneumonia.
Infected cells may be multinucleated or bear intranuclear inclusions, but pathologic changes are minor. The virus inhibits T-cell responses, and there is a temporary reduction in their immune reactivity to other antigens.
Although specific antibodies and CMI responses are generated, these fail to clear the virus (see Ch. 16), which often continues to be shed in saliva and urine for many months. The infection is, however, eventually controlled by CMI mechanisms, although infected cells remain in the body throughout life and can be a source of reactivation and disease when CMI defences are impaired.
CMV owes its success in our species to its ability to evade immune defences. For instance, it presents a poor target for cytotoxic T (Tc) cells by interfering with the transport of major histocompatibility complex (MHC) class I molecules to the cell surface (see Ch. 10), and it induces Fc receptors on infected cells (see Ch. 16).
Cytomegalovirus infection can cause fetal malformations and pneumonia in immunodeficient patients
In the natural host, the human infant or child, CMV causes no illness, and in general it causes a mild illness in adults. However, as with all infections, there is a spectrum of clinical disease ranging from asymptomatic to severely ill. A glandular fever-type illness can occur in adolescents which is similar to Epstein–Barr virus (EBV) infection, with fever, lethargy and abnormal lymphocytes and mononucleosis in blood smears. Primary infection during pregnancy allows the spread of virus from the blood to the placenta and then to the fetus, resulting in symptomatic CMV infection at birth in 18%, and detection of other sequelae in 25% by just under 5 years of age, as described in Chapter 23. Reactivation of infection during pregnancy also occurs, which may be asymptomatic at birth but up to 8% will have symptoms by 5 years of age. CMV is second only to Down’s syndrome as a cause of mental retardation in babies.
In immunodeficient patients such as bone marrow or solid organ transplant recipients (see Ch. 30), CMV infection can cause an interstitial pneumonitis with infiltrating infected mononuclear cells. Other sites affected include the CNS, with focal cerebral ‘micronodular’ lesions with infected mononuclear cells, together with a variety of other complications, including retinitis in HIV-infected individuals with AIDS. This was a major complication before the advent of highly active antiretroviral therapy (HAART). In addition, the gastrointestinal tract may be involved, with a colitis and hepatitis.
Clinical diagnosis of primary infection is rarely possible, because it is often asymptomatic. However, in symptomatic immunocompetent individuals, the diagnosis is made by detecting CMV IgM in blood samples. In those with possible CMV pneumonitis, a bronchoalveolar lavage sample is collected by passing a bronchoscope into the lungs and collecting washings, and CMV antigen or CMV DNA detection methods are used to make the diagnosis. Multinucleated cells or cells with prominent intranuclear inclusions may be seen in lung biopsy material. CMV IgM and IgG serology is available but is unlikely to be of diagnostic help in immunosuppressed patients. The management of post-transplant recipients involves CMV DNA monitoring of whole blood or plasma samples and giving pre-emptive therapy, having detected a CMV viraemia (see Ch. 30).
Antiviral treatment options in CMV infection
While ganciclovir, foscarnet or cidofovir are effective treatments, aciclovir is ineffective. These antiviral drugs reduce viral replication, do not eliminate the virus and can be used in specific clinical situations as pre-emptive therapy (see Ch. 30). As CMV pneumonitis is an immunopathological disease, CMV specific or human normal immunoglobulin is given in addition to the antiviral agent to potentially block the Tc-cell response to pneumocytes expressing the target antigens.
Prevention of CMV infection
There is no vaccine, but trials of live, inactivated and recombinant vaccines have been carried out. Bearing in mind that it is the second most common cause of mental retardation in babies, immunization is a major consideration once a number of practical issues have been resolved. The results of a recombinant CMV glycoprotein B vaccine trial reported in 2011 involving solid organ transplant recipients suggested that antibody levels generated in response to vaccine led to reduced viraemia and duration of antiviral use. Transmission can be reduced in various settings by avoiding contact between congenitally infected children and susceptible pregnant women or maintaining good hand hygiene if this is not possible. Blood for transfusion of newborns, and solid organ and bone marrow transplants, should preferably come from CMV antibody-negative donors.
Epstein–Barr virus infection
Epstein–Barr virus is transmitted in saliva
Epstein–Barr virus (EBV), like CMV, is species specific. EBV is structurally and morphologically identical to other herpesviruses (see Ch. 3), but is antigenically distinct. Major antigens include the viral capsid antigen (VCA) and the EBV-associated nuclear antigens (EBNA) that are used in diagnostic tests. Humans are the natural hosts.
EBV is transmitted by the exchange of saliva, for instance during kissing, and is a ubiquitous infection. In resource-poor countries, infection probably occurs via close contact in early childhood and is subclinical. Elsewhere, infection occurs in two peaks at 1–6 years and 14–20 years of age, and in most cases, causes illness.
The clinical features of EBV infection are immunologically mediated
Clinical and immunologic events in EBV infection are illustrated in Figure 18.6. EBV replicates in B lymphocytes, after making a specific attachment to the C3d receptor (CD21) on these cells, and also in certain epithelial cells. The pathogenesis of the disease and the clinical features can be accounted for on this basis. Virus is shed in saliva from infected epithelial cells and possibly lymphocytes in salivary glands, and from the oropharynx, with clinically silent spread to B lymphocytes in local lymphoid tissues and elsewhere in the body (lymph nodes, spleen).
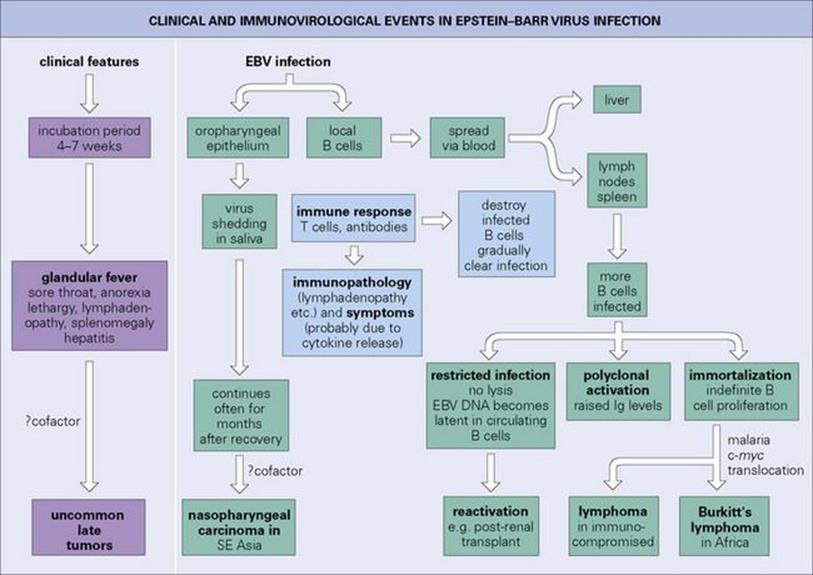
Figure 18.6 Clinical and immunovirologic events in Epstein–Barr virus (EBV) infection in adolescents or adults. A milder, often subclinical infection, occurs in children.
T lymphocytes respond immunologically to the infected B cells (outnumbering the latter by about 50 to 1) and appear in peripheral blood as ‘atypical lymphocytes’ (Fig. 18.7). Much of the disease is attributable to an immunologic civil war, as specifically activated T cells respond to the infected B cells. In the naturally infected infant or small child, these immune responses are weak and there is generally no clinical disease. Older children, however, become unwell, and young adults especially develop infectious mononucleosis or glandular fever 4–7 weeks after initial infection. This is characterized by fever, sore throat (see Fig. 18.3), often with petechiae on the hard palate, lymphadenopathy and splenomegaly, with anorexia and lethargy as prominent features. Hepatitis may occur, with mild elevations of hepatocellular enzymes in 90% of cases and jaundice in 9%. Splenic rupture may occur.
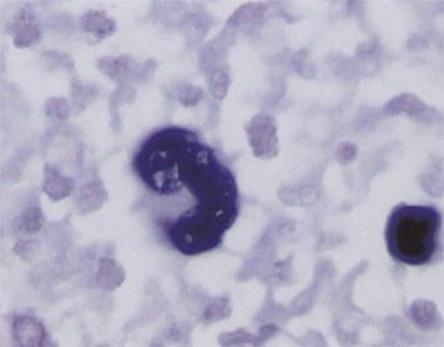
Figure 18.7 An atypical lymphocyte characteristic of Epstein–Barr virus infection.
(From Valbuena et al: Classical Hodgkin lymphoma arising in the rectum, Annals of Diagnostic Pathology 9:38-42, 2005.)
Complications are seen in about 1% of acute EBV infections and may be due to virus invading the tissue or to immune-mediated damage. These include aseptic meningitis and encephalitis, nearly always with complete recovery, haemolytic anaemia, airway obstruction due to oropharyngeal swelling, haemophagocytic syndrome and splenic rupture.
The symptoms are presumably due to the action of cytokines released during the intense immunologic activity. High levels of interferon-gamma, produced by activated T cells and NK cells, are likely to contribute to the symptoms as it causes headache, tiredness and fever. The infected B cells are stimulated to differentiate and produce antibodies; this polyclonal activation of B cells is responsible for the production of heterophil antibodies (reacting with erythrocytes of sheep or horses) and a variety of autoantibodies. Spontaneous recovery usually occurs in 2–3 weeks, but the symptoms may persist for a few months. The virus remains as a latent infection in spite of antibody and CMI responses, and saliva often remains infectious for months after clinical recovery.
The autoantibodies produced in response to EBV infection include IgM antibodies to erythrocytes (cold agglutinins), which are present in most cases. About 1% of cases develop an autoimmune haemolytic anaemia, which subsides within 1–2 months.
A ‘hairy tongue’ condition caused by EBV replication in squamous epithelial cells in the tongue occurs in immunodeficient patients.
Epstein–Barr virus remains latent in a small proportion of B lymphocytes
Epstein–Barr virus is well equipped to evade immune defences (see Ch. 16). It acts against complement and interferon, and produces a fake interleukin 10 (IL-10) molecule that interferes with the action of the host’s own IL-10 (an important immunoregulatory cytokine). EBV also prevents apoptosis (lysis) of infected cells, and the boldness of its strategy has enabled it to take up permanent residence within the immune system.
EBV DNA is present in episomal form in a small proportion of B lymphocytes, and a few copies may be integrated into the cell genome. Later in life, immunodeficiency can lead to reactivation of infection so that EBV reappears in the saliva, usually with no clinical symptoms.
Laboratory tests for diagnosing infectious mononucleosis should include viral capsid antigen IgM detection
Infectious mononucleosis is diagnosed clinically by the characteristic syndrome and the appearance of palatal petechiae in the throat. Laboratory diagnosis is made by detecting VCA IgM in the serum. However, there are other tests that help and these include:
• demonstrating atypical lymphocytes, comprising up to 30% of nucleated cells, in a blood smear. A number of viral infections cause an atypical lymphocytosis; therefore this is not specific to EBV.
• demonstrating heterophil antibodies to horse (or sheep) erythrocytes in the ‘monospot’ test. These are present in 90% of cases, but may not be detected in those less than 14 years of age and the response is short lived.
• EBV-specific antibody is the mainstay of diagnosis, in particular detecting VCA IgM indicates current infection. VCA IgG can be detected soon after VCA IgM and EBNA IgG appears a few weeks later after symptom onset.
Treatment of EBV infection is limited
Antiviral agents are not used to treat EBV-infected immunocompetent individuals. In immunosuppressed people in specific clinical settings there are some data on using specific antivirals to reduce viral replication, but they are only effective in the lytic part of the life cycle. In addition, an anti-CD20 receptor humanized monoclonal antibody called rituximab has been used to target EBV-infected B cells in specific clinical settings. There is no licensed vaccine, but placebo-controlled clinical trials have been carried out involving an envelope glycoprotein subunit vaccine and a CD8 T-cell peptide vaccine. The subunit vaccine was shown to have a significant effect on clinical disease but did not prevent infection.
Cancers associated with EBV
Epstein–Barr virus is closely associated with Burkitt’s lymphoma in African children
Burkitt’s lymphoma (Fig. 18.8) is virtually restricted to parts of Africa and Papua New Guinea, so it is clear that EBV alone is not enough to cause the lymphoma. The most likely co-carcinogen is malaria, which acts by weakening T-cell control of EBV infection and perhaps by causing polyclonal activation of B cells, the increased turnover rendering them more susceptible to neoplastic transformation.
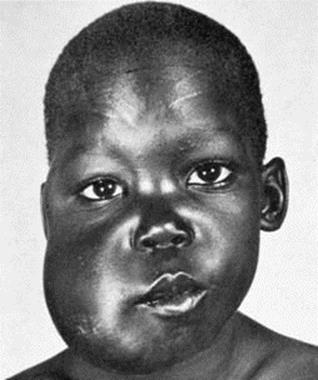
Figure 18.8 Burkitt’s lymphoma affecting the maxilla in an African child.
(Courtesy I. Magrath, MD, Bethesda, Md. From Zitelli B, Davis H: Atlas of Pediatric Physical Diagnosis, 2007, Mosby Elsevier.)
Epstein–Barr virus is closely associated with other B-cell lymphomas in immunodeficient patients
For example, B-cell lymphomas occur in 1–10% of solid organ transplant recipients, especially children, when primary EBV infection occurs post-transplantation. EBV DNA and RNA transcripts are found in the tumour cells, which also show a translocation of the c-myc oncogene on chromosome 8 to the immunoglobulin heavy chain locus on chromosome 14 (see Ch. 17). Post-transplant lymphoproliferative disorders (PTLD) are due to uncontrolled B-cell proliferation. In addition, there is the rare X-linked lymphoproliferative disease (XLP) that is associated with EBV infection. This inherited disorder involves mutations in the gene that codes for the signalling lymphocyte activation molecule associated protein. The latter is key to B-cell activation of T cells and NK cells which control EBV-infected B cells. Therefore, individuals with this X-linked disorder can develop fatal infectious mononucleosis and lymphomas and it can only be prevented by having an allogeneic bone marrow transplant.
Epstein–Barr virus infection is also closely associated with nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is a very common cancer in China and South-East Asia. EBV DNA is detectable in the tumour cells, and a co-carcinogen, possibly ingested nitrosamines from preserved fish, is likely. Host genetic factors controlling human leukocyte antigens (HLA) and immune responses may confer susceptibility to NPC.
Bacterial infections
Bacteria responsible for pharyngitis include:
• Strep. pyogenes (group A β-haemolytic, Fig. 18.9), the commonest and most important to diagnose because it can lead to complications (see below), but can be readily treated with penicillin
• Corynebacterium diphtheriae
• Haemophilus influenzae (type B), which occasionally causes severe epiglottitis with obstruction of the airways, especially in young children
• Borrelia vincentii together with certain fusiform bacilli, which can cause throat or gingival ulcers
• Neisseria gonorrhoeae.
Each of these types of bacteria attach to the mucosal surface, sometimes invading local tissues.
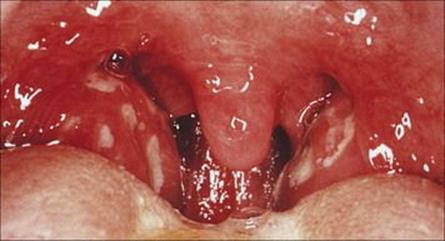
Figure 18.9 Streptococcal tonsillitis due to group A β-haemolytic Streptococcus pyogenes with intense erythema of the tonsils and a creamy-yellow exudate.
(Courtesy of J.A. Innes.)
Complications of Strep. pyogenes infection
Complications of Strep. pyogenes throat infection include quinsy, scarlet fever, rheumatic fever, rheumatic heart disease and glomerulonephritis
These complications are important enough to be listed separately, although most are uncommon in resource-rich countries where there is good access to medical care and probably less exposure to streptococci. The complications include:
• Peritonsillar abscess (‘quinsy’) is an uncommon complication of untreated streptococcal sore throat.
• Otitis media, sinusitis, mastoiditis (see below) is caused by local spread of Strep. pyogenes.
• Scarlet fever. Certain strains of Strep. pyogenes produce an erythrogenic toxin coded for by a lysogenic phage. The toxin spreads through the body and localizes in the skin to induce a punctate erythematous rash (scarlet fever; Fig. 18.10). The tongue is initially furred, but later red. The rash begins as facial erythema and then spreads to involve most of the body except the palms and soles. The face is generally flushed with circumoral pallor. The rash fades over the course of 1 week and is followed by extensive desquamation. The skin lesions themselves are not serious, but they signal infection by a potentially harmful streptococcus, which in pre-antibiotic days could sometimes spread through the body to cause cellulitis and septicaemia.
• Rheumatic fever. This is an indirect complication. Antibodies formed to antigens in the streptococcal cell wall cross-react with the sarcolemma of human heart, and with tissues elsewhere. Granulomas are formed in the heart (Aschoff’s nodules), and 2–4 weeks after the sore throat the patient (usually a child) develops myocarditis or pericarditis, which may be associated with subcutaneous nodules, polyarthritis and, rarely, chorea. Chorea is a disease of the central nervous system resulting from streptococcal antibodies reacting with neurones.
• Rheumatic heart disease. Repeated attacks of Strep. pyogenes with different M types can lead to damage to the heart valves. Certain children have a genetic predisposition to this immune-mediated disease. If a primary attack is accompanied by rising or high antistreptolysin O (ASO) antibody levels, future attacks must be prevented by penicillin prophylaxis throughout childhood. In many developing countries, rheumatic heart disease is the most common type of heart disease.
• Acute glomerulonephritis. Antibodies to streptococcal components combine with them to form circulating immune complexes, which are then deposited in glomeruli, together, probably, with autoantibodies to glomerular components. Here, the complement and coagulation systems are activated, resulting in local inflammation. Blood appears in the urine (red cells, protein) and there are signs of an acute nephritis syndrome (oedema, hypertension) 1–2 weeks after the sore throat. ASO antibodies are usually elevated. Only four to five of the 65 M types of Strep. pyogenesgive rise to this condition, and repeated infection with different ‘nephritogenic’ types is unlikely. Penicillin prophylaxis is therefore not given. In contrast to rheumatic fever, second attacks are rare.
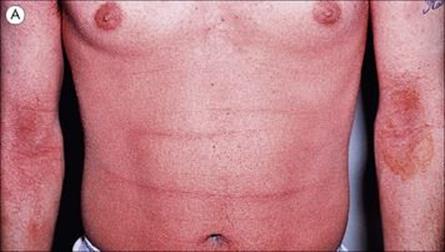
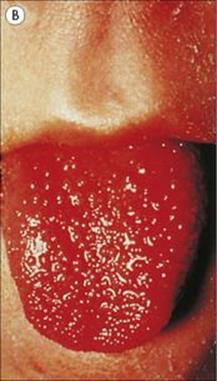
Figure 18.10 Scarlet fever. (A) Punctate erythema is followed by peeling for 2–3 weeks.
(From James et al: Andrews’ Diseases of the Skin, 2006, Saunders Elsevier.) (B) The tongue is furred at first and then becomes raw with prominent papillae. (Courtesy of W.E. Farrar.)
Diagnosis
A laboratory diagnosis is not generally necessary for pharyngitis and tonsillitis
There are many possible viral causes of pharyngitis and tonsillitis, and the clinical condition is generally not serious enough to seek laboratory help. The diagnosis of EBV or CMV infection is helped by detecting a lymphocytosis and atypical lymphocytes in a blood film. EBV is distinguished from CMV by detecting VCA IgM, although the less specific tests such as the Paul–Bunnell or monospot test may be used, whereas CMV diagnosis is made by detecting CMV IgM. HSV is readily isolated or the DNA detected in swabs from the lesions sent to the laboratory, but clinical diagnosis is usually adequate. Bacteria are identified by culturing throat swabs (see Ch. 32). It is especially important to diagnose Strep. pyogenes infection by culture because of the possible complications (see above) and because, unlike Strep. pneumoniae, it remains susceptible to penicillin. Resistance to erythromycin and tetracycline, however, is increasing. Although during the winter months up to 16% of schoolchildren carry group A streptococci in the throat without symptoms, treatment is recommended.
Parotitis
Mumps virus is spread by airborne droplets and infects the salivary glands
There is only one serotype of this single-stranded RNA paramyxovirus. It spreads by airborne droplets, salivary secretions and possibly urine. Close contact is necessary, for example, at school, as the peak incidence is at 5–14 years of age. However, susceptible adults are at risk of complications of mumps such as orchitis.
After entering the body, the primary site of replication is the epithelium of the upper respiratory tract or eye. The virus spreads, undergoing further multiplication in local lymphoid tissues (lymphocytes and monocytes) and reticuloendothelial cells. After approximately 7–10 days the virus enters the blood, a primary viraemia, and localizes in salivary and other glands and body sites including the central nervous system, testis, pancreas and ovary (Fig. 18.11) and is excreted in the urine. Infected cells lining the parotid ducts degenerate and finally, after an incubation period of 16–18 days, the inflammation, with lymphocyte infiltration and often oedema, results in disease. After a prodromal period of malaise and anorexia lasting 1–2 days, the parotid gland becomes painful, tender and swollen, and is sometimes accompanied by submandibular gland involvement (Fig. 18.12). This is the classic sign of mumps, and parotitis is the most common clinical sign. Other sites may be invaded, with clinical consequences such as inflammation of the testis and pancreas, resulting respectively in orchitis and pancreatitis (Table 18.7). CMI as well as antibody responses appear, and the patient usually recovers within 1 week. There is life-long resistance to reinfection.
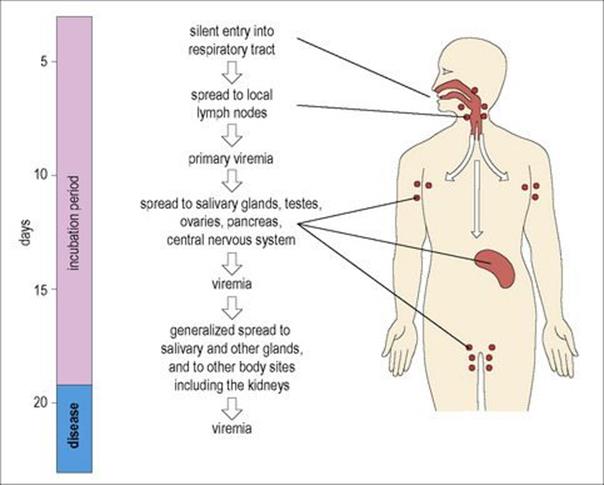
Figure 18.11 The pathogenesis of mumps. Understanding the pathogenesis of this infection helps to explain the disease picture, sites of shedding and the complications that can arise, but little is known about the events that occur during the first week of infection.
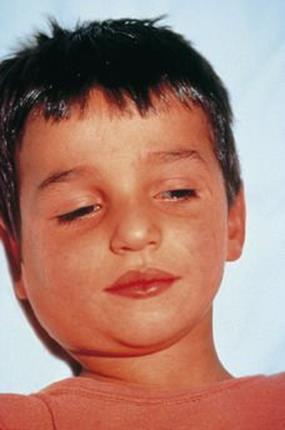
Figure 18.12 Enlarged submandibular glands in a child with mumps.
(From Heumann et al: Klinische Infektiologie, 2008, Elsevier.)
Table 18.7 Clinical consequences of mumps virus invasion of different body tissues
|
Site of growth |
Result |
Comment |
|
Salivary glands |
Inflammation, parotitis |
Often absent; can be unilateral |
|
Meninges |
Meningitis |
Common (in about 10% cases) |
|
Kidney |
Virus present in urine |
No clinical consequences |
|
Testis, ovary |
Epididymo-orchitis; rigid tunica albuginea around testis make orchitis more painful and more damaging in male |
Common in adults (20% in adult males); often unilateral; not a significant cause of sterility |
|
Pancreas |
Pancreatitis |
Rare complication (possible role in juvenile diabetes mellitus) |
|
Mammary gland |
Virus detectable in milk; mastitis in 10% post-pubertal females |
|
|
Thyroid |
Thyroiditis |
Rare |
|
Myocardium |
Myocarditis |
Rare |
|
Joints |
Arthritis |
Rare |
Laboratory diagnosis is made:
• by detecting viral RNA in throat swabs, cerebrospinal fluid (CSF) or urine or by isolating virus in cell culture
• by detecting mumps-specific IgM antibody.
Treatment and prevention
There is no specific treatment, but mumps is prevented by using the attenuated live virus vaccine, which is safe and effective. This is usually given in combination with measles and rubella vaccines (MMR vaccine).
Combined MMR has been a controversial issue in the UK after autism and bowel disorders were reported as being possibly associated with immunization. However, despite a series of epidemiological studies showing no association with immunization, there was a fall in MMR uptake rates and subsequent outbreaks of mumps and measles around the UK. These rates had improved by 2011; however, increasing numbers of outbreaks of measles were seen in other parts of Europe.
Otitis and sinusitis
Otitis and sinusitis can be caused by many viruses and a range of secondary bacterial invaders
Many viruses are capable of invading the air spaces associated with the upper respiratory tract (sinuses, middle ear, mastoid). Mumps virus or respiratory syncytial virus (RSV), for instance, can cause vestibulitis or deafness, which is generally temporary. The range of secondary bacterial invaders is the same as for other upper respiratory tract infections, i.e. Strep. pneumoniae and H. influenzae and sometimes anaerobes, such as Bacteroides fragilis. Brain abscess is a major complication (see Ch. 24). Blockage of the Eustachian (auditory) tube or the opening of sinuses, caused by allergic swelling of the mucosa, prevents mucociliary clearance of infection, and the local accumulation of inflammatory bacterial products causes further swelling and blockage.
Acute otitis media
Common causes of acute otitis media are viruses, Strep. pneumoniae and H. influenzae
This condition is extremely common in infants and small children, partly because the eustachian (auditory) tube is open more widely at this age. A study in Boston showed that 83% of 3-year-olds had had at least one episode, and 46% had had three or more episodes since birth. At least 50% of the attacks are viral in origin (especially RSV), and the bacterial invaders are nasopharyngeal residents, most commonly Strep. pneumoniae or H. influenzae, and sometimes Strep. pyogenes or Staph. aureus. There may be general symptoms, and acute otitis media should be considered in any child with unexplained fever, diarrhea or vomiting. The ear drum shows dilated vessels with bulging of the drum at a later stage (Fig. 18.13). Fluid often persists in the middle ear for weeks or months (‘glue ear’), regardless of therapy, and contributes to impaired hearing and learning difficulties in infants and small children.
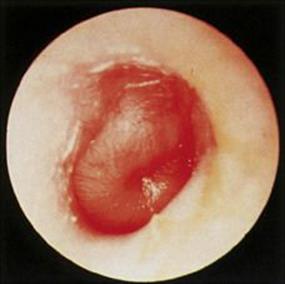
Figure 18.13 Acute otitis media with bulging ear drum.
(Courtesy of M. Chaput de Saintonge.)
If acute attacks are inadequately treated, there may be continued infection with a chronic discharge through a perforated drum and impaired hearing. This is ‘chronic suppurative otitis media’.
Otitis externa
Causes of otitis externa are Staph. aureus, Candida albicans and Gram-negative opportunists
Infections of the outer ear can cause irritation and pain, and must be distinguished from otitis media. In contrast to the middle ear, the external canal has a bacterial flora similar to that of the skin (staphylococci, corynebacteria and, to a lesser extent, propionibacteria), and the pathogens responsible for otitis media are rarely found in otitis externa. The warm moist environment favours Staph. aureus, Candida albicans and Gram-negative opportunists such as Proteus and Pseudomonas aeruginosa.
Ear drops containing polymyxin or other antibiotics are usually an effective treatment.
Acute sinusitis
The aetiology and pathogenesis of acute sinusitis are similar to those of otitis media. Clinical features include facial pain and localized tenderness. It may be possible to identify the causative bacteria by microscopy and culture of pus aspirated from the sinus, but sinus puncture is not often carried out. In addition, as is the case for otitis media, the patient can be treated empirically with ampicillin or amoxicillin, or with the newer oral cephalosporins (e.g. cefixime) to deal with beta-lactamase-producing organisms.
Acute epiglottitis
Acute epiglottitis is generally due to H. influenzae capsular type B infection
Acute epiglottitis is most often seen in young children. For unknown reasons, H. influenzae capsular type B spreads from the nasopharynx to the epiglottis, causing severe inflammation and oedema. There is usually a bacteraemia.
Acute epiglottitis is an emergency and necessitates intubation and treatment with antibiotics
Acute epiglottitis is characterized by difficulty in breathing because of respiratory obstruction and, until the airway has been secured by intubation, extreme care must be taken when examining the throat in case the swollen epiglottis is sucked into the oedematous airway and causes total obstruction. Treatment is begun immediately with antibiotics effective against H. influenzae (cefotaxime, chloramphenicol). The clinical diagnosis is confirmed by isolating bacteria from the blood and possibly the epiglottis. The H. influenzae type B (Hib) vaccine greatly reduces the frequency of this and other infections due to H. influenzae type B.
Respiratory obstruction due to diphtheria (see below) is rare in resource-rich countries, but the characteristic false membrane and local swelling can extend from the pharynx to involve the uvula.
Oral cavity infections
Saliva flushes the mouth and contains a variety of antibacterial substances
The oral cavity is continuous with the pharynx, but is dealt with separately because of the presence of teeth, which are subject to a particular set of microbiologic problems. The normal mouth contains commensal microorganisms, some of which are to a large extent restricted to the mouth (see Table 18.1). Most of them make specific attachments to teeth or mucosal surfaces and are shed into the saliva as they multiply. The litre or so of saliva secreted each day mechanically flushes the mouth. It also contains secretory antibodies, polymorphs, desquamated mucosal cells and antibacterial substances such as lysozyme and lactoperoxidase. When salivary flow is decreased for a few hours, as between meals, there is a fourfold increase in the number of bacteria in saliva, and, in dehydrated patients or in severe illnesses such as typhoid or pneumonia, the mouth becomes foul because of microbial overgrowth.
Oral candidiasis
Changes in the oral flora produced by broad-spectrum antibiotics and impaired immunity predispose to thrush
The presence of commensal bacteria in the mouth makes it difficult for invading microorganisms to become established, but changes in oral flora upset this balance. For instance, prolonged administration of broad-spectrum antibiotics allows the normally harmless C. albicans to flourish, penetrating the epithelium with its pseudomycelia, and causing thrush. Oral thrush (candidiasis, Fig. 18.14) is also seen when immunity is impaired, as in HIV infection and malignancy, and occasionally in newborn infants and the elderly. It sometimes spreads to involve the oesophagus. The diagnosis is readily confirmed by Gram stain and culture of scraped material, which shows large Gram-positive budding yeasts.
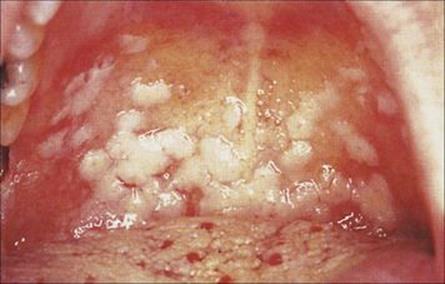
Figure 18.14 Oral candidiasis.
(Courtesy of J.A. Innes.)
Topical antifungal agents (e.g. nystatin or clotrimazole) or oral fluconazole (see Ch. 33) are effective treatments for thrush, together with attention to any predisposing factors.
Another example of the shifting boundary between harmless coexistence and tissue invasion by resident microbes is seen with vitamin C deficiency, which reduces mucosal resistance and allows residents to cause gum infections.
Caries
In the USA and Western Europe, 80–90% of people are colonized by Streptococcus mutans, which causes dental caries
The microorganisms specifically adapted for life on teeth form a film called dental plaque on the tooth surface. This is a complex mass containing about 109 bacteria/g embedded in a polysaccharide matrix (Fig. 18.15). The film, visible as a red layer when a dye such as Erythrocin is taken into the mouth, is largely removed by thorough brushing, but re-establishes itself within a few hours. The clean teeth become covered with salivary glycoproteins to which certain streptococci (especially Strep. mutans and Strep. sobrinus) become attached and multiply. In the USA and Western Europe, 80–90% of people are colonized by Strep. mutans. Strep. mutans itself synthesizes glucan (a sticky high molecular weight polysaccharide) from sucrose and this forms a matrix between these streptococci. Certain other bacteria, including anaerobic filamentous fusobacteria and actinomycetes, are also present. When the teeth are not cleaned for several days, plaque becomes thicker and more extensive – a tangled forest of microorganisms.
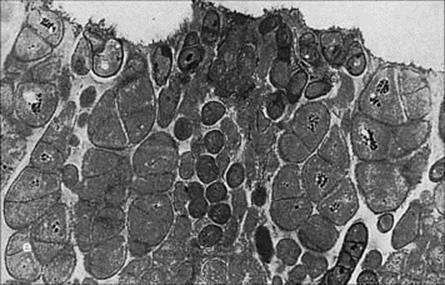
Figure 18.15 Dental plaque on the deep surface of a child’s tooth. e, enamel. (× 20 000)
(Courtesy of H.N. Newman.)
The bacteria in plaque use dietary sugar and form lactic acid, which decalcifies the tooth locally. Proteolytic enzymes from the bacteria help to break down other components of the enamel to give rise to a painful cavity in the tooth (caries). Infection may then spread into the pulp of the tooth to form a pulp or root abscess, and from here to the maxillary or mandibular spaces.
The pH in an active caries lesion may be as low as 4.0. Therefore, caries usually develops in crevices on the tooth when suitable bacteria (Strep. mutans) are in the plaque and there is a regular supply of sucrose. It may legitimately be regarded as an infectious disease – one of the most prevalent infectious diseases in resource-rich countries due to closely placed bacteria-coated teeth and a sugary, often fluoride-deficient, diet.
Periodontal disease
Actinomyces viscosus, Actinobacillus and Bacteroides spp. are commonly involved in periodontal disease
A space (the gingival crevice) readily forms between the gums and tooth margin, and it may be considered as an oral backwater. It contains polymorphs, complement, IgG and IgM antibodies, and easily becomes infected. Gingival crevices normally contain an average of 2.7 × 1011microbes/g, and 75% of them are anaerobes. Bacteria such as Actinomyces viscosus, Actinobacillus and Bacteroides spp. are commonly involved. In periodontal disease, the space enlarges to become a ‘pocket’, with local inflammation, an increasing number of polymorphs and a serum exudate. The inflamed gum bleeds readily and later recedes, while the multiplying bacteria cause halitosis. Finally, the structures supporting the teeth are affected, with reabsorption of ligaments and weakening of bone, causing the teeth to loosen. Periodontal disease with gingivitis is almost universal, although its severity varies greatly. It is a major cause of tooth loss in adults.
![]()
 Key Facts
Key Facts
• The respiratory tract from the nose to the alveoli is a continuum, and any given microbe can cause disease in more than one segment.
• Some respiratory infections are restricted to the surface epithelium (influenza, diphtheria, pertussis), while others spread throughout the body (measles, rubella, mumps, CMV, EBV).
• ‘Professional’ invaders infect the healthy respiratory tract (e.g. common cold viruses, influenza viruses, mumps, CMV, EBV, M. tuberculosis), whereas ‘secondary’ invaders cause disease when host defences are impaired (e.g. Staph. aureus, Pneumocystis jiroveii, Pseudomonas).
• Common diseases of the teeth and neighbouring structures – caries, periodontal disease – are of microbial aetiology.
• Diphtheria is a life-threatening disease caused by a biochemically defined bacterial toxin, and is completely preventable by vaccination.
![]()