Medical Microbiology
Section 4 Clinical manifestation and diagnosis of infections by body system
19 Lower respiratory tract infections
Introduction
Although the respiratory tract is continuous from the nose to the alveoli, it is convenient to distinguish between infections of the upper and lower respiratory tract, even though the same microorganisms might be implicated in infections of both. Infections of the upper respiratory tract and associated structures are the subject of Chapter 18. Here, we discuss infections of the lower respiratory tract. These infections tend to be more severe than infections of the upper respiratory tract, and the choice of appropriate antimicrobial therapy is important and may be life saving.
Laryngitis and tracheitis
Parainfluenza viruses are common causes of laryngitis
Viral infections of the upper respiratory tract may spread downwards to involve the larynx and the trachea. Usually the cause is a parainfluenza virus, but sometimes it is RSV, influenza virus or an adenovirus. Diphtheria (see below) may involve the larynx or trachea.
In adults, laryngeal infection (laryngitis) and tracheitis cause hoarseness and a burning retrosternal pain. The larynx and trachea have non-expandable rings of cartilage in the wall, and are easily obstructed in children, due to the narrow passages, leading to hospital admission. Swelling of the mucous membrane may lead to a dry cough and inspiratory stridor (‘crowing’) known as croup. Bacteria such as group A streptococci, Haemophilus influenzae and Staphylococcus aureus are less common causes of laryngitis and tracheitis.
Diphtheria
Diphtheria is caused by toxin-producing strains of Corynebacterium diphtheriae and can cause life-threatening respiratory obstruction
Diphtheria is now rare in resource-rich countries due to widespread immunization with toxoid (see Ch. 34), but it is still common in resource-poor countries. Non-toxigenic strains occur in the normal pharynx, but bacteria producing the extracellular toxin (exotoxin; see Ch. 2) must be present to cause disease. They can colonize the pharynx (especially the tonsillar regions), the larynx, the nose and occasionally the genital tract, and in the tropics or in indigent people with poor skin hygiene, the skin.
Adhesion is mediated by pili or fimbriae covalently attached to the bacterial cell wall. The bacteria multiply locally without invading deeper tissues or spreading through the body. The toxin destroys epithelial cells and polymorphs, and an ulcer forms, which is covered with a necrotic exudate forming a ‘false membrane’. This soon becomes dark and malodorous, and bleeding occurs on attempting to remove it. There is extensive inflammation and swelling (Fig. 19.1) and the cervical lymph nodes may be enlarged to give a ‘bull neck’ appearance.
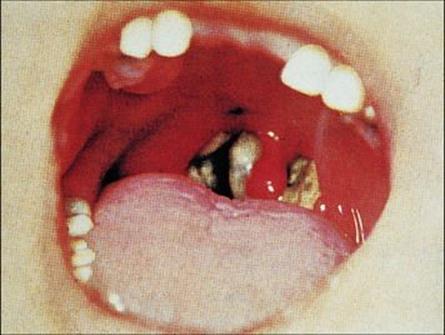
Figure 19.1 Pharyngeal diphtheria. Characteristic diphtheria ‘false membrane’ in a child, with local inflammation.
(Courtesy of Norman Begg.)
Nasopharyngeal diphtheria is the most severe form of the disease. When the larynx is involved, it can result in life-threatening respiratory obstruction. Anterior nasal diphtheria is a mild form of the disease if it occurs on its own, because the toxin is less well absorbed from this site, and a nasal discharge may be the main symptom. The patient will, however, be highly infectious.
Diphtheria toxin can cause fatal heart failure and a polyneuritis
The toxin (Box 19.1 and Fig. 19.2) is absorbed into the lymphatics and blood, and has several effects:
• Constitutional upset, with fever, pallor, exhaustion.
• Myocarditis, usually within the first 2 weeks. Electrocardiographic changes are common and cardiac failure can occur. If this is not lethal, complete recovery is usual.
• Polyneuritis, which may occur after the onset of illness, due to demyelination. It may, for instance, affect the 9th cranial nerve, resulting in paralysis of the soft palate and regurgitation of fluids.
![]()
Box 19.1  Lessons in Microbiology
Lessons in Microbiology
Diphtheria toxin
The genes encoding toxin production are carried by a temperate bacteriophage which, during the lysogenic phase, is integrated into the bacterial chromosome. The toxin is synthesized as a single polypeptide (molecular weight 62 000; 535 amino acids) consisting of:
• fragment B (binding) at the carboxy terminal end, which attaches the toxin to the host cells (or to any eukaryotic cell)
• fragment A (active) at the amino terminal end, which is the toxic fragment.
Toxic fragment A is only formed by protease cleavage and reduction of disulfide bonds after cellular uptake of the toxin. Fragment A inactivates elongation factor-2 (EF-2) by adenosine diphosphate (ADP) ribosylation and thereby inhibits protein synthesis (Fig. 19.2). Prokaryotic and mitochondrial protein synthesis is not affected because a different EF is involved. A single bacterium can produce 5000 toxin molecules/h and the toxic fragment is so stable within the cell that a single molecule can kill a cell. For unknown reasons, myocardial and peripheral nerve cells are particularly susceptible.
![]()
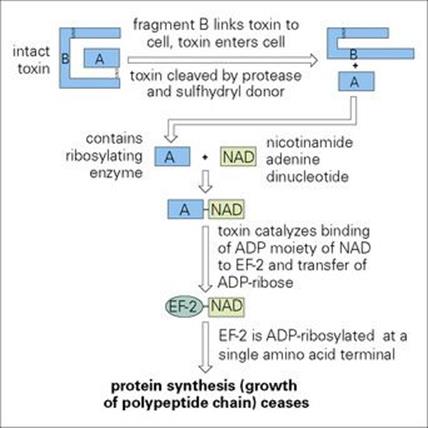
Figure 19.2 Mechanism of action of diphtheria toxin. ADP, adenosine diphosphate; EF-2, elongation factor-2.
Diphtheria is managed by immediate treatment with antitoxin and antibiotic
Diphtheria is a life-threatening disease, and clinical diagnosis is a matter of urgency. As soon as the diagnosis is suspected clinically, the patient is isolated to reduce the risk of the toxigenic strain spreading to other susceptible individuals, and antitoxin treatment is started. The antitoxin is produced in horses, and tests for hypersensitivity to horse serum should be carried out. Penicillin or erythromycin is given as well. Laryngeal diphtheria may result in an obstructed airway and require a tracheotomy to assist with respiration.
The diagnosis is confirmed in the laboratory by isolation and identification of the organism (Ch. 32) and demonstrating toxin production by a gel-diffusion precipitin reaction (Elek test). PCR can be carried out in some reference laboratories to detect the tox gene responsible for producing the toxin.
Contacts may need chemoprophylaxis or immunization
Contacts of diphtheria patients should be tested for carriage of toxigenic C. diphtheriae and if necessary be given chemoprophylaxis or immunization. Toxigenic bacteria may be carried and transmitted by asymptomatic convalescents or by apparently healthy individuals.
Diphtheria is prevented by immunization
Diphtheria has almost disappeared from resource-rich countries as a result of the immunization of children with a safe, effective toxoid vaccine (see Ch. 34). However, the disease reappears when immunization is neglected. In 1990, epidemics began in the Russian Federation, and by 1994, all 15 of the newly independent states of the former Soviet Union were involved, with 157 000 reported cases by 1997. The World Health Organization (WHO) website reported in 2011 that the incidence of diphtheria ranged from 0.5–1/100 000 population in Armenia, Estonia, Lithuania and Uzbekistan, to 27–32/100 000 in Russia and Tajikistan. Case fatality rates ranged from 2–3% in Russia to 17–23% in Azerbaijan, Georgia and Turkmenistan. Worldwide, in 2004, the World Health Organization estimated there were 5000 deaths and in 2009, 857 cases were reported.
Whooping cough
Whooping cough is caused by the bacterium Bordetella pertussis
Whooping cough or pertussis is a severe disease of childhood. Bordetella pertussis is confined to humans and is spread from person to person by airborne droplets. The organisms attach to, and multiply in, the ciliated respiratory mucosa, but do not invade deeper structures. Surface components such as filamentous haemagglutinin and fimbrial agglutinogens play an important role in specific attachment to respiratory epithelium.
B. pertussis infection is associated with the production of a variety of toxic factors
Some of these toxic factors affect inflammatory processes, while others damage ciliary epithelium. They are:
• Pertussis toxin, which resembles diphtheria and other toxins (see Chs 17 and 18) in being a subunit toxin with an active (A) unit and a binding (B) unit. The A unit is an adenosine diphosphate (ADP)-ribosyl transferase, which catalyses the transfer of ADP-ribose from nicotinamide adenine dinucleotide (NAD) to host cell proteins. The functional consequence of this is a disruption of signal transduction to the affected cell, but the toxin probably has other effects on the cell surface as well.
• Adenylate cyclase toxin, which is a single peptide that can enter host cells and cause them to increase their cyclic adenosine monophosphate (cAMP) to supraphysiologic levels. In neutrophils, this results in an inhibition of defence functions such as chemotaxis, phagocytosis and bactericidal killing. This toxin may also be responsible for the haemolytic properties of B. pertussis.
• Tracheal cytotoxin, which is a cell wall component derived from the peptidoglycan of B. pertussis that specifically kills tracheal epithelial cells (see Ch. 2).
• Endotoxin, which differs from the classic endotoxin of other Gram-negative rods, but has functional similarities and may play a role in the pathogenesis of infection.
B. pertussis infection is characterized by paroxysms of coughs followed by a ‘whoop’. After an incubation period of 7–10 days (range 5–21 days), B. pertussis infection is manifest first as a catarrhal illness with little to distinguish it from other upper respiratory tract infections. This is followed up to 1 week later by a dry non-productive cough, which becomes paroxysmal. A paroxysm is characterized by a series of short coughs producing copious mucus, followed by a ‘whoop’, which is a characteristic sound produced by an inspiratory gasp of air. Despite the severity of the cough, the symptoms are confined to the respiratory tract, and lobar or segmental collapse of the lungs can occur (Fig. 19.3).

Figure 19.3 Chest radiograph showing patchy consolidation and collapse of the right middle lobe in whooping cough.
(Courtesy of J.A. Innes.)
Complications include central nervous system (CNS) anoxia, exhaustion and secondary pneumonia due to invasion of the damaged respiratory tract by other pathogens.
The early clinical picture is non-specific, and the true diagnosis may not be suspected until the paroxysmal phase. The organisms can be isolated on suitable media from throat swabs or on ‘cough plates’ (see Ch. 32), but they are fastidious and do not survive well outside the host’s environment.
Whooping cough is managed with supportive care and erythromycin
Supportive care is of prime importance. Infants are at greatest risk of complications, and admission to hospital should be considered for children less than 1 year of age. For specific antibacterial treatment to be effective it must penetrate the respiratory mucosa and inhibit or kill the infecting organism. Treatment with macrolide antibiotics such as erythromycin, clarithromycin or azithromycin is recommended. Although the treatment is often only started when the disease is recognized in the paroxysmal phase, it does appear to reduce its severity and duration. It also reduces the bacterial load in the throat, thereby helping to reduce both the infectivity of the patient and the risk of secondary infections.
Prophylaxis with macrolide antibiotics of close contacts of active cases is helpful in controlling the spread of infection.
Whooping cough can be prevented by active immunization
For many years, a whole cell vaccine comprising a killed suspension of B. pertussis cells was used, combined with purified diphtheria and tetanus toxoids and administered as ‘DPT’ or ‘triple’ vaccine. Although an effective vaccine, there were major concerns about side effects. These included fever, malaise and pain at the site of administration in up to 20% of infants; convulsions, thought to be associated with the vaccine in about 0.5% of vaccinees; and encephalopathy and permanent neurologic sequelae associated with vaccination, with an estimated rate of 1 in 100 000 vaccinations (< 0.001%).
Concern about side effects led to a marked fall in uptake of the vaccine and subsequently to a marked increase in the incidence of whooping cough (see Ch. 31).
Acellular pertussis vaccines became the dominant vaccine preparation as they provide the same or better protection against whooping cough and cause fewer side effects as they are highly purified with much reduced levels of endotoxin compared with whole cell vaccines. The acellular vaccines contain pertussis toxoid and other bacterial components, including the filamentous haemagglutinin and fimbriae, and are given in combination with other vaccines such as diphtheria, tetanus, polio and Haemophilus influenzae type B. In 2008, about 82% of all infants worldwide received three doses of pertussis vaccine. WHO estimated that global pertussis immunization prevented about 687 000 deaths that year and that about 16 million cases of pertussis occurred worldwide. Ninety-five per cent were in resource-poor countries and whooping cough led to about 195 000 childhood deaths.
Acute bronchitis
Acute bronchitis is an inflammatory condition of the tracheobronchial tree, usually due to infection
Causative agents include rhinoviruses and coronaviruses, which also infect the upper respiratory tract, and lower tract pathogens such as influenza viruses, adenoviruses and Mycoplasma pneumoniae. Secondary bacterial infection with Streptococcus pneumoniae and Haemophilus influenzaemay also play a role in pathogenesis. The degree of damage to the respiratory epithelium varies with the infecting agent:
• With influenza virus infection, it may be extensive and leave the host prone to secondary bacterial invasion (post-influenza pneumonia; see below).
• With Mycoplasma pneumoniae infection, specific attachment of the organism to receptors on the bronchial mucosal epithelium (Fig. 19.4) and the release of toxic substances by the organism results in sloughing of affected cells. There is a 4-yearly epidemic cycle that normally occurs 2 years after the Olympic Games. A dry cough is the most prominent presentation, and treatment is largely symptomatic. However, it can cause pneumonia and complications involving other organs, such as hepatitis, encephalitis, arthralgia, skin lesions and haemolytic anaemia. Treatment involves antibiotics such as tetracyclines or macrolides.
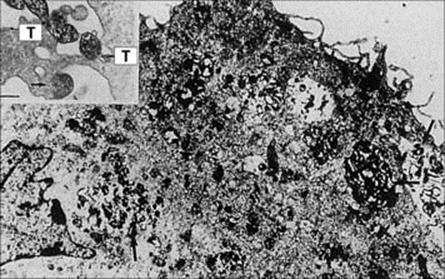
Figure 19.4 Opsonized Mycoplasma pneumoniae cells (arrowed) phagocytosed by an alveolar macrophage (bar, 2 μm). The insert shows M. pneumoniae cells adhering with the tip organelle (T) to macrophage surfaces.
(From Jacobs E. Rev Med Microbiol 2:83–90, © 1991.)
Acute exacerbations of chronic bronchitis
Infection is only one component of chronic bronchitis
Chronic bronchitis is a condition characterized by cough and excessive mucus secretion in the tracheobronchial tree that is not attributable to specific diseases such as bronchiectasis, asthma or tuberculosis. Infection appears to be only one component of the syndrome, the others being cigarette smoking and inhalation of dust or fumes from the workplace. Bacterial infection does not appear to initiate the disease, but is probably significant in perpetuating it and in producing the characteristic acute exacerbations. Streptococcus pneumoniae and unencapsulated strains ofHaemophilus influenzae are the organisms most frequently isolated, but interpretation of the significance of their presence in sputum is difficult because they are also commonly found in the normal throat flora and can therefore contaminate expectorated sputum. Other bacteria such asStaphylococcus aureus and Mycoplasma pneumoniae are less commonly associated with infection and exacerbation. Viruses are frequent causes of acute infection.
Antibiotic therapy may be helpful in the treatment of acute exacerbations, although its efficacy is difficult to assess.
Bronchiolitis
Around 75% of bronchiolitis presentations are caused by respiratory syncytial virus infection
Bronchiolitis is a disease restricted to childhood, and usually to children less than 2 years of age. The bronchioles of a young child have such a fine bore that if their lining cells are swollen by inflammation the passage of air to and from the alveoli can be severely restricted. Infection results in necrosis of the epithelial cells lining the bronchioles and leads to peribronchial infiltration, which may spread into the lung fields to give an interstitial pneumonia (see below). As many as 75% of these infections are caused by respiratory syncytial virus (RSV) and the other 25% are also of viral aetiology, although Mycoplasma pneumoniae is implicated occasionally.
Respiratory syncytial virus infection
Respiratory syncytial virus is the most important cause of bronchiolitis and pneumonia in infants
Respiratory syncytial virus (RSV) is a typical paramyxovirus, and two major strains have been identified: group A and group B. Its surface spikes bear G protein (not haemagglutinin or neuraminidase) for attachment to the cell, and fusion (F) protein. The latter initiates viral entry by fusing the viral envelope to the cell membrane, and also fuses host cells to form syncytia.
RSV infection is transmitted by droplets and to some extent by hands. Outbreaks occur each winter, and during the RSV season, infection can spread in hospitals as well as in the community. Nearly all individuals have been infected by 2 years of age. About 1 in every 100 infants with RSV bronchiolitis or pneumonia requires admission to hospital.
Respiratory syncytial virus infection can be particularly severe in young infants
After inhalation, the virus establishes infection in the nasopharynx and lower respiratory tract. Clinical illness appears after an incubation period of 4–5 days. The illness can be particularly severe in young infants, with peak mortality at 3 months of age, the virus invading the lower respiratory tract by direct surface spread to cause bronchiolitis or pneumonia. Young infants develop a cough, rapid respiratory rate and cyanosis. In young children and adults, however, the virus is restricted to the upper respiratory tract, causing a less severe common cold-type illness. Otitis media is quite common. Secondary bacterial infection is rare.
The manifestations of RSV infection appear to have an immunopathologic basis
Maternal antibodies in the infant react with virus antigens, perhaps with the liberation of histamine and other mediators from the host’s cells. In early trials, a killed vaccine was used and, during subsequent natural RSV infection, the vaccinees had more frequent and severe lower respiratory tract disease compared with unimmunized children, supporting an immune-mediated pathogenesis.
Neutralizing antibodies are formed, at lower levels in younger infants, but cell-mediated immunity (CMI) is needed to terminate the infection. The virus continues to be shed from the lungs of children lacking CMI for many months. Apparently healthy children may continue to show depressed pulmonary function or wheeze even 1–2 years after apparent recovery.
Recurrent infections are common, but are less severe. The reason for recurrence, which is also a feature of parainfluenza virus infection, is unknown.
Respiratory syncytial virus RNA is detectable in throat swab specimens, and ribavirin is indicated for severe disease
Molecular methods, such as PCR, used to detect RSV RNA in throat swab specimens, have a higher diagnostic sensitivity than immunofluorescence (Fig. 19.5) or enzyme-linked immunosorbent assay (ELISA) methods (see Ch. 32), detecting RSV-specific antigens in smears of exfoliated cells obtained by nasopharyngeal aspiration. However, virus isolation is less helpful due to the time taken to detect a cytopathic effect, and success depends on inoculating respiratory secretions as soon as possible into cell cultures.
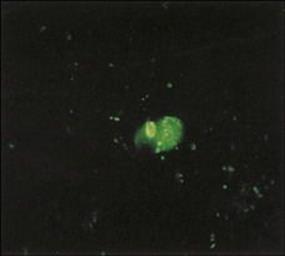
Figure 19.5 Immunofluorescent preparation from the nasopharynx showing respiratory syncytial virus-infected cells (bright green).
(Courtesy of H. Stern.)
In most children, treatment is supportive, involving rehydration, bronchodilators and, if needing admission to hospital, oxygen. The antiviral agent ribavirin, given as an aerosol, has been used successfully in a number of clinical settings, including children with severe infection and immunosuppressed individuals at risk of severe disease. A monoclonal antibody, palivizumab, can be used as prophylaxis to prevent RSV infection in infants less than 2 years old at risk of severe disease such as those with chronic lung disease, congenital heart disease or those born at less than 32 weeks of age. At present, there is no vaccine available.
Hantavirus pulmonary syndrome (HPS)
The reservoir host for Sin Nombre virus (SNV), a New World hantavirus, is the deer mouse found commonly in North America. In 1993, individuals were infected in south-west USA and developed severe cardiopulmonary disease. HPS followed flu-like symptoms as viral invasion of the pulmonary capillary endothelium led to fluid pouring into the lungs due to increased vascular permeability, and at least 26 deaths were reported secondary to pulmonary oedema, hypotension and cardiogenic shock. The route of transmission is by inhaling SNV-infected rodent faeces, saliva or urine. The Old World hantaviruses cause haemorrhagic fever with renal syndrome. The pathogenesis of both diseases is thought to involve aberrant immune responses by SNV-infected endothelial cells that are also involved in regulating vascular permeability. By 2009, 510 individuals with HPS had been reported in the USA, with a 35% mortality rate.
Ribavirin treatment may be successful if initiated at an early stage in the disease course.
Pneumonia
Pneumonia has long been known as ‘the old person’s friend’ as it is the most common cause of infection-related death in the USA and Europe. It is caused by a wide range of microorganisms giving rise to indistinguishable symptoms. The challenge lies not in the clinical diagnosis of pneumonia, except perhaps in children, in whom it may be more difficult to diagnose, but in the laboratory identification of the microbial cause.
Microorganisms reach the lungs by inhalation, aspiration or via the blood
Microorganisms gain access to the lower respiratory tract by inhalation of aerosolized material or by aspiration of the normal flora of the upper respiratory tract. The size of inhaled particles is important in determining how far they travel down the respiratory tract; only those less than about 5 mm in diameter reach the alveoli. Less frequently, the lungs become seeded with organisms as a result of spread via the blood from other infected sites. Healthy individuals are susceptible to infection by a range of pathogens possessing adhesins, which allow the pathogens to attach specifically to the respiratory epithelium. In addition, people with impaired defences, for example, if immunocompromised, with preceding viral damage, or with cystic fibrosis, may develop infections with organisms that do not cause infections in health. An example is Pneumocystis jirovecii, an important cause of pneumonia in individuals with AIDS.
The respiratory tract has a limited number of ways in which it can respond to infection
The host’s response can be defined by the pathologic and radiologic findings, but the terms can be confusing because they are applied differently in different situations. However, four descriptive terms are in common use (Fig. 19.6):
• Lobar pneumonia refers to involvement of a distinct region of the lung. The polymorph exudate formed in response to infection clots in the alveoli and renders them solid. Infection may spread to adjacent alveoli until constrained by anatomic barriers between segments or lobes of the lung. Thus one lobe may show complete consolidation.
• Bronchopneumonia refers to a more diffuse patchy consolidation, which may spread throughout the lung as a result of the original pathologic process in the small airways.
• Interstitial pneumonia involves invasion of the lung interstitium and is particularly characteristic of viral infections of the lungs.
• Lung abscess, sometimes referred to as necrotizing pneumonia, is a condition in which there is cavitation and destruction of the lung parenchyma.
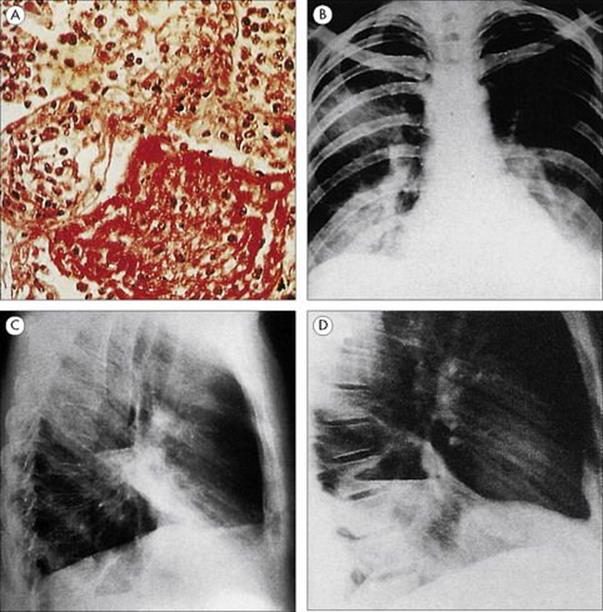
Figure 19.6 Four types of pneumonia. (A) Pneumococcal lobar pneumonia, showing consolidated alveoli filled with neutrophils and fibrin. (H&E stain) (Courtesy of I.D. Starke and M.E. Hodson.) (B) Mycoplasma bronchopneumonia, with patchy consolidation in several areas of both lungs. (Courtesy of J.A. Innes.) (C) Interstitial pneumonia due to influenza virus. (Courtesy of I.D. Starke and M.E. Hodson.) (D) Lung abscess, showing an abscess cavity in the lower lobe of the right lung.
(Courtesy of J.A. Innes.)
The outcomes common to all these conditions are respiratory distress resulting from the interference with air exchange in the lungs, and systemic effects as a result of infection in any part of the body.
A wide range of microorganisms can cause pneumonia
Age is an important determinant (Table 19.1):
• Most childhood pneumonia is caused either by viruses or by bacteria invading the respiratory tract secondary to viral infection, e.g. after measles infection. Neonates born to mothers with genital Chlamydia trachomatis infection may develop a chlamydial interstitial pneumonitis (see Ch. 21) resulting from colonization of the respiratory tract during birth.
• In the absence of an underlying disorder such as cystic fibrosis, pneumonia is unusual in older children. Children and young adults with cystic fibrosis are very prone to lower respiratory tract infection, caused characteristically by Staphylococcus aureus, Haemophilus influenzae andPseudomonas aeruginosa.
• The cause of pneumonia in adults depends upon a number of risk factors such as age, underlying disease and exposure to pathogens through occupation, travel or contact with animals.
Table 19.1 Causes of pneumonia related to age
|
Children |
Adults |
|
Mainly viral (e.g. respiratory syncytial virus, parainfluenza) or bacterial secondary to viral respiratory infection (e.g. after measles) |
Bacterial causes more common than viral |
|
Neonates may develop interstitial pneumonitis caused by Chlamydia trachomatis acquired from the mother at birth |
Aetiology varies with age, underlying disease, occupational and geographic risk factors |
Pneumonia in children is more often viral in origin or bacterial secondary to a viral respiratory infection. In adults, bacterial pneumonia is more common.
Pneumonia acquired in hospital tends to be caused by a different spectrum of organisms, particularly Gram-negative bacteria. The causative agents of adult pneumonia are summarized in Figure 19.7. Although clinical and epidemiologic clues help to suggest the likely cause, microbiologic investigations are essential to confirm the diagnosis and ensure optimal antimicrobial therapy.
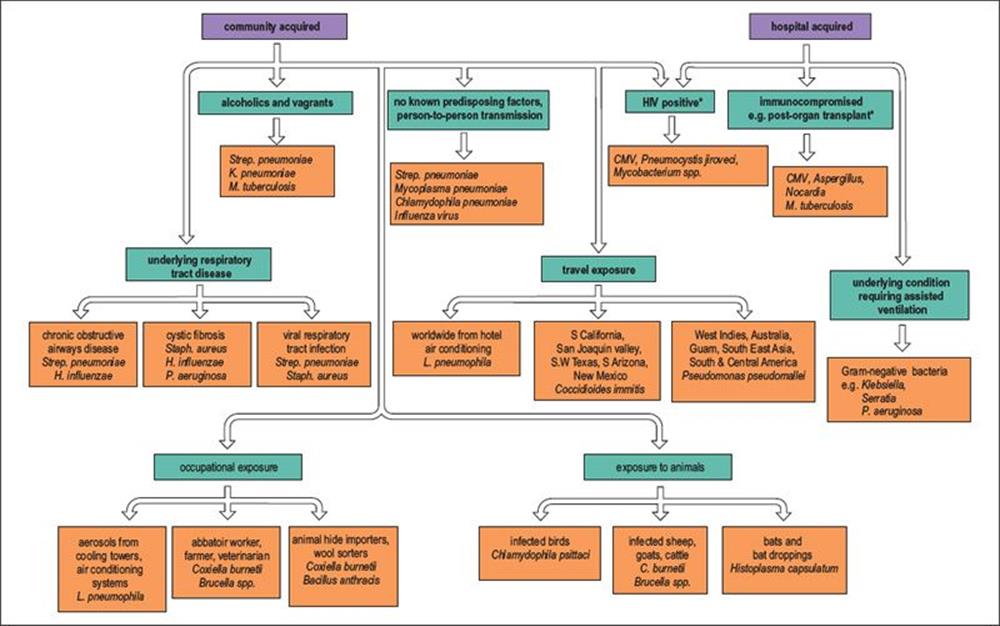
Figure 19.7 Many pathogens are capable of causing pneumonia in adults, and the aetiology is related to risk factors such as the exposure to pathogens through occupation, travel and contact with animals. The elderly are more likely to be infected and tend to have a more severe illness than young adults. * These infections are often reactivating endogenous infections rather than community or hospital acquired. C., Coxiella; CMV, cytomegalovirus; H., Haemophilus; K., Klebsiella; L., Legionella; M., Mycobacterium; P., Pseudomonas; Staph., Staphylococcus; Strep.,Streptococcus.
Viral pneumonias show a characteristic interstitial pneumonia on chest radiography more often than bacterial pneumonias (see Fig. 19.6C), and for the sake of clarity are described separately below. Infections with RSV have been described earlier in this chapter, and opportunist pathogens, such as Pneumocystis jirovecii, associated specifically with pneumonia in the immunocompromised, are described in Chapter 30.
Bacterial pneumonia
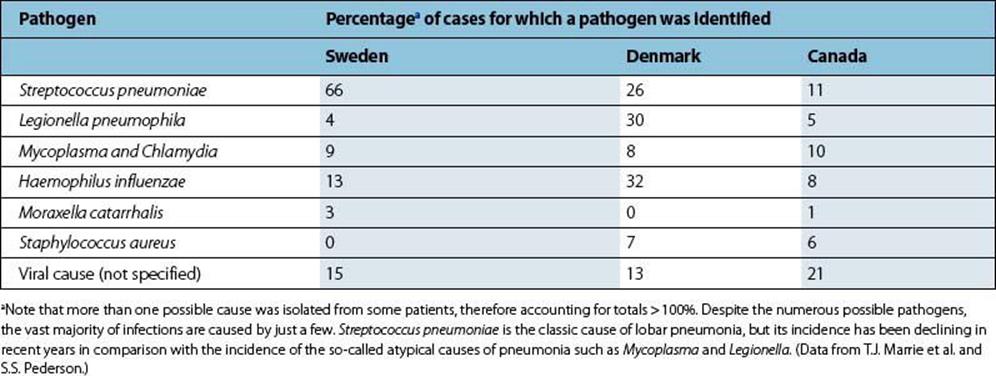
Streptococcus pneumoniae is the classic bacterial cause of acute community-acquired pneumonia
In the past, 50–90% of pneumonias were caused by Streptococcus pneumoniae (the ‘pneumococcus’), but the relative importance of this pathogen has decreased and it now causes only 25–60% of cases (Table 19.2). Haemophilus influenzae is estimated to be the cause of 5–15% of cases, but the true incidence is difficult to determine because this organism frequently colonizes the upper respiratory tract of bronchitic patients (see above).
Table 19.2 Common causes of pneumonia in community-based studies in three countries
A variety of bacteria cause primary atypical pneumonia
When penicillin, an effective antibiotic treatment for pneumococcal infection, became widely available, a significant proportion of cases of pneumonia failed to respond to this treatment and were labelled ‘primary atypical pneumonia’. ‘Primary’ refers to pneumonia occurring as a new event, not secondary to influenza, for example, and ‘atypical’ to the fact that Strep. pneumoniae is not isolated from sputum from such patients, the symptoms are often general as well as respiratory, and the pneumonia fails to respond to penicillin or ampicillin. The causes of atypical pneumonia include Mycoplasma pneumoniae, Chlamydophila (formerly Chlamydia) pneumoniae and Chlamydophila (formerly Chlamydia) psittaci, Legionella pneumophila and Coxiella burnetii. The relative importance of these pathogens varies in different studies (Table 19.2). Infection withChlamydophila pneumoniae is common. About 50% of adults have antibodies, and in the USA it causes up to 300 000 cases of pneumonia each year in adults. Mycoplasma pneumoniae and Chlamydophila pneumoniae appear to be solely human pathogens, whereas Chlamydophila psittaciand Coxiella burnetii are acquired from infected animals, and Legionella pneumophila is acquired from contaminated environmental sources (see Fig. 19.7).
Moraxella catarrhalis (previously Branhamella catarrhalis) is recognized increasingly as a cause of pneumonia, particularly in patients with carcinoma of the lung or other underlying lung disease. Other aetiologic agents of pneumonia associated with particular underlying diseases, occupations or exposure to animals and travel are summarized in Figure 19.7 and described in other chapters. It is important to note that a causative organism is not isolated in up to 35% of lower respiratory tract infections.
Patients with pneumonia usually present feeling unwell and with a fever
Signs and symptoms of a chest infection include:
• chest pain, which may be pleuritic in nature (pain on inspiration)
• a cough, which may produce sputum
• shortness of breath (dyspnoea).
Some infections result in symptoms confined mainly to the chest, whereas others such as Legionnaires’ disease caused by Legionella pneumophila have a much wider systemic involvement, and the patient may present with mental confusion, diarrhea and evidence of renal or liver dysfunction. However, the distinction between localized and systemic symptoms is not usually reliable enough for an accurate diagnosis.
Chest examination may reveal abnormal crackling sounds, called ‘rales’, and evidence of consolidation, even before changes become evident on radiography.
Patients with pneumonia usually have shadows in one or more areas of the lung
The chest radiograph is an important adjunct to the clinical diagnosis. Patients with pneumonia usually have shadows indicating consolidation (see above for descriptions of lobar, broncho- and interstitial pneumonia). However, careful interpretation is required to differentiate between infection and non-infective processes such as tumours.
Pneumonia is the most common cause of death from infection in the elderly
It is also an important cause of death in the young and previously healthy. Complications of infection include spread of the infecting organisms:
• directly to extrapulmonary sites such as the pleural space, giving rise to empyema (see below)
• indirectly, via the blood to other parts of the body.
For example, the majority of patients with pneumococcal pneumonia have positive blood cultures, and pneumococcal meningitis not infrequently follows pneumonia in the elderly.
Sputum samples are best collected in the morning and before breakfast
Microscopic examination and culture of expectorated sputum remain the mainstays of respiratory bacteriology, despite doubts about the value of these procedures. Collection of sputum is non-invasive, but more invasive techniques, such as transtracheal aspiration, bronchoscopy and bronchoalveolar lavage and open lung biopsy, may yield more useful results.
Sputum samples are best collected in the morning because sputum tends to accumulate while the patient is lying in bed, and before breakfast to reduce contamination by food particles and bacteria from food. It is important that the specimen submitted for examination is truly sputum and not simply saliva. A physiotherapist can be of great assistance to ill patients who may be unable to cough unaided.
The usual laboratory procedures on sputum specimens from patients with pneumonia are Gram stain and culture
Examination of the Gram-stained sputum (see Ch. 32) can give a presumptive diagnosis within minutes if the film reveals a host response in the form of abundant polymorphs and the putative pathogen, e.g. Gram-positive diplococci characteristic of Streptococcus pneumoniae (Fig. 19.8). The presence of organisms in the absence of polymorphs is suggestive of contamination of the specimen rather than infection, but it is important to remember that immunocompromised patients may not be able to mount a polymorph leukocyte response. Also, remember that the causative agents of atypical pneumonia, with the exception of Legionella pneumophila (Fig. 19.9), will not be seen in Gram-stained smears.
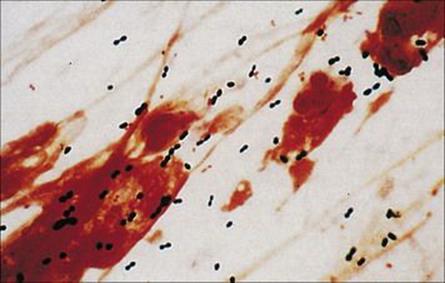
Figure 19.8 Gram-stained smears of sputum can help the physician make a rapid diagnosis if, like this, they contain abundant Gram-positive diplococci characteristic of pneumococci, as well as polymorphs. However, many of the important causes of pneumonia will not be stained by Gram stain.
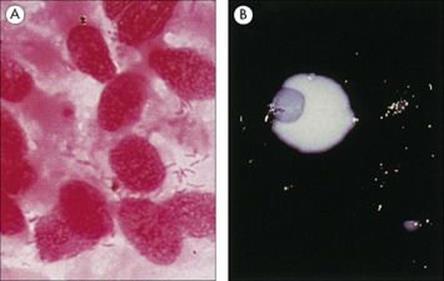
Figure 19.9 Legionella pneumophila. (A) Gram stain of a bronchial biopsy specimen in a patient with fulminant Legionnaires’ disease. (Courtesy of S. Fisher-Hoch.) (B) Culture plate showing white colonies on buffered charcoal yeast extract medium.
(Courtesy of I. Farrell.)
Standard culture techniques will allow the growth of the bacterial pathogens such as Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae and Klebsiella pneumoniae and other non-fastidious Gram-negative rods. Special media or conditions are required for the causative agents of atypical pneumonia, including Legionella pneumophila (Fig. 19.9).
Rapid non-cultural techniques have been applied successfully to the diagnosis of pneumococcal pneumonia. Detection of pneumococcal antigen by agglutination of antibody-coated latex particles (see Ch. 32) can be used with both sputum and urine specimens, as antigen is excreted in the urine. Use of this technique means the result is available within 1 h of receipt of the specimen, but antibiotic susceptibility tests cannot be performed unless the organisms are isolated.
Microbiologic diagnosis of atypical pneumonia is usually confirmed by serology
As mentioned above, several important causes of pneumonia will not be revealed in Gram-stained sputum smears and cannot be grown on simple routine culture media. For these reasons, the diagnosis is usually confirmed by serologic tests rather than by culture. In some infections, IgM, antigen or genome detection is being used to make the diagnosis at an early stage. The classic techniques involve detection of a single high titre of specific antibodies, or preferably demonstration of a rising titre between the acute and convalescent phase of the disease, but the diagnosis is often made retrospectively. The important serologic tests are shown in Table 19.3.
Table 19.3 Serological diagnosis of ‘atypical’ pneumonia
|
Pathogen |
Test |
|
Mycoplasma pneumoniae |
Complement fixation test (CFT), IgM by latex agglutination or ELISA |
|
Legionella pneumophila |
Urinary antigen test or rapid microagglutination test |
|
Chlamydophila pneumonia |
Microimmunofluorescence or ELISA using species-specific antigens |
|
Coxiella burnetii |
CFT (phase I and phase II antigens) |
Several of the bacterial causes of pneumonia are difficult to grow in the laboratory, so examination of the patient’s serum for specific antibodies is the usual method of diagnosis. It is always better to demonstrate a rising titre between acute- and convalescent-phase sera than to rely on a single sample. ELISA, enzyme-linked immunosorbent assay.
Pneumonia is treated with appropriate antimicrobial therapy
Once the cause of the pneumonia has been identified, selection of the appropriate antimicrobial therapy is relatively straightforward (Table 19.4), though the incidence of penicillin and other antibiotics resistance in pneumococci has increased in some countries.
Table 19.4 Antibacterial agents for pneumonia
|
Initial treatment of community-acquired pneumonia |
|
|
First choice |
Ampicillin + erythromycin |
|
Unless clinical picture clearly indicates lobar pneumonia; if so |
Ampicillin |
|
Pneumonia secondary to viral respiratory tract infection |
Ampicillin + flucloxacillin |
|
Pneumonia in chronic bronchitis |
Co-amoxiclav or cefuroxime |
|
Pneumonia in an alcoholic, drug user or a patient who may have aspirated |
Ampicillin + gentamicin |
|
Treatment of choice when pathogen has been identified |
|
|
Streptococcus pneumoniae |
Ampicillin or penicillin (erythromycin if allergic to beta-lactams) |
|
Mycoplasma pneumoniae |
|
|
Legionella pneumoniae |
|
|
Chlamydophila pneumoniae |
|
|
Chlamydophila psittaci |
|
|
Coxiella burnetii |
|
|
Staphylococcus aureus |
Flucloxacillin |
|
Haemophilus influenzae |
Ampicillina, augmentin or cefuroxime |
|
Klebsiella pneumoniae |
Gentamicin, chloramphenicol or ciprofloxacin |
Penicillin (or ampicillin) remains the agent of choice for pneumococcal infections as long as the isolates are susceptible. Penicillin-resistant pneumococci now occur in many countries, and in some it is no longer safe to assume susceptibility to penicillin (or ampicillin). Many of the resistant strains are still susceptible to cephalosporins, and in countries with a high incidence of resistance, these agents may replace penicillin, at least until the results of antibiotic susceptibility are known. It is important to recognize that penicillin (and ampicillin and cephalosporins) is not active against the other common causes of pneumonia. Therefore, a combination is often recommended for initial therapy.
a If non-beta-lactamase producer.
The choice of treatment is more difficult when sputum is not produced or does not reveal the pathogen. It is therefore important to take a full history and use invasive diagnostic techniques if appropriate to help establish the cause.
Prevention of pneumonia involves measures to minimize exposure, and pneumococcal immunization post-splenectomy and for those with sickle cell disease
Respiratory infections are usually transmitted by airborne droplets, so person-to-person spread is virtually impossible to prevent, although less crowding and better ventilation help to reduce the chances of acquiring infection. Infections acquired from sources other than humans may be more amenable to prevention, for example, by avoiding contact with sick animals (Q fever) or birds (psittacosis). The contamination of cooling systems and hot water supplies by Legionella has been the subject of intense study, and regulations are now in force in the UK and elsewhere to provide guidance for maintenance engineers.
Immunization is available for a few respiratory pathogens. A pneumococcal vaccine incorporating the polysaccharide capsular antigens of the most common types of Strep. pneumoniae is recommended for those at particular risk, e.g. post-splenectomy or individuals with sickle cell disease who are unable to deal effectively with capsulate organisms.
Viral pneumonia
Viruses can invade the lung from the bloodstream as well as directly from the respiratory tract
Many viruses cause pneumonia (Table 19.5) and, as with viral infections of the upper respiratory tract, generally accomplish infection in the face of normal host defences. Perfectly healthy individuals are susceptible, and most of these viruses have surface molecules that attach specifically to the respiratory epithelium. RSV can cause pneumonia in infants and is described earlier in this chapter.
Table 19.5 Viral pneumonia
|
Virus |
Clinical condition |
Comments |
|
Influenza A or B |
Primary viral pneumonia or pneumonia associated with secondary bacterial infection |
Pandemics (type A) and epidemics (type A or B); increased susceptibility in elderly or in certain chronic diseases; antivirals and vaccine available |
|
Parainfluenza (types 1–4) |
Croup, pneumonia in children < 5 years of age; upper respiratory illness (often subclinical) in older children and adults |
Antiviral (ribavirin) available but is of limited effectiveness vaccines not available |
|
Measles |
Secondary bacterial pneumonia common; primary viral (giant cell) pneumonia in those with immunodeficiency |
Adult infection rare but severe; King and Queen of Hawaii both died of measles when they visited London in 1824; antivirals and vaccine available |
|
Respiratory syncytial virus |
Bronchiolitis (infants); common cold syndrome (adults) |
Peak mortality in 3–4 month old infants; secondary bacterial infection rare; antivirals available |
|
Adenovirus |
Pharyngoconjunctival fever, pharyngitis, atypical pneumonia (military recruits) |
Vaccines not available, cidofovir or ribavirin could be used in specific clinical settings |
|
Cytomegalovirus |
Interstitial pneumonitis |
In immunocompromised patients (e.g. bone marrow transplant recipients); antivirals and immunoglobulin available |
|
Varicella-zoster virus |
Pneumonia in young adults suffering primary infection |
Uncommon; recognized 1–6 days after rash; lung lesions may eventually calcify; antivirals and vaccine available |
Several different groups of viruses cause infection of the lower respiratory tract, particularly in children. Some, such as influenza and measles, leave the patient particularly susceptible to secondary bacterial infection.
Even when viruses of this group do not themselves cause pneumonia, they may damage respiratory defences, laying the ground for secondary bacterial pneumonia. Sometimes the virus fails to spread significantly to air spaces, but remains in interstitial tissues to cause interstitial pneumonitis. An example is cytomegalovirus (CMV) pneumonitis in immunodeficient patients, particularly bone marrow transplant recipients.
Parainfluenza virus infection
As with RSV, parainfluenza viruses are most likely to cause lower respiratory tract disease, croup and pneumonia, in children.
There are four types of parainfluenza viruses with differing clinical effects
The surface spikes of parainfluenza viruses are composed of haemagglutinin plus neuraminidase on one type of spike and fusion proteins on another. The four types of virus have different antigens. After infection by respiratory droplets, these viruses spread locally on respiratory epithelium.
Parainfluenza viruses 1–3 cause pharyngitis, croup, otitis media, bronchiolitis and pneumonia. Croup is seen in children less than 5 years of age, and consists of acute laryngotracheobronchitis with a harsh cough and hoarseness. Parainfluenza virus 4 is less common and generally causes a common-cold-type illness.
Real-time PCR methods detecting parainfluenza RNA in throat swabs have revolutionized the diagnosis of these and other respiratory virus infections due to the increased sensitivity and time to diagnosis using these tests. Virus-specific antigens can be detected in cells from respiratory washings, and virus culture can be carried out, in settings where molecular analysis is not available. Ribavirin may be given in severe infections or in immunosuppressed individuals but there is no vaccine.
Adenovirus infection
Adenoviruses cause about 5% of acute respiratory tract illness overall
There are 41 antigenic types of adenovirus, some of which cause upper respiratory tract infections such as pharyngoconjunctival fever and sore throat (see Ch. 18) and lower respiratory tract infections. Adenovirus respiratory tract infections generally cause non-specific symptoms in children less than 5 years of age. As maternal antibody wanes, lower respiratory tract illnesses become more frequent, especially with adenovirus 7.
Types 3, 4 and 7 have caused outbreaks of respiratory illness ranging from pharyngitis to atypical pneumonia in military recruits, with crowding and stress as possible co-factors.
Recovery is generally uneventful, but adenoviruses may persist in the body, because they can be recovered from at least 50% of surgically removed tonsils. An enteric-coated vaccine for types 4 and 7 has been used to prevent outbreaks of infection in military recruits. In 2011, the FDA approved a new version of this vaccine that was planned to be offered to all military trainees in the USA.
Human metapneumovirus
Human metapneumovirus (hMPV), discovered in Holland in 2001, is a respiratory pathogen closely related to RSV, peaks in the winter months and accounts for up to 15% of respiratory tract infections. It is associated with a spectrum of illness from mild infection to bronchiolitis and pneumonia. Symptoms may include a fever, runny nose, cough, sore throat and wheeze. Infection occurs in infants and young children, with some reports that by 5 years of age most children have had an hMPV infection. In addition, hMPV has also been detected in older children and adults, suggesting that reinfection may occur. Archived sera have been tested and it seems that humans have been exposed to hMPV for at least 50 years.
Human bocavirus
Human bocavirus (HBoV), discovered in 2005, is a member of the Parvoviridae subfamily. A report from Sweden compared the results of testing 258 children with lower respiratory tract infections with, and 282 without, a diagnosis. HBoV was detected by PCR in samples from 17 children (3.1%), 14 of whom were negative for other respiratory viruses and the children had lower respiratory tract symptoms. The remainder had two RSV and one adenovirus co-infection. The virus has been detected in faecal and serum samples and as a co-infection in respiratory samples. The clinical importance of HBoV has been difficult to determine, especially as it can be detected in ill as well as healthy control subjects. However, when quantifying the HBoV load, it has been shown to be significantly higher in those patients with HBoV alone compared with those co-infected.
Influenza virus infection
Influenza viruses are classic respiratory viruses and cause endemic, epidemic and pandemic influenza
The structure of a typical orthomyxovirus single-stranded RNA is shown in Figure 19.10, and the budding process in Figure 19.11.
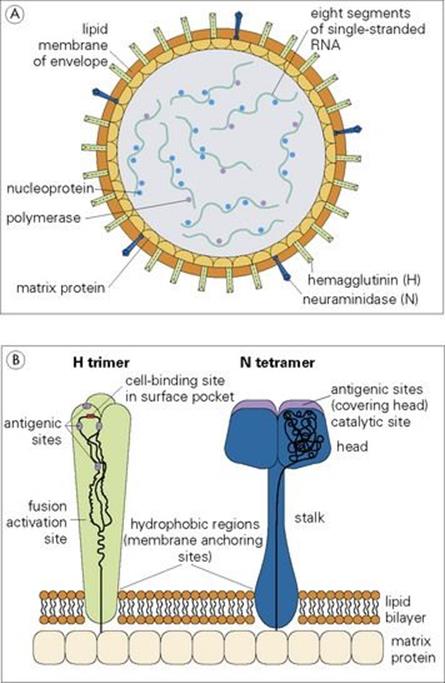
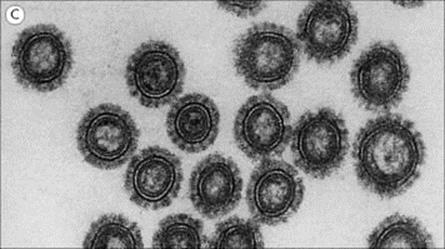
Figure 19.10 The influenza A virus particle (A), with detail enlarged (B) to show surface haemagglutinin (H) and neuraminidase (N). Each particle has approximately 500 H spikes, which bind to the host cell and fuse the viral envelope to the cell’s plasma membrane to initiate infection, and approximately 100 N spikes, which release the virus from the cell surface. Nucleoprotein and polymerase proteins are closely associated with RNA segments to form ribonucleoprotein (RNP). The N tetramer is propeller-shaped as viewed from the end. Detail of only one unit of H trimer and N tetramer is shown. The three-dimensional structure is known from X-ray crystallographic analysis. Electron micrograph (C) shows sectioned influenza virus particles. × 300 000.
(Courtesy of D. Hockley.)

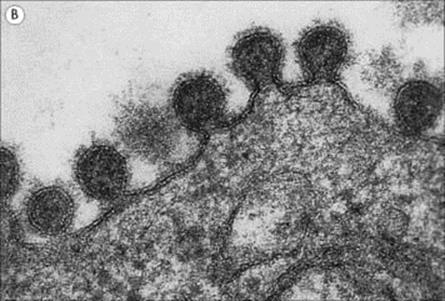
Figure 19.11 Influenza virus budding from the surface of an infected cell. (A) Scanning electron micrograph (× 27 000). (B) In section (× 350 000).
(Courtesy of D. Hockley.)
There are three types of influenza virus: A, B and C
The internal ribonucleoprotein (RNP) is a group-specific antigen that distinguishes influenza A, B and C viruses:
• Influenza A viruses cause epidemics and occasionally pandemics, and there is an animal reservoir, notably in birds.
• Influenza B viruses only cause epidemics and do not involve animal hosts.
• Influenza C viruses do not cause epidemics and give rise to only minor respiratory illness.
The influenza virus envelope has haemagglutinin and neuraminidase spikes
These are shown in Figure 19.10. In the case of influenza A, the haemagglutinin (H) and neuraminidase (N) are type-specific antigens and are used to characterize different strains of influenza A virus (Table 19.6). Circulating strains are H3N2, H1N1 and H1N2. In giving the full nomenclature, the influenza type, the location and year of isolation is also included (e.g. A/Philippines/82/H3N2).
Table 19.6 Pandemic human influenza viruses
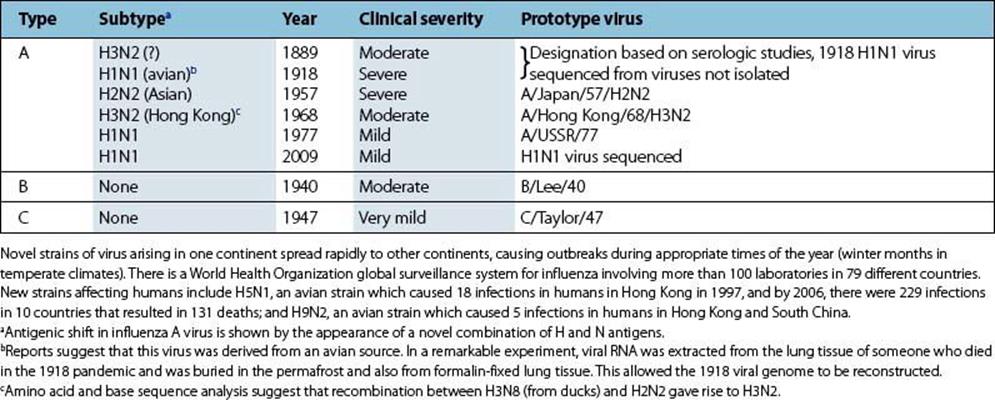
The single-stranded RNA genome is segmented, and these segments can be reassorted during virus replication to give a progeny virus with a novel combination of H and N antigens when virus particles of more than one strain infect a cell simultaneously.
Influenza viruses undergo genetic change as they spread through the host species
These changes are of two types:
1. Antigenic drift. Small mutations affecting the H and N antigens occur constantly. When changes in these antigens enable the virus to multiply significantly in individuals with immunity to preceding strains, the new subtype can reinfect the community. Antigenic drift is seen with all types of influenza.
2. Antigenic shift. Less commonly, and only with influenza A, there is a sudden major change referred to as shift, in the antigenicity of the H or N antigens. This is based on recombination between different virus strains when they infect the same cell. The major change in H or N means that the new strain can spread through populations immune to pre-existing strains and the stage is set for a new pandemic (Table 19.6). Associated with the change in H and N are other genetic changes, which may or may not confer increased pathogenicity or change the ability to spread rapidly from person to person.
Influenza is a highly infectious, acute viral infection that has affected both humans and animals over the centuries. It was so named after an outbreak of a respiratory disease in Italy in the fifteenth century that was thought to have developed under the influence of the stars.
The mixing vessel hypothesis for the production of new influenza strains came about as a result of influenza A viruses infecting pigs, horses, seals and other mammals, and the ability of the virus to reassort. For example, pigs in some countries live in the same dwellings as the farmers, allowing the potential mixing of influenza viruses and emergence of new strains.
The 1918 Spanish influenza pandemic (H1N1) was estimated to have led to 50–100 million deaths around the world and was followed in 1957 and 1968 by the less severe Asian (H2N2) and Hong Kong (H3N2) influenza pandemics, respectively. These were examples of antigenic shift, whereas antigenic drift resulted in frequent epidemics between the pandemic years. In 1976, there was a swine influenza scare in Fort Dix, USA, and in 1997, 18 people in Hong Kong became ill having had an H5N1 avian influenza A virus infection. Six of the infected people subsequently died. The outbreak ceased after public health authorities ordered the slaughter of all live chickens in Hong Kong.
Five human infections were reported in 1999 in Hong Kong and South China with the avian influenza A virus, H9N2. There was neither evidence of wider spread nor human-to-human transmission with either strain although it had circulated widely among birds in Hong Kong and China (Fig. 19.12). WHO has reported avian influenza A (H5N1) virus infections with a 60% mortality, mostly in children and young adults, with 560 cases and 330 deaths worldwide between 2003 and 2011. Most infections have been documented especially in Indonesia and Vietnam, but also in Asia, Africa, the Pacific and a few in Europe.
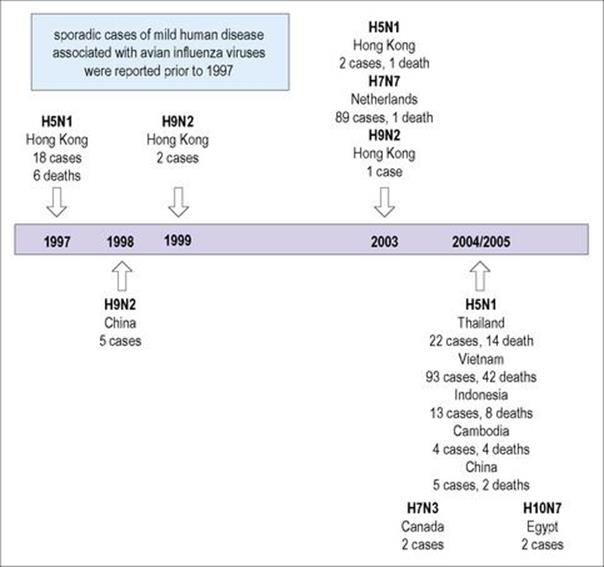
Figure 19.12 Deaths due to avian flu. Hong Kong, 1997–2003; Thailand, 2004–2005.
(Redrawn from: Fauci AS. Pandemic influenza threat and preparedness. Emerg Infect Dis 2006; 12:73–7.)
Another avian influenza virus, H7N7, is highly pathogenic in birds and may be more transmissible between humans. During an outbreak of highly pathogenic avian influenza in Holland in 2003, an H7N7 virus infected 86 poultry workers and three family members who had no contact with chickens. They developed conjunctivitis and/or flu-like symptoms. A veterinarian who handled infected chickens died of pneumonia and acute respiratory distress.
The 16 antigenically distinct H subtypes (H1–16) of influenza A virus reservoirs include wild birds, especially waterfowl. These include the H5 and H7 subtypes. There are nine N subtypes (N1–9). Outbreaks of H5N1 avian influenza in migratory waterfowl, domestic poultry, and humans in Asia have occurred. Over time, the host range has increased, with infections in waterfowl, ferrets, members of the cat family and humans. The virus has become more virulent, as seen by the mortality rate in the human population together with neurological clinical features.
Descriptive molecular epidemiology has shown that the precursor of the 1997 Hong Kong H5N1 virus was first seen in geese in 1996 in Guangdong, China. In turn, the goose virus had RNA segments from influenza viruses found in quail and the N segment from a duck virus. Subsequent evolution of the goose virus resulted in a predecessor of the Z genotype that caused the death of many waterfowl in Hong Kong nature parks and infected humans in that area in 2002. The Z genotype then predominated and spread across South-East Asia and killed, or resulted in culling of, millions of domestic fowl.
The 1918 pandemic H1N1 strain is believed to have resulted from spontaneous mutations in an avian H1N1 virus after sequence analysis was carried out on viral RNA recovered from people who had died and had been buried in the Scandinavian permafrost. However, the other pandemic viruses mentioned above, including the 2009 pandemic H1N1 strain, were due to genetic reassortment of the viral segmented RNA genomes after a host was infected by avian and human influenza A viruses at the same time.
In April 2009, there were reports from Mexico and the USA, in southern California, of a respiratory illness caused by a novel swine influenza A H1N1 virus. These were worrying times as it was thought that the new influenza virus could cause a pandemic with high morbidity and mortality. Pandemic influenza response plans had been developed and refined in many countries for the expected and overdue influenza outbreak. Viral sequence analysis showed that it was composed of a combination of genes most closely related to North American and Eurasian swine-lineage H1N1 influenza viruses. Exposure to pigs was not seen when investigating those infected. In addition, the new virus was circulating among humans and not among pig herds. Within weeks there were reports of people with influenza in a number of American states and also Canada and other parts of the world. The influenza pandemic alert was raised to phase 4 on the basis of human-to-human spread and outbreaks in the community. This became phase 5 by the end of April and countries started to activate their pandemic response plans as the pandemic had started. Diagnostic real-time polymerase chain reaction (PCR) tests were developed in days in order to confirm the diagnosis and a vaccine virus chosen for high-yield preparation in case it was needed. National stockpiles of antiviral drugs (oseltamivir and zanamivir) and personal protective equipment were activated
By June 2009, WHO changed its alert level to pandemic phase 6 as pandemic H1N1 was reported in more than 70 countries and community outbreaks were happening globally. This virus contained gene reassortments from Eurasian and North American swine influenza, North American avian influenza and North American human influenza virus infections. The seasonal aspect of influenza virus infections had altered as laboratories experienced huge workloads over the northern hemisphere summer months.
Confirmed and probable infections occurred mainly among 5–24-year-olds. Mostly older children and young adults were admitted to hospital as well as those in the at-risk groups identified in previous influenza pandemics, including women who were pregnant. In addition, increased risk of complications was seen in obese people and those with chronic neurological conditions. There were few influenza infections seen in the 65 year and older age groups, which was unusual. Studies showed that children and young adults had no pre-existing cross-reactive antibody to the 2009 H1N1 influenza virus compared with over 30% of adults 60 years of age or older who had been exposed previously.
Networks were set up worldwide to ensure that the experiences managing influenza-infected individuals in critical care facilities and elsewhere in the southern hemisphere were shared and lessons learnt. In addition, the circulating influenza viruses were monitored closely for any antigenic variation as well as the development of antiviral resistance. Influenza-infected patients on critical care units in acute respiratory failure received mechanical ventilation with intermittent positive-pressure ventilation in which the lungs receive air enriched with oxygen at high pressure. However, another technique called extracorporeal membrane oxygenation (ECMO) treatment improved recovery by providing gas exchange outside the body using heart–lung bypass equipment and obviating the deleterious effects of providing direct oxygenation at high pressure.
Across the northern hemisphere, the 2009 H1N1 influenza A summer activity peaked and declined during the summer but levels of influenza activity remained above normal with small community outbreaks. On 10 August 2010, the WHO International Health Regulations (IHR) Emergency Committee declared an end to the 2009 H1N1 pandemic globally.
There was concern about a second wave of infection and preparations were made to offer the recently prepared vaccine to specific groups of individuals, those at-risk and healthcare workers. The anticipated second wave started in the autumn and the amount of influenza activity fell quite quickly and remained at lower levels until the spring.
The WHO Global Influenza Surveillance and Response System monitoring circulating influenza viruses detected an avian influenza A H5N1 virus that was reported as H5N1 clade 2.3.2.1 that circulated in poultry in parts of Asia in February 2011. This was not detected in humans and was not seen as a public health threat, more as a marker of the continual evolution of these viruses.
Epidemics and pandemics are due to the appearance of new strains of viruses so that a given individual is regularly reinfected with different strains. This is in contrast to viruses that undergo minimal antigenic variation (monotypic viruses), such as measles or mumps, for which one infection confers life-long immunity. Monitoring avian influenza viruses such as H5N1 and H7N7 is therefore critical in determining their potential to become more pathogenic and spread. Reassortment between H5N1 or H7N7 and human H1N1 or H3N2 influenza viruses may result in efficient transmissibility together with retention of viral pathogenicity. An influenza pandemic could then evolve.
Transmission of influenza is by droplet inhalation
Influenza infections occur throughout the world. Except in the tropics, the infection is almost entirely restricted to the coldest months of the year. This is largely because, during cold weather, people spend more time inside buildings with limited air space, which favours transmission by droplet inhalation, and perhaps also because of decreased host resistance. Influenza activity within a community is reflected not only in the numbers of people becoming ill and consulting doctors, but also in excess mortality due to acute respiratory disease, such as pneumonia, which particularly affects the elderly (Fig. 19.13).
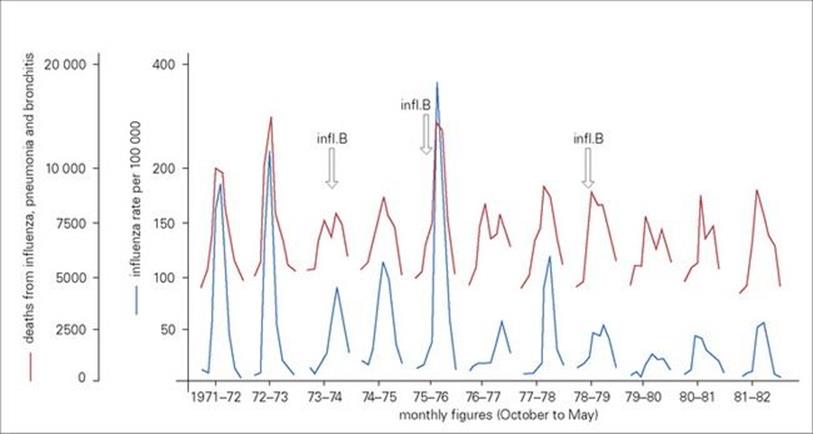
Figure 19.13 Outbreaks of influenza within a community are reflected by a general increase in deaths from acute respiratory disease. Notifications of new cases of clinical influenza are paralleled by an increase in deaths attributed to influenza, pneumonia and bronchitis. Monthly figures from October to May for England and Wales (1971–83) are shown. The peaks are due to the spread of different strains of influenza A (H3N2 and H1N1) and influenza B (arrows) viruses in the community.
(Data from the Office of Population, Censuses and Surveys.)
With respect to the avian influenza viruses, they are spread by movement of poultry and poultry products, live poultry markets and unhygienic practices, and backyard flocks that are not controlled.
The initial symptoms of influenza are due to direct viral damage and associated inflammatory responses. The virus enters the respiratory tract in droplets and attaches to sialic acid receptors on epithelial cells via the H glycoprotein of the virus envelope. Just 1–3 days after infection, the cytokines liberated from damaged cells and from infiltrating leukocytes cause symptoms such as chills, malaise, fever and muscular aches. There are also respiratory symptoms such as a runny nose and cough. Most people feel better within 1 week. The direct viral damage and associated inflammatory responses can be severe enough to cause bronchitis and interstitial pneumonia.
Influenzal damage to the respiratory epithelium predisposes to secondary bacterial infection
Secondary bacterial invaders include staphylococci, pneumococci and Haemophilus influenzae. Life-threatening influenza is often due to secondary bacterial infection, especially with Staphylococcus aureus, the viral infection being brought under control by antibody and cell-mediated immune responses to the infecting virus. Although antiviral antibodies may not be detected within the serum for 1–2 weeks, they are produced at an earlier stage, but are complexed with viral antigens in the respiratory tract.
Mortality due to secondary bacterial pneumonia is higher in apparently healthy individuals over 60 years of age and in those with impaired resistance due to, for example, chronic cardiorespiratory disease or renal disease. Pregnant women are also more vulnerable.
Rarely, influenza causes CNS complications
Central nervous system (CNS) complications include meningitis, encephalomyelitis and polyneuritis. These appear to be indirect immunopathologic complications rather than due to CNS invasion by the virus. Guillain–Barré syndrome, a polyneuropathy with proximal, distal or generalized motor weakness, occurred as a significant but rare (1/100 000) sequel to the widespread vaccination of citizens in the USA with inactivated H3N2 influenza virus in 1976. However, subsequent vaccines have not been associated with this syndrome.
During influenza epidemics a diagnosis can generally be made clinically
Rapid diagnosis can be made by collecting samples from the respiratory tract, such as throat swabs that can be tested by real-time PCR for influenza viral RNA, and the viruses can be typed simultaneously. Antiviral resistance can also be detected using PCR as well as sequence analysis. Alternatively, if these methods are not available, influenza-infected cells can be detected using immunofluorescence techniques, but nasopharyngeal aspirates are usually required to improve the yield of cellular material for testing. Virus isolation can also be used but there may be a delay of at least 7 days until identification. Finally, a rise in specific antibodies can be detected by complement fixation test or ELISA (see Ch. 32) in paired serum samples, taken within a few days of illness and 7–10 days later. However, this is only helpful retrospectively.
Vaccines and antiviral agents can be used to prevent influenza
The aim of immunization is to help prevent infection, and those at risk of complications from influenza infection should be offered vaccine before the ‘flu season’.
Influenza virus vaccines in regular use are:
• those consisting of egg-grown virus, which are then purified, formalin-inactivated and extracted with ether
• the less reactogenic purified H and N antigens prepared from virus that has been disrupted (‘split’) by lipid solvents.
Studies investigating the protective efficacy of cell culture-derived influenza virus vaccines have demonstrated similar results to the egg-grown virus vaccine. Influenza A (H3N2 and H1N1 strains) and influenza B are included in the vaccine. The exact virus strains are reviewed annually in relation to the viruses circulating the previous year. The vaccines are given by parenteral injection, and provide protection against disease in up to 70% of individuals for about 1 year. Vaccination of individuals at high risk, especially those over 65 years of age and those with chronic cardiopulmonary disease, is recommended. It might be expected that the respiratory route would be a better way of inducing respiratory immunity, and live attenuated virus vaccines administered intranasally have been investigated.
The antiviral agents rimantadine and amantadine are M2 ion channel blockers, stop hydrogen ion efflux by altering the pH as they are basic compounds and affect intracellular viral uncoating. They only inhibit the replication of influenza A viruses and were superseded in 1999 by the neuraminidase inhibitors, zanamivir and oseltamivir, which act on both influenza A and B. Oseltamivir (Tamiflu) is easier to administer as it is given orally as opposed to zanamivir that is given by inhaler. These antivirals can reduce the severity of the infection, but should be given within 1–2 days of disease onset. They have also been shown to be effective when used for prophylaxis if given within 48 hours of symptom onset.
Oseltamivir resistance has been widely reported and transmission of oseltamivir resistance has occurred without direct selective drug pressure. This did not affect virulence or viral replication. During the 2009 influenza A H1N1 pandemic, intravenous oseltamivir and zanamivir preparations were made available together with peramivir and laninamivir, also NA inhibitors that had been developed, the latter has a longer half-life.
Finally, another therapeutic option from last century involved using hyperimmune plasma made from blood collected from human donors who had recovered from the 1918 Spanish influenza pandemic. This was given to patients with severe influenza infections who subsequently recovered. Some individuals with severe pandemic H1N1 infections recovered, having received hyperimmune plasma infusions collected from individuals with pandemic H1N1 infection or from vaccinated donors.
With an eye to a future pandemic, nations have been developing stockpiles of anti-influenza drugs that include oseltamivir. New drug targets focusing on entry, replication, and maturation as well as novel approaches to rapid vaccine production are being investigated.
Culling domestic poultry has contained the spread of the H5N1 virus. However, rapid detection and increased biosecurity together with the use of vaccines are critical in controlling the infection. In addition, after the SARS-associated coronavirus (SARS CoV) outbreak (see next section) there are questions as to what lessons were learnt for any future influenza epidemic or pandemic. The problem is that influenza viruses are more easily transmitted than SARS CoV. Together with the reduced transmissibility, early detection and containment that were successful in controlling the SARS CoV may not be effective in preventing an influenza pandemic.
Severe acute respiratory syndrome-associated coronavirus infection
An outbreak of severe respiratory disease with no identifiable cause was reported from Guangdong Province in the People’s Republic of China in November 2002. The agent spread to mainly parts of east and southeast Asia, as well as Toronto in Canada, and was eventually reported in 30 countries. The WHO issued a global health alert in March 2003 concerning severe acute respiratory syndrome (SARS). The main symptoms were high fever > 38°C, cough, shortness of breath or difficulty in breathing. Chest X-rays consistent with pneumonia were also seen. Close contact with someone infected with the SARS agent was the highest risk of the infection spreading from person to person and occurred mostly in family members and hospital staff caring for SARS patients. The incubation period was generally between 2 and 7 days, with a 10-day maximum.
The SARS-associated coronavirus (SARS CoV), a new member of the coronavirus family, was identified by virus isolation in cell culture and electron microscopy in conjunction with molecular methods. Diagnostic methods included PCR detection and serology. The rapid identification of the SARS-associated coronavirus, implementation of infection control on a scale not seen previously involving face masks, checking for fever in the community and at airports, which resulted in rapid isolation on detecting symptom onset, international scientific networking, and immediate availability of data set a global standard for investigation of disease outbreaks.
By July 2003, just slightly more than 4 months since the virus began moving between countries via international air travel, WHO reported that all known chains of person-to-person transmission of the SARS virus had been broken. The largest outbreaks occurred in mainland China, with 5327 cases and 348 deaths, and Hong Kong, where 1755 cases and 298 deaths were reported. Overall, there were 8437 SARS diagnoses in 29 countries and nearly 10% case fatality rate. The predecessor of the SARS-associated coronavirus crossed species barriers over the years when changes in the viral reservoir and humans’ eating habits resulted in an ability to transmit to, and between, humans. In China, the quality of the food is considered to be best if it is prepared freshly from live animals in wet-markets found close to residential areas. In addition, eating a range of exotic wild animals, including bats and civet cats, is popular in south China as it is thought to improve both health and sexual performance. Although the natural reservoir for the SARS-CoV has not been discovered, a number of SARS-CoV-like viruses have been detected in various wildlife species including Himalayan masked palm civet cats, Chinese ferret badgers, raccoon dogs and horseshoe bats. Angiotensin-converting enzyme 2 (ACE2) is the SARS-CoV receptor on host cells that binds the viral spike protein. With respect to transmissibility, it is interesting to note that there is a large difference in the binding affinity of the palm civet and human SARS-CoV strains spike proteins to the human ACE2 receptor despite there being only four amino acid differences between them. Sequencing studies have shown that during the outbreaks various genes evolved quite rapidly in the animal reservoirs, which would have improved the transmissibility between animals and humans and between humans as well (Fig. 19.14).
Figure 19.14 Chinese wet-markets and SARS.
(Redrawn from Woo PC et al. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr Opin Infect Dis 2006; 19:401–7.)
From a management perspective, the relatively poor transmissibility of the virus spreading mainly by respiratory droplets over a short distance was helpful in controlling infections.
However, transmission also occurred by direct and indirect contact with respiratory secretions, faeces or infected animals. The virus was shown to be stable at room temperature, surviving for up to 2 days on surfaces and up to 4 days in faeces. Protection was afforded by face masks, including the N95 masks. SARS-CoV spread more efficiently in hospitals, especially in intensive care unit settings, and clusters of cases occurred in hotel and apartment buildings in Hong Kong. Attack rates as high as 50% or more were seen. Isolation of infected individuals and stringent infection control measures were observed.
Laboratory diagnosis was carried out using methods including SARS-CoV RNA detection by PCR in clinical specimens including respiratory samples and faeces.
No specific antiviral treatment was available, although ribavirin was used to treat some individuals, although little effect was seen in vitro. Corticosteroids damped down the effect of virally induced cytokine responses that could damage lung tissue. Interferons were reported to inhibit the virus. Intriguingly, some reports suggested some antiretroviral therapies might help, such as protease inhibitors, and further work has focused on these agents and looks promising.
Finally, with regard to potential vaccines and the correlates of protection, neutralizing antibodies are found in convalescent human serum. As these antibodies to the viral spike protein prevent virus entry and neutralize virus infectivity in vitro, whole inactivated virus and recombinant protein vaccines have been developed that elicit neutralizing antibody responses. These have been shown to prevent SARS although cell-mediated immunity may also assist viral clearance and disease resolution.
Measles
Secondary bacterial pneumonia is a frequent complication of measles in developing countries
Measles is dealt with in detail as a multisystem infection in Chapter 26. It is mentioned here because:
• It can cause ‘giant cell’ pneumonia in those with impaired immune responses.
• The virus replicates in the lower respiratory tract and, under certain circumstances, causes sufficient damage to lead to secondary bacterial pneumonia.
Secondary bacterial pneumonia is now uncommon in resource-rich countries, but is a frequent complication among children in resource-poor countries, and measles remains a major cause of death in childhood. Depressed immune responsiveness, inadequate vaccination programmes, malnutrition (especially vitamin A) and poor medical care to deal with complications tip the host–parasite balance markedly in favour of the virus.
After an incubation period of 10–14 days, symptoms include fever, a runny nose, conjunctivitis and cough. Koplik’s spots and then the characteristic rash appear 1–2 days later. The virus replicates in the epithelium of the nasopharynx, middle ear and lung, interfering with host defences and enabling bacteria such as pneumococci, staphylococci and meningococci to establish infection. Pneumonia generally results in those with measles being admitted to hospital, but otitis media is also common. Virus replication continues unchecked in children with severely impaired cell-mediated immune responses, giving rise to a giant cell pneumonia, which is a rare and usually fatal manifestation (Fig. 19.15). Other complications are referred to in Chapter 26, and the neurologic complications in Chapter 24.
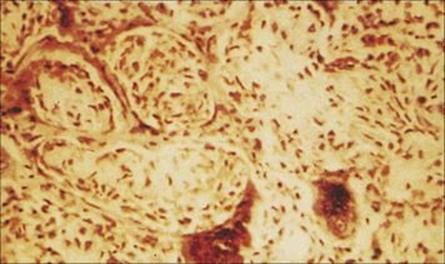
Figure 19.15 Lung biopsy in measles pneumonia showing inflammatory cell infiltrate, proliferation of the alveolar lining cells and large, darkly staining, multinucleate giant cells. (H&E stain.)
(Courtesy of I.D. Starke and M.E. Hodson.)
Measles is diagnosed clinically, but detection of specific IgM responses, measles viral RNA detection and virus isolation are helpful.
Antibiotics are needed for secondary bacterial complications of measles, but the disease can be prevented by immunization
If severe, ribavirin treatment is available, but antibiotics are needed for bacterial complications. Children with severe measles generally have very low levels of serum retinol; recovery is hastened and death is made less likely when they are given 400 000 IU vitamin A.
Measles is prevented by a highly effective, live, attenuated vaccine, given with mumps and rubella vaccines (MMR, see Ch. 34). Since immunization began, the number of cases has declined by 70%. In the USA, after a rise to nearly 30 000 cases in 1990, the number fell to 488 (47 of them imported) in 1996. It was planned to eliminate the disease in the Americas by the year 2000, by which time a group of scientists convened by the Centers for Disease Control (CDC) decided that measles was no longer endemic in the USA. The WHO is hoping for global eradication by 2010–2015. However, due to an unfounded MMR vaccine scare in the UK, the number of individuals with measles rose considerably due to a fall in vaccine uptake. Before the vaccine was available in the 1960s, there were 135 million cases and 7–8 million deaths each year worldwide. Measles is still a killer, but deaths had already been reduced to an estimated 164 000 a year by 2008.
Cytomegalovirus infection
Cytomegalovirus (CMV) infection can cause an interstitial pneumonitis in immunocompromised patients
As discussed in Chapter 18, the virus does not normally replicate in respiratory epithelium or cause respiratory illness; but in immunocompromised patients, in particular bone marrow transplant recipients, it can give rise to an interstitial pneumonia. CMV monitoring in specific groups of immunosuppressed patients is critical, especially in the first few months post transplantation. In a number of different types of sample, CMV DNA can be detected and quantified, the virus can be isolated, and characteristic inclusions demonstrated in lung tissue (Fig. 19.16).
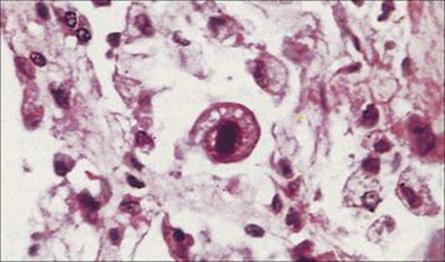
Figure 19.16 Owl’s eye inclusion body in cytomegalovirus infection. Large numbers of virus particles accumulate in the nucleus of the enlarged infected cell to produce a single dense inclusion. (H&E stain.)
(Courtesy of I.D. Starke and M.E. Hodson.)
Tuberculosis
Tuberculosis is one of the most serious infectious diseases of the resource-poor world
Tuberculosis kills about 3 million people and infects almost 9 million others every year wherever poverty, malnutrition and poor housing prevail. It affects the apparently healthy as well as being a serious disease of the immunocompromised, and has become particularly obvious in patients with AIDS. Tuberculosis is primarily a disease of the lungs, but may spread to other sites or proceed to a generalized infection (‘miliary’ tuberculosis). It is also referred to in Chapters 2024 and 26.
Tuberculosis is caused by Mycobacterium tuberculosis
Other species of mycobacteria, referred to as atypical mycobacteria, mycobacteria other than tuberculosis (MOTT) or non-tuberculous mycobacteria (NTM) also cause infection in the lungs (Table 19.7).
Table 19.7 Mycobacteria associated with human disease
|
Species |
Clinical disease |
|
Slow growersa |
|
|
M. tuberculosis |
Tuberculosis |
|
M. bovis |
Bovine tuberculosis |
|
M. leprae |
Leprosy |
|
M. aviumb |
|
|
M. intracellulareb |
|
|
M. kansasii |
Lung infections |
|
M. marinum |
Skin infections and deeper infections (e.g. arthritis, osteomyelitis) associated with aquatic activity |
|
M. scrofulaceum |
Cervical adenitis in children |
|
M. simiae |
Lung, bone and kidney infections |
|
M. szulgai |
Lung, skin and bone infections |
|
M. ulcerans |
Skin infections |
|
M. xenopi |
Lung infections |
|
M. paratuberculosis |
? Association with Crohn’s disease |
|
Rapid growersa |
|
|
M. fortuitum |
Opportunist infections with introduction of organisms into deep subcutaneous tissues; usually associated with trauma or invasive procedures |
|
M. chelonae |
|
Many species of mycobacteria are associated with occasional disease, but the major pathogens of the genus are M. tuberculosis, M. bovis and M. leprae.
a Slow growers require > 7 days for visible growth from a dilute inoculum; rapid growers require < 7 days from a dilute inoculum.
b M. avium complex; recent studies show that the two species are distinct. Of the M. avium complex, serotypes 1–6 and 8–11 are assigned to M. avium, serotypes 7,12–17,19,20 and 25 assigned to M. intracellulare.
Infection is acquired by inhalation of Mycobacterium tuberculosis in aerosols and dust. Airborne transmission of tuberculosis is efficient because infected people cough up enormous numbers of mycobacteria, projecting them into the environment, where their waxy outer coat (see Ch. 2) allows them to withstand drying and therefore survive for long periods of time in air and house dust.
The pathogenesis of tuberculosis depends upon the history of previous exposure to the organism
In primary infection, i.e. infection in individuals encountering Mycobacterium tuberculosis for the first time, the organisms are engulfed by the alveolar macrophages in which they can both survive and multiply. Non-resident macrophages are attracted to the site, ingest the mycobacteria and carry them via the lymphatics to the local hilar lymph nodes. In the lymph nodes, the immune response, predominantly a CMI response, is stimulated. The CMI response is detectable 4–6 weeks after infection by introducing purified protein derivative (PPD) of Mycobacterium tuberculosisinto the skin. A positive result is shown by local induration and erythema, 48–72 h later.
The CMI response helps to curb further spread of Mycobacterium tuberculosis
However, some Mycobacterium tuberculosis organisms may have already escaped to set up foci of infection in other body sites. Sensitized T cells release lymphokines that activate macrophages and increase their ability to destroy the mycobacteria. The body reacts to contain the organisms within ‘tubercles’, which are small granulomas consisting of epithelioid cells and giant cells (Fig. 19.17). The lung lesion plus the enlarged lymph nodes is often called the Ghon or primary complex. After a time, the material within the granulomas becomes necrotic and caseous or cheesy in appearance.
Figure 19.17 Histopathology showing dense inflammatory infiltration, granuloma formation and caseous necrosis in pulmonary tuberculosis.
(Courtesy of R. Bryan.)
The tubercles may heal spontaneously, become fibrotic or calcified, and persist as such for a lifetime in people who are otherwise healthy. They will show up on a chest radiograph as radiopaque nodules (Fig. 19.18). However, in a small percentage of people with primary infection, and particularly in the immunocompromised, the mycobacteria are not contained within the tubercles, but invade the bloodstream and cause disseminated disease (‘miliary’ tuberculosis, Fig. 19.19).
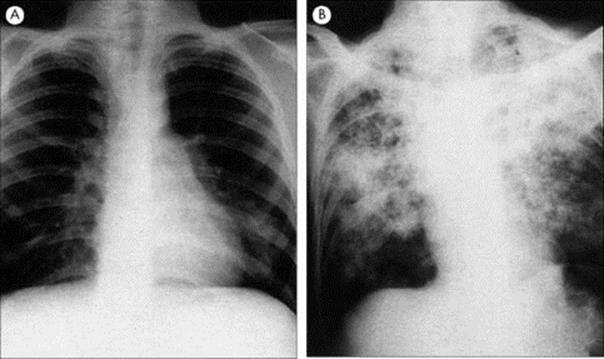
Figure 19.18 Chest radiographs of (A) primary tuberculosis, showing the Ghon focus in the lower left lung, and (B) post-primary pulmonary tuberculosis showing advanced disease.
(Courtesy of J.A. Innes.)
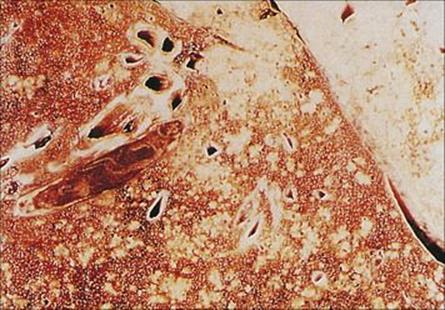
Figure 19.19 Miliary tuberculosis. Gross specimen of lung showing the cut surface covered with white nodules, which are the miliary foci of tuberculosis.
(Courtesy of J.A. Innes.)
Secondary tuberculosis is due to reactivation of dormant mycobacteria, and is usually a consequence of impaired immune function resulting from some other cause such as malnutrition, infection (e.g. AIDS), chemotherapy for treatment of malignancy, or corticosteroids for the treatment of inflammatory diseases.
Tuberculosis illustrates the dual role of the immune response in infectious disease
On the one hand, the CMI response controls the infection and, when it is inadequate, the infection disseminates or reactivates. On the other hand, nearly all the pathology and disease is a consequence of this CMI response, as Mycobacterium tuberculosis causes little or no direct or toxin-mediated damage.
Reactivation occurs most commonly in the apex of the lungs. This site is more highly oxygenated than elsewhere, allowing the mycobacteria to multiply more rapidly to produce caseous necrotic lesions, which spill over into other sites in the lung, and from where organisms spread to more distant sites in the body.
Primary tuberculosis is often asymptomatic
In contrast to pneumonia, which is usually an acute infection, the onset of tuberculosis is insidious, the infection proceeding for some time before the patient becomes sufficiently ill to seek medical attention. Primary tuberculosis is usually mild and asymptomatic and in 90% of cases does not proceed further. However, clinical disease develops in the remaining 10%.
Mycobacteria have the ability to colonize almost any site in the body. The clinical manifestations are variable: fatigue, weight loss, weakness and fever are all associated with tuberculosis. Infection in the lungs characteristically causes a chronic productive cough, and the sputum may be blood-stained as a result of tissue destruction. Necrosis may erode blood vessels, which can rupture and cause death through haemorrhage.
Complications of Mycobacterium tuberculosis infection arise from local spread or dissemination
The organism may disseminate via the lymphatics and bloodstream to other parts of the body. This usually occurs at the time of primary infection, and in this way chronic foci are established, which may proceed to necrosis and destruction in, for example, the kidney. Alternatively, spread may be by extension to a neighbouring part of the lung, for instance when a tubercle erodes into a bronchus and discharges its contents, or into the pleural cavity, resulting in a pleural effusion.
Although the number of cases of pulmonary tuberculosis has been declining in resource-rich countries since the beginning of the twentieth century, hastened by the advent of specific antimicrobial drugs, the incidence of extrapulmonary tuberculosis has stayed roughly constant for many years and therefore makes up a greater proportion of the tuberculosis caseload in resource-rich countries than in resource-poor countries.
The Ziehl–Neelsen stain of sputum can provide a diagnosis of tuberculosis within 1 h, whereas culture can take 6 weeks
A diagnosis of tuberculosis is suggested by the clinical signs and symptoms referred to above, supported by characteristic changes on chest radiography (see Fig. 19.18) and positive skin test reactivity in the tuberculin (Mantoux) test. These tests are confirmed by microscopic demonstration of acid-fast rods and culture of Mycobacterium tuberculosis. Microscopic examination of a smear of sputum stained by Ziehl–Neelsen’s method or by auramine (see Ch. 32) often reveals acid-fast rods (Fig. 19.20). This result can be obtained within 1 h of receipt of the specimen in the laboratory. This is important because Mycobacterium tuberculosis can take up to 6 weeks to grow in culture, although radiometric methods may reduce the time required for detection, and therefore confirmation of the diagnosis is necessarily delayed. Rapid non-culture tests to detectMycobacterium tuberculosis (e.g., using the polymerase chain reaction [PCR, see Ch. 32]; the new automated Xpert MTB-RIF molecular test) are becoming increasingly available. Further tests are required to identify the mycobacterial species and to establish susceptibility to antituberculosis drugs.
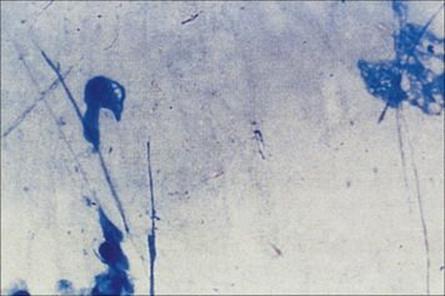
Figure 19.20 Pulmonary tuberculosis. Sputum preparation showing pink-stained, acid-fast tubercle bacilli. (Ziehl-Neelsen stain.)
(Courtesy of J.A. Innes.)
Specific antituberculosis drugs and prolonged therapy are needed to treat tuberculosis
Mycobacteria are innately resistant to most antibacterial agents, and specific antituberculosis drugs have to be used; these are reviewed in Chapter 33. The key features of treatment are the use of:
• combination therapy – usually three drugs such as isoniazid, rifampicin, ethambutol to prevent emergence of resistance
• prolonged therapy – minimum 6 months period which is necessary to eradicate these slow-growing intracellular organisms.
The number of strains resistant to the first-line antituberculosis drugs has increased and has stimulated health agencies to monitor treatment more carefully (e.g. DOTS, directly observed treatment, short-course) and rekindled research interest in finding new agents. Extensively, drug resistant TB, referred to as XDR tuberculosis, has been described in strains in Asia, the Americas and Europe. Between 2000 and 2004, a survey of 17 000 TB isolates from these areas revealed that 2% of multidrug resistant (MDR) TB was also XDR. In an outbreak in an HIV-infected population attending a rural hospital in South Africa, 24% of MDR TB was also resistant to at least three of the six classes of second-line agents. A high mortality rate within a median of 25 days was reported. It seems that this is not a single strain and may arise regularly in many different places due to badly managed care, i.e. non-compliance and poor prescribing practices, and disease control. As a result, methods detecting drug resistant TB at the point of care, together with new antituberculosis agents and strategies to reduce transmission, need to be established.
Tuberculosis is prevented by improved social conditions, immunization and chemoprophylaxis
The steady decline in incidence of tuberculosis since the beginning of the twentieth century, and before specific preventive measures were available, underlines the importance of improvements in social conditions in the prevention of this and many other infectious diseases. However, there has been an increase in the number of cases associated with AIDS; in some countries in the resource-poor world, HIV infection and AIDS are threatening to overwhelm tuberculosis control programmes.
Immunization with a live attenuated BCG (bacille Calmette–Guérin) vaccine, has been used effectively in situations where tuberculosis is prevalent. Immunization, which confers positive skin test reactivity, does not prevent infection, but it allows the body to react quickly to limit proliferation of the organisms. In areas where there is a low prevalence of disease, immunization has been largely replaced by chemoprophylaxis.
Prophylaxis with isoniazid for 1 year is recommended for people who have had close contact with a case of tuberculosis. It is also advocated for individuals who show recent conversion to skin test positivity, when it is essentially early treatment of subclinical infection rather than prophylaxis.
Cystic fibrosis
Individuals with cystic fibrosis are predisposed to develop lower respiratory tract infections
Cystic fibrosis is the most common lethal inherited disorder among Caucasians, with an incidence of approximately 1 in 2500 live births. The disease is characterized by pancreatic insufficiency, abnormal sweat electrolyte concentrations and production of very viscid bronchial secretions. The latter tend to lead to stasis in the lungs and this predisposes to infection.
P. aeruginosa colonizes the lungs of almost all 15–20-year-olds with cystic fibrosis
The respiratory mucosa of individuals with cystic fibrosis presents a different environment for potential pathogens from that found in healthy individuals, and the common infecting organisms and the nature of infections differ from other lung infections. These invaders include:
• Staph. aureus, which causes respiratory distress and lung damage, but can be well controlled by specific antistaphylococcal chemotherapy
• Pseudomonas aeruginosa, which is the pathogen of paramount importance (see below)
• in recent years P. cepacia, another member of the genus Pseudomonas, which has become an increasing problem
• H. influenzae, typically non-encapsulated strains, which may be found in association with Staph. aureus and P. aeruginosa; their pathogenic significance is unclear, but they appear to contribute to respiratory exacerbations.
P. aeruginosa infection is uncommon in those under 5 years of age, but colonizes the lungs of almost all patients aged 15–20 years, often encouraged by its intrinsic resistance to antistaphylococcal agents. Early in the course of infection, normal colony types are grown from sputum cultures, but as infection progresses, the organism changes to a highly mucoid form, almost mimicking the mucoid secretions of the patient (Fig. 19.21). These mucoid forms are thought to grow in microcolonies in the lung, but most of the lung damage is due to immunologic responses to the organisms and to the alginate, which forms the mucoid material (Fig. 19.22). P. aeruginosa rarely invades beyond the lung even in the most severely infected individuals.
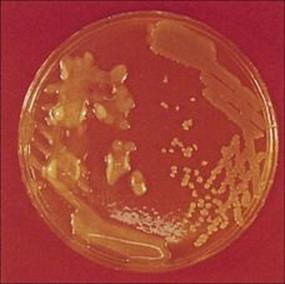
Figure 19.21 Pseudomonas aeruginosa isolated from the sputum of patients with cystic fibrosis characteristically grows in a very mucoid colonial form, shown here on the left of the picture, with the normal colonial form on the right for comparison.
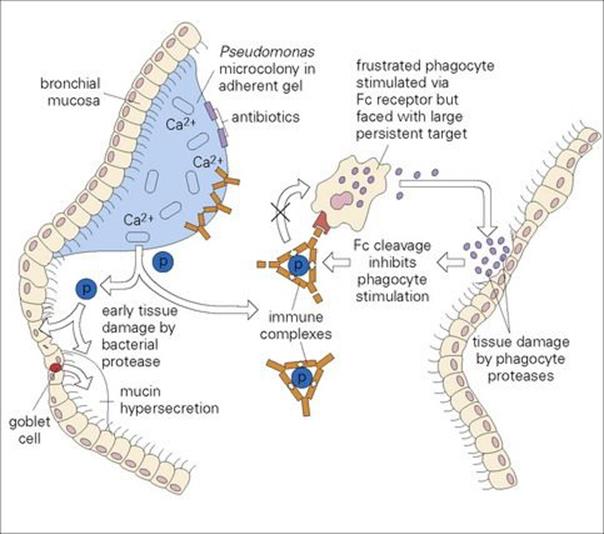
Figure 19.22 Pseudomonas infection in the lung of cystic fibrosis is chronic, but rarely invasive beyond the bronchial mucosa. The organisms are thought to grow in microcolonies embedded in a calcium (Ca2 +)-dependent mucoid alginate gel, which contains DNA and tracheobronchial mucin, and attaches to the bronchial mucosa. This protects the organisms from the host defences and provides a physical and electrolyte barrier to antibiotics. Much of the damage to tissue is thought to be due to the slow release of bacterial proteases (which disrupt the mucosa and cause mucin hypersecretion), immunopathologic mechanisms exacerbated by the size, antigenicity and persistence of the alginate matrix, and the indirect action of immune complexes associated with Pseudomonas antigens (p). Tissue damage is also caused by phagocyte proteases. Intermittent exacerbations can be explained by the cleavage of the Fc of immune complexes by these proteases and consequent inhibition of further phagocyte stimulation.
(Redrawn from Govan JRW. Rev Med Microbiol 1:19–28, © 1990.)
Although specific antibacterial chemotherapy can reduce the symptoms of infection and improve the quality of life, infections, particularly with P. aeruginosa and P. cepacia, are impossible to eradicate and are frequently a cause of death. Heart–lung transplantation is a successful alternative treatment for some patients.
Lung abscess
Lung abscesses usually contain a mixture of bacteria including anaerobes
This is a suppurative infection of the lung, sometimes referred to as ‘necrotizing pneumonia’. The most common predisposing cause is aspiration of respiratory or gastric secretions as a result of altered consciousness. The infection is therefore endogenous in origin and cultures often reveal a mixture of bacteria, with anaerobes such as Bacteroides and Fusobacterium playing an important role (Fig. 19.23).
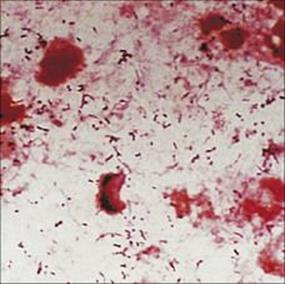
Figure 19.23 Gram stain of pus from a lung abscess showing Gram-positive cocci and both Gram-negative and Gram-positive rods.
(Courtesy of J.R. Cantey.)
Patients with lung abscesses may be ill for at least 2 weeks before presentation, with possible swinging fever, and usually produce large amounts of sputum, which, if foul smelling, gives a strong hint of the presence of anaerobes and often suggests the diagnosis. Most diagnoses are made from chest radiographs (see Fig. 19.7D) and the cause confirmed by microbiologic investigation.
Treatment of lung abscess should include an anti-anaerobic drug and last 2–4 months
Because of the likely presence of anaerobes, a suitable anti-anaerobic agent such as metronidazole should be part of the treatment regimen, and treatment may be needed for 2–4 months to prevent relapse. If diagnosis and treatment are delayed, infection may spread to the pleural space, giving rise to empyema (see below).
Pleural effusion and empyema
Up to 50% of patients with pneumonia have a pleural effusion
Pleural effusions arise in a variety of different diseases. Sometimes the organisms infecting the lung spread to the pleural space and give rise to a purulent exudate or ‘empyema’.
Pleural effusions can be demonstrated radiologically, but detection of empyema can be difficult, particularly in a patient with extensive pneumonia.
Aspiration of pleural fluid provides material for microbiologic examination, and Staph. aureus, Gram-negative rods and anaerobes are commonly involved.
Treatment should be directed at drainage of pus, eradication of infection and expansion of the lung.
Fungal infections
Disease associated with fungal infection is most commonly seen in patients with defective immunity, either as a consequence of immune suppressive treatment or of concomitant disease. A number of species can cause opportunistic infections, and two are of particular importance:Aspergillus fumigatus and Pneumocystis jirovecii (formerly P. carinii).
Aspergillus
The most important species are Aspergillus fumigatus and Aspergillus flavus.
Aspergillus can cause allergic bronchopulmonary aspergillosis, aspergilloma or disseminated aspergillosis
The genus Aspergillus contains many species and these are ubiquitous in the environment. They do not form part of the normal flora. Their spores are regularly inhaled without harmful consequences, but some species, notably A. fumigatus, are able to cause a range of diseases, including:
• Allergic bronchopulmonary aspergillosis (ABPA), which is, as its name suggests, an allergic response to the presence of Aspergillus antigen in the lungs and occurs in patients with asthma. ABPA occurs in some 10% of cystic fibrosis patients.
• Aspergilloma in patients with pre-existing lung cavities or chronic pulmonary disorders. Aspergillus colonizes a cavity and grows to produce a fungal ball, a mass of entangled hyphae – the aspergilloma (Fig. 19.24). In this case, fungi do not invade the lung tissue, but the presence of a large aspergilloma can cause respiratory problems. Aspergillomas can be related, however, to chronic pulmonary aspergillosis where invasion of lung tissue does occur.
• Disseminated disease in the immunosuppressed patient when the fungus spreads from the lungs.
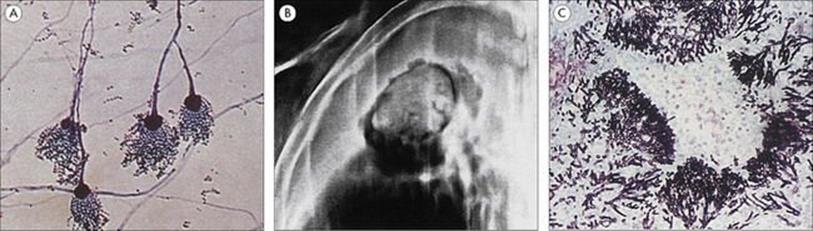
Figure 19.24 Aspergillus fumigatus. (A) Lactophenol cotton blue stained preparation showing the characteristic conidiophores. (B) Aspergilloma. Tomogram showing fungus ball contained within the lung cavity, outlined by air space. (Courtesy of J.A. Innes.) (C) Invasive aspergillosis. Histologic section showing fungal hyphae invading the lung parenchyma and blood vessels. (Grocott stain.)
(Courtesy of C. Kibbler.)
Invasive aspergillosis carries a high mortality as treatment is very difficult due to the limited number and toxic nature of antifungal agents active against Aspergillus (see Ch. 33) plus the lack of functional host defences. Treatment is with an intravenous lipid formulation of amphotericin B. Voriconazole or caspofungin are alternatives. A primary aim of therapy is to improve the neutrophil count.
Pneumocystis jirovecii (formerly P. carinii )
Pneumocystis pneumonia is an important opportunistic infection in AIDS
P. jiroveciii is a fungus commonly found in immunocompetent humans and in rodents. There is strong host specificity, so Pneumocystis infection of humans is not a zoonosis. Infection probably spreads by droplet though airborne transmission has only been directly demonstrated in animal models. Disease occurs in debilitated and immune-deficient individuals. Before the advent of highly active antiretroviral therapy (HAART), a high proportion of AIDS patients developed Pneumocystis pneumonia, which could be fatal.
Pneumocystis occurs as three developmental forms, the trophozoite, up to 5 μm diameter, precyst and cyst. Spores are released when the cysts rupture. Disease is associated with an interstitial pneumonitis, with plasma cell infiltration. Infections of internal organs other than the lung (e.g. lymph nodes, spleen, liver) have also been reported at post-mortem examination.
Treatment is dealt with in Chapter 30.
Parasitic infections
A variety of parasites localize to the lung or involve the lung at some stage in their development
Such parasites include:
• Nematodes such as Ascaris, Strongyloides and the hookworms (see Chs 6 and 22), which migrate through the lungs as they move to the small intestine, breaking out of the capillaries around the alveoli to enter the bronchioles. The damage caused by this process, and the development of inflammatory responses, can lead to a transient pneumonitis with cough, wheeze, dyspnoea and pulmonary infiltrates.
• Schistosome larvae, which may cause mild respiratory symptoms as they migrate through the lungs (see Chs 6 and 22). Heavy acute infections may produce pneumonitis with poorly defined nodular lesions or reticulonodular appearances.
• The microfilariae of filarial nematodes such as Wuchereria or Brugia, which appear in the peripheral circulation with a regular diurnal or nocturnal periodicity, their appearance coinciding with the time at which the vector blood-sucking insects are likely to feed. Outside these periods, the larvae become sequestered in the capillaries of the lung. Under certain conditions, as yet undefined, and in certain individuals, the presence of the larvae triggers a condition known as ‘tropical pulmonary eosinophilia’ (TPE or Weingarten’s syndrome). This is characterized by the onset over several months of cough, dyspnoea and wheeze, worse at night and marked peripheral blood eosinophilia. Microfilariae are absent from the peripheral blood. Antifilarial antibody tests are strongly positive. Chest X-ray examination shows bilateral fine nodular or reticulonodular shadowing.
• Ascaris and Strongyloides infections, which may also trigger a pulmonary eosinophilia, although the condition is distinct from TPE.
• Echinococcus granulosus infection, which leads to the development of hydatid cysts in a proportion (20–30%) of cases due to localization of the larvae of the tapeworm in the lungs (see Chs 6 and 22). These cysts may reach a considerable size, causing respiratory distress, largely as a consequence of the mechanical pressure exerted on lung tissue. Spontaneous rupture may occur and result in acute anaphylaxis.
• Entamoeba histolytica infection, which may rarely involve the lung.
• Paragonimus westermani, the oriental lung fluke, which is the most important example of one of the very few adult parasites that live in the lung, infecting an estimated 22 million people, mainly in Asia. Infection is acquired by eating crustaceans containing the infective metacercariae. These migrate from the intestine across the body cavity and penetrate into the lungs. The adults develop within fibrous cysts, which connect with the bronchi to provide an exit for the eggs (Fig. 19.25). Infections cause chest pain and difficulty in breathing, and can cause bronchopneumonia when large numbers of parasites are present. Single lesions can be confused with lung cancer, tuberculosis and fungal lesions. Praziquantel is an efficient anthelmintic for paragonimiasis.
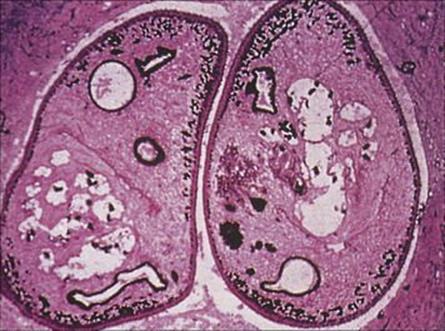
Figure 19.25 Two adult Paragonimus contained within a fibrous cyst in the lung.
(Courtesy of H. Zaiman.)
![]()
 Key Facts
Key Facts
• Although continuous from nose to alveoli, the respiratory tract is divided into ‘upper’ and ‘lower’ from the viewpoint of infection.
• Infections in the lower respiratory tract are spread by the airborne route (except parasites), are acute or chronic, tend to be severe and may be fatal without correct treatment. They are caused by a wide range of organisms – usually bacteria or viruses, but also fungi and parasites.
• Bronchitis, an inflammatory condition of the tracheobronchial tree, is usually chronic with acute exacerbations associated with infection by viruses and bacteria. The disease is characterized by cough and excessive mucus production, and the diagnosis is clinical. Antibiotics are often given, but their efficacy is uncertain.
• Bronchiolitis, usually caused by RSV, is acute and severe in young children. RSV causes outbreaks in the community and in hospitals. The disease has an immunopathologic basis, and specific treatment (ribavirin) may be considered. No vaccine is available.
• Pneumonia is caused by a variety of pathogens depending upon the patients’ age, previous or underlying disease, and occupational and geographic factors. Correct microbiologic diagnosis is essential to optimize therapy. Mortality from pneumonia remains significant.
• B. pertussis colonizes the ciliated respiratory epithelium causing the specifically human infection whooping cough. Pertussis toxin and other toxic factors are important for virulence. Diagnosis is clinical, alerted by the characteristic paroxysmal cough. Supportive care is paramount; antibiotics play a peripheral role. Prevention by immunization is effective, and new safer vaccines are becoming available.
• Influenza viruses cause endemic, epidemic and pandemic infections as a result of the capacity of the virus for antigenic drift and shift. The disease is acute in onset and can be clinically severe. Viral damage to the respiratory mucosa predisposes to secondary bacterial pneumonia. Antiviral agents are available. Immunization is important, but needs to be kept up to date due to the frequent antigenic changes in the circulating virus.
• Tuberculosis, a major killer, is becoming more common because of its association with AIDS. Infection is usually chronic. Primary infection with M. tuberculosis results in a localized pulmonary lesion, while secondary disease arises from reactivation as a result of an impairment of immune function. Clinical diagnosis is supported by demonstrating the acid-fast M. tuberculosis in sputum. Effective treatment is available, but long courses of drug combinations are essential. Chemoprophylaxis and BCG immunoprophylaxis are important in prevention.
• Aspergillus causes disease in the lung ranging from invasive disease in the immunocompromised to allergic conditions in the otherwise healthy. Effective treatment is difficult because of the limited number of active antifungals and lack of host defences.
• Cystic fibrosis is an inherited disease that predisposes to a particular pattern of lung disease characterized by infection with P. aeruginosa. Infection can be controlled by antibacterials, but rarely eradicated.
• Various species of parasites pass through or localize in the lungs at some stage in their life cycle. Damage is limited unless the parasite load is high, and is usually immunopathologic in nature.
![]()