Medical Microbiology
Section 4 Clinical manifestation and diagnosis of infections by body system
24 Central nervous system infections
Introduction
Central nervous system infections are usually blood-borne or infectious agents invading via peripheral nerves
The brain and spinal cord are protected from mechanical pressure or deformation by enclosure in rigid containers, the skull and vertebral column, which also act as barriers to the spread of infection. The blood vessels and nerves that traverse the walls of the skull and vertebral column are the main routes of invasion. Blood-borne invasion is the most common route of infection, for example, by polioviruses or Neisseria meningitidis. Invasion via peripheral nerves is less common; examples include herpes simplex, varicella-zoster and rabies viruses. Local invasion from infected ears or sinuses, local injury or congenital defects such as spina bifida, also occurs, while invasion from the olfactory tract leading to amoebic meningitis is rare.
Here, we discuss the main routes of central nervous system invasion by microorganisms (see also Ch. 13) and the body’s response, followed by a more detailed discussion of the diseases that result.
Invasion of the central nervous system
Natural barriers act to prevent blood-borne invasion
Blood-borne invasion takes place across:
• the blood–brain barrier to cause encephalitis
• the blood–cerebrospinal fluid (CSF) barrier to cause meningitis (Fig. 24.1).
The blood–brain barrier consists of tightly joined endothelial cells surrounded by glial processes, while the brain–CSF barrier at the choroid plexus consists of endothelium with fenestrations, and tightly joined choroid plexus epithelial cells. Microbes can traverse these barriers by:
• growing across, infecting the cells that comprise the barrier
• being passively transported across in intracellular vacuoles
• being carried across by infected white blood cells.
Examples of each route are seen in viral infections. Poliovirus, for instance, invades the central nervous system (CNS) across the blood–brain barrier. After oral ingestion of virus, a complex stepwise series of events leads to CNS invasion (Fig. 24.2). Poliovirus also invades the meninges after localizing in vascular endothelial cells, and can cross the blood–CSF barrier. Mumps virus behaves in the same way, as do circulating Haemophilus influenzae, meningococci or pneumococci. Once infection has reached the meninges and CSF, the brain substance can in turn be invaded if the infection crosses the pia. In poliomyelitis, for instance, a meningitic phase often precedes encephalitis and paralysis.

Figure 24.1 Structures of the blood–brain and blood–cerebrospinal fluid (CSF) barriers.
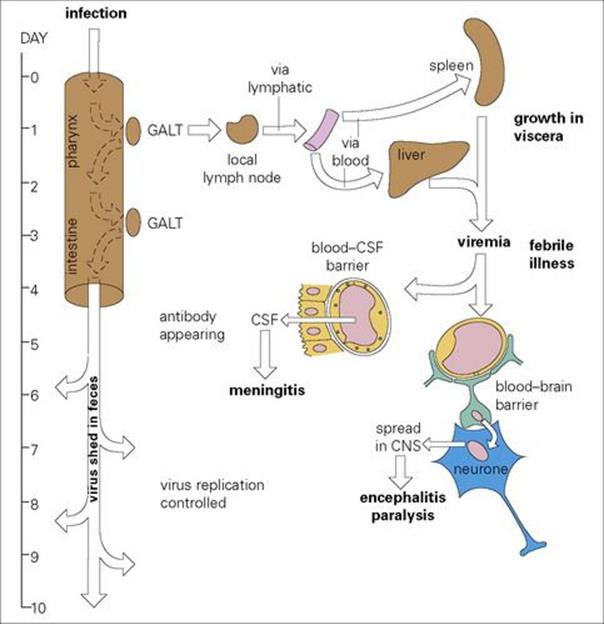
Figure 24.2 The mechanism of central nervous system (CNS) invasion by poliovirus. CSF, cerebrospinal fluid; GALT, gut-associated lymphoid tissue.
CNS invasion, however, is a rare event because most microorganisms fail to pass from blood to the CNS across the natural barriers. A large variety of viruses can grow and cause disease if introduced directly into the brain, but circulating viruses generally fail to invade, and CNS involvement by polio, mumps, rubella or measles viruses is seen in only a very small proportion of infected individuals. The factors that determine such CNS invasion are unknown.
Invasion of the CNS via peripheral nerves is a feature of herpes simplex, varicella-zoster and rabies virus infections
Herpes simplex virus (HSV) and varicella-zoster virus (VZV) present in skin or mucosal lesions (see Ch. 26), travel up axons using the normal retrograde transport mechanisms that can move virus particles (as well as foreign molecules such as tetanus toxin) at a rate of about 200 mm/day, to reach the dorsal root ganglia. Rabies virus, introduced into muscle or subcutaneous tissues by the bite of a rabid animal, infects muscle fibres and muscle spindles after the virus binds to the nicotinic acetylcholine receptor. It then enters peripheral nerves and travels to the CNS, to reach glial cells and neurones, where it multiplies.
The body’s response to invasion
CSF cell counts increase in response to infection
The response to invading viruses is reflected by an increase in lymphocytes, mostly T cells, and monocytes in the CSF (Table 24.1). A slight increase in protein also occurs, the CSF remaining clear. This condition is termed ‘aseptic’ meningitis. The response to pyogenic bacteria shows a more spectacular and more rapid increase in polymorphonuclear leukocytes and proteins (Fig. 24.3), so that the CSF becomes visibly turbid. This condition is termed ‘septic’ meningitis. Certain slower growing or less pyogenic microorganisms induce less dramatic changes, such as in tuberculous or listerial meningitis.
Table 24.1 Changes in cerebrospinal fluid (CSF) in response to invading microbes
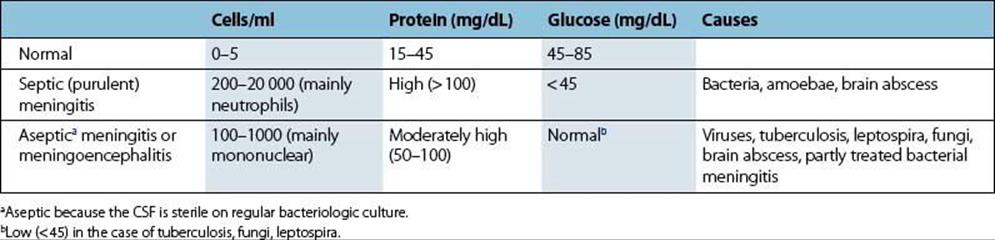
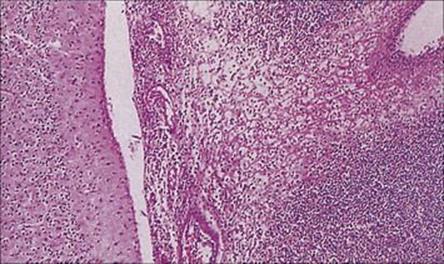
Figure 24.3 Bacterial meningitis. Exudate of acute inflammatory cells in the subarachnoid space (H&E stain).
(Courtesy of P. Garen.)
The pathologic consequences of CNS infection depend upon the microorganism
In the CNS itself, viruses can infect neural cells, sometimes showing a marked preference. Polio and rabies viruses, for instance, invade neurones, whereas JC virus invades oligodendrocytes. Because there is very little extracellular space, spread is mostly direct from cell to cell along established nervous pathways. Invading bacteria and protozoa generally induce more dramatic inflammatory events, which limit local spread so that infection is soon localized to form abscesses.
Viruses induce perivascular infiltration of lymphocytes and monocytes, sometimes, as in the case of polio, with direct damage to infected cells. (The pathogenesis of viral encephalomyelitis is shown later, in Fig. 24.7.) Associated immune responses not only to viral, but also often to host CNS components, play a part in postvaccinial encephalitis. Infiltrating B cells produce antibody to the invading microorganism, and T cells react with microbial antigens to release cytokines that attract and activate other T cells and macrophages. The pathologic condition evolves over the course of several days and occasionally, when partly controlled by host defences, over the course of years, e.g. subacute sclerosing panencephalitis (SSPE) caused by measles, which has both a virological and immunological pathogenesis. Bacteria cause more rapidly evolving pathologic changes, with local responses to bacterial antigens and toxins playing an important part.
In all cases, a degree of inflammation and oedema that would be trivial in striated muscle, skin or liver may be life-threatening when it occurs in the vulnerable ‘closed box’ containing the leptomeninges, brain and spinal cord. It may be several weeks after clinical recovery before cellular infiltrations are removed and histologic appearances are restored to normal.
CNS invasion only rarely assists in the transmission of infection
From the point of view of a parasitic microorganism that needs to be transmitted to a fresh host, invasion of the CNS is generally foolish because it damages the host. The only occasions on which it makes sense are:
• When dorsal root ganglion neurones are invaded as an essential step in establishing latency (HSV and VZV). This gives a mechanism for reactivation and further episodes of shedding from mucosal or skin lesions.
• In the case of rabies (see below), where CNS invasion in the animal host is necessary for two reasons. First, it enables the virus to spread from the CNS down peripheral nerves to the salivary glands, from which transmission takes place. Second, invasion of the limbic system of the brain causes a change in behaviour of the infected animal so that it becomes less retiring, more aggressive and more likely to bite, thus transmitting the infection. Invasion of the limbic system can be regarded as a fiendish strategy on the part of rabies virus to promote its own transmission and survival.
Meningitis
Bacterial meningitis
Acute bacterial meningitis is a life-threatening infection, needing urgent specific treatment
Bacterial meningitis is more severe, but less common, than viral meningitis and may be caused by a variety of agents (Table 24.2). Prior to the 1990s, Haemophilus influenzae type b (Hib) was responsible for most cases of bacterial meningitis. However, the introduction of the Hib vaccine into childhood immunization regimens has lowered overall Hib incidence in favour of Neisseria meningitidis and Streptococcus pneumoniae, which are now responsible for most bacterial meningitis. These three pathogens have several virulence factors in common (Table 24.3), including possession of a polysaccharide capsule (Table 24.4).
Table 24.2 The important causative agents of non-viral meningitis, their treatment and prevention
|
Pathogen |
Treatmenta |
Prevention |
|
Neisseria meningitidis |
Penicillin (or chloramphenicol) |
Rifampicin prophylaxis for close contacts; polysaccharide vaccine (poor protection against group B) |
|
Haemophilus influenzae |
Ampicillinb, ceftriaxone or cefotaxime (or chloramphenicol) |
Polysaccharide vaccine against type b (Hib) |
|
Streptococcus pneumoniae |
Penicillinc (or ceftriaxone or chloramphenicol) |
Prompt treatment of otitis media and respiratory infections; polyvalent (23 serotypes) polysaccharide vaccine |
|
Escherichia coli (and other coliforms), group B streptococci |
Gentamicin + cefotaxime or ceftriaxone (or chloramphenicol)b |
No vaccines available |
|
Listeria monocytogenes |
Penicillin or ampicillin + gentamicin |
No vaccines available |
|
Mycobacterium tuberculosis |
Isoniazid and rifampin and pyrazinamide ± streptomycin |
BCG vaccination; isoniazid prophylaxis for contacts recommended in USA |
|
Cryptococcus neoformans |
Amphotericin B and flucytosine |
No vaccines available |
a Treatment should be initiated immediately and the susceptibility of the infecting isolate confirmed in the laboratory.
b If isolate is shown to be susceptible (10–20% of isolates are resistant because they produce a plasmid coded beta-lactamase).
c In areas of high prevalence of penicillin resistant pneumococci initial treatment with ceftriaxone may be advised until susceptibility of isolate is known. BCG, bacille Calmette–Guérin.
Table 24.3 Virulence factors in bacterial meningitis
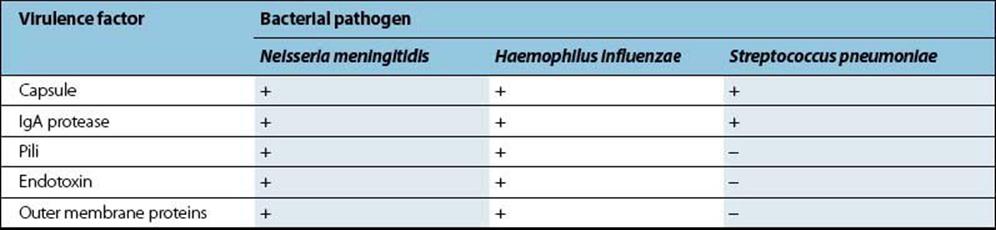
Table 24.4 Polysaccharide capsules are important virulence factors in the pathogenesis of bacterial meningitis
Meningococcal meningitis
Neisseria meningitidis is carried by about 20% of the population, but higher rates are seen in epidemics
Neisseria meningitidis is a Gram-negative diplococcus which closely resembles N. gonorrhoeae in structure (see Ch. 21), but with an additional polysaccharide capsule that is antigenic and by which the serotype of N. meningitidis can be recognized. The bacteria are carried asymptomatically in the population, up to 20% depending on geographic location, and are attached by their pili to the epithelial cells in the nasopharynx. Invasion of the blood and meninges is a rare and poorly understood event. The known virulence factors are summarized in Table 24.3. People possessing specific complement-dependent bacterial antibodies to capsular antigens are protected against invasion. Those with C5–C9 complement deficiencies show increased susceptibility to bacteraemia (as they do to N. gonorrhoeae bacteraemia; see Ch. 21). Young children who have lost the antibodies acquired from their mother, and adolescents who have not previously encountered the infecting serotype, and therefore have no type-specific immunity, are those most often infected.
Person-to-person spread takes place by droplet infection, and is facilitated by other respiratory infections, often viral, that cause increased respiratory secretions. Thus, conditions of overcrowding and confinement such as prisons, military barracks and college dormitories contribute to the frequency of infection in populations. During outbreaks of meningococcal meningitis, which most frequently occur in late winter and early spring, the carrier rate may reach 60–80%. Specific serotypes associated with infection exhibit some geographic variation. However, serotypes B, C and Y tend to predominate in more resource-rich countries, whereas serotypes A and W-135 are more common in less developed regions. Available vaccines target serotypes A, C, Y and W-135 but not B (Table 24.4). The UK was the first country to introduce the meningitis C conjugate vaccine. It has been part of routine childhood immunization since November 1999. The UK Department of Health recommends that all first-year university and college students and others between 20 and 24 years old should be immunized against meningitis C. The US Centers for Disease Control guidance is similar.
Group B meningococcal disease is diagnosed in more than 50% of meningitis cases; however, vaccine development has been hindered because a potential target, the group B capsule, is an autoantigen. Vaccines have been prepared using the bacterial outer membrane and recombinant proteins which may be protective.
Clinical features of meningococcal meningitis include a haemorrhagic skin rash
After an incubation period of 1–3 days, the onset of meningococcal meningitis is sudden with a sore throat, headache, drowsiness and signs of meningitis which include fever, irritability, neck stiffness and photophobia. There is often a haemorrhagic skin rash with petechiae, reflecting the associated septicaemia (Fig. 24.4). In about 35% of patients, this septicaemia is fulminating, with complications due to disseminated intravascular coagulation, endotoxaemia and shock, and renal failure. In the most severe cases there is an acute Addisonian crisis, with bleeding into the brain and adrenal glands referred to as Waterhouse–Friedrichsen syndrome. Mortality from meningococcal meningitis reaches 100% if untreated, but remains around 10% even if treated. In addition, serious sequelae such as permanent hearing loss may occur in some survivors (Table 24.5).

Figure 24.4 Meningococcal septicaemia showing a mixed petechial and maculopapular rash on the extremities and exterior surfaces.
(Courtesy of W.E. Farrar.)
Table 24.5 Clinical features of bacterial meningitis
A diagnosis of acute meningitis is usually suspected on clinical examination
Laboratory identification of the bacterial cause of acute meningitis is essential so that appropriate antibiotic therapy can be given and prophylaxis of contacts initiated. Preliminary microscopy results involving white cell counts and Gram staining for bacteria should be available within an hour of receipt of the CSF sample in the laboratory. Results of culture of CSF and blood should follow after 24 h (see Ch. 32). Molecular diagnosis of meningococcal infection can also be carried out, and may be of clinical assistance as early treatment saves lives, but makes culture of viable organisms from specimens more difficult.
Serology is not helpful in the diagnosis because the infection is too acute for an antibody response to be detectable. Bacterial meningitis is a medical emergency and antibiotic therapy (penicillin or ampicillin) must be instigated if the diagnosis is suspected (Table 24.2).
Close contacts in the family, referred to as ‘kissing contacts’, should be given single-dose ciprofloxacin. Note that penicillin is not used for prophylaxis because it does not eliminate nasopharyngeal carriage of meningococci. Rifampicin used to be recommended but it is associated with rapid induction of resistance, has to be taken for a longer time period and interacts with oral contraceptives.
Haemophilus meningitis
Type b H. influenzae causes meningitis in infants and young children
H. influenzae is a Gram-negative coccobacillus. ‘Haemophilus’ means ‘blood-loving’, and the name ‘influenzae’ was given because it was originally thought to be the cause of influenza, but is now known to be a common secondary invader in the lower respiratory tract. There are six types (a–f) of H. influenzae, distinguishable serologically by their capsular polysaccharides:
• Unencapsulated strains are common and are present in the throat of most healthy people.
• The capsulated type b, a common inhabitant of the respiratory tract of infants and young children (where it may cause infection: see Ch. 18), very occasionally invades the blood and reaches the meninges.
Maternal antibody protects the infant up to 3–4 months of age, but as it wanes, there is a ‘window of susceptibility’ until the child produces his/her own antibody. Anticapsular antibodies are good opsonins (see Ch. 14), which allow the bacteria to be phagocytosed and killed, but children do not generally produce them until 2–3 years of age, possibly because these antibodies are T independent. In addition to the capsule, H. influenzae has several other virulence factors, as shown in Table 24.3.
Acute H. influenzae meningitis is commonly complicated by severe neurologic sequelae
The incubation period of H. influenzae meningitis is 5–6 days, and the onset is often more insidious than that of meningococcal or pneumococcal meningitis (Table 24.5). The condition is less frequently fatal, but, as with meningococcal infection, serious sequelae such as hearing loss, delayed language development, and mental retardation and seizures may occur (Table 24.5).
General diagnostic features are the same as for meningococcal meningitis, as explained above. For laboratory diagnosis, see Chapter 32. It is important to note that the organisms may be difficult to see in Gram-stained smears of CSF, particularly if they are present in small numbers.
H. influenzae type b (Hib) vaccine is effective for children from 2 months of age
General features of treatment are referred to above under meningococcal meningitis; details are summarized in Table 24.2. An effective Hib vaccine, suitable for children 2 months of age and upwards, is available. Rifampicin prophylaxis is recommended for close contacts of patients with invasive Hib disease.
Pneumococcal meningitis
Streptococcus pneumoniae is a common cause of bacterial meningitis, particularly in children and the elderly
Strep. pneumoniae was first isolated more than 100 years ago but relatively little is known about its virulence attributes apart from its polysaccharide capsule (Tables 24.3, 24.4), and the pneumococcus remains a major cause of morbidity and mortality. (Pneumococcal respiratory tract infections are reviewed in Ch. 19.)
Strep. pneumoniae is a capsulate Gram-positive coccus carried in the throats of many healthy individuals. Invasion of the blood and meninges is a rare event, but is more common in the very young (< 2 years of age), in the elderly, in those with sickle cell disease, in debilitated or splenectomized patients and following head trauma. Susceptibility to infection is associated with low levels of antibodies to capsular polysaccharide antigens: antibody opsonizes the organism and promotes phagocytosis, thereby protecting the host from invasion. However, this protection is type specific and there are more than 85 different capsular types of Strep. pneumoniae.
The clinical features of pneumococcal meningitis are generally worse than with N. meningitidis and H. influenzae and are summarized in Table 24.5. The general diagnostic features are the same as for meningococcal meningitis described above. Details are referred to in Chapter 32.
Treatment and prevention of pneumococcal meningitis are summarized in Table 24.2. Since penicillin-resistant pneumococci have been observed worldwide, attention must be paid to the antibiotic susceptibility of the infecting strain, and empiric chemotherapy usually involves a combination of vancomycin and either cefotaxime or ceftriaxone.
An effective heptavalent protein-conjugate pneumococcal vaccine is available which the US Centers for Disease Control recommends for all children from 2 to 23 months of age (i.e. to be given with other recommended childhood vaccines) and for older children (24–59 months) who are at high risk (e.g. sickle cell disease, HIV infection, chronic illness or weakened immune systems) for serious pneumococcal infection. The older 23-valent polysaccharide vaccine remains available for children older than 5 years of age.
Listeria monocytogenes meningitis
Listeria monocytogenes causes meningitis in immunocompromised adults
Listeria monocytogenes is a Gram-positive coccobacillus and an important cause of meningitis in immunocompromised adults, especially in renal transplant and cancer patients. It also causes intrauterine infections and infections of the newborn, as summarized in Chapter 23. L. monocytogenes is less susceptible than Strep. pneumoniae to penicillin, and the recommended treatment is a combination of penicillin or ampicillin with gentamicin.
Neonatal meningitis
In general, neonates, especially those with low birth weight, are at increased risk for meningitis because of their immature immunological status. This is illustrated by problems with, for example, humoral and cellular immunity, phagocytic capability and inefficient alternative complement pathway. This is especially true as a result of medical advances that have contributed to the increased survival of pre-term infants.
Although mortality rates due to neonatal meningitis in resource-rich countries are declining, the problem is still serious
Neonatal meningitis can be caused by a wide range of bacteria, but the most frequent are group B haemolytic streptococci (GBS) and E. coli (Table 24.6; see also Ch. 23). This may occur by routes such as nosocomial infection. However, the infant may also be infected from the mother. For example, with women vaginally colonized by GBS, the infant may swallow maternal secretions such as infected amniotic fluid during delivery.
Table 24.6 Group B streptococci are a major cause of neonatal meningitis
|
Group B streptococci (Streptococcus agalactiae) are normal inhabitants of the female genital tract and may be acquired by the neonate |
||
|
At or soon after birth |
In the nursery |
|
|
Early onset disease |
Late onset disease |
|
|
Age |
< 7 days |
1 week–3 months |
|
Risk factors |
Heavily colonized mother lacking specific antibody |
Lack of maternal antibody |
|
Premature rupture of membranes |
Exposure to cross-infection from heavily colonized babies |
|
|
Pre-term delivery |
Poor hygiene in nursery |
|
|
Prolonged labour, obstetric complications |
||
|
Type of disease |
Generalized infection including bacteraemia, pneumonia and meningitis |
Predominantly meningitis |
|
Type of group B streptococcus |
All serotypes but meningitis mostly due to type III |
90% type III |
|
Outcome |
Approximately 60% fatal; serious sequelae in many survivors |
Approximately 20% fatal |
|
Treatment |
Take blood and CSF for culture |
Treat on suspicion |
|
Treat on suspicion |
Take blood and CSF for culture |
|
|
Gentamicin and ampicillin or cefotaxime/ceftriaxone |
Gentamicin and ampicillin or cefotaxime/ceftriaxone |
|
|
Prevention |
Antibiotic treatment does not reliably abolish carriage in mother; not recommended |
Good hygiene practices in nursery |
|
‘Blind’ treatment of sick baby who has risk factors |
Do not allow mothers to handle other babies |
|
CSF, cerebrospinal fluid.
Neonatal meningitis often leads to permanent neurologic sequelae such as cerebral or cranial nerve palsy, epilepsy, mental retardation or hydrocephalus. This is partly because the clinical diagnosis of meningitis in the neonate is difficult, perhaps with no more specific signs than fever, poor feeding, vomiting, respiratory distress or diarrhea. In addition, due to the possible range of aetiological agents, ‘blind’ antibiotic therapy in the absence of susceptibility tests may not be optimal, and adequate penetration of the antibiotic into the CSF is also an issue.
Tuberculous meningitis
Patients with tuberculous meningitis always have a focus of infection elsewhere, but approximately 25% may have no clinical or historic evidence of such an infection. In > 50% of cases, meningitis is associated with acute miliary tuberculosis (Fig. 24.5). In areas with a high prevalence of tuberculosis, meningitis tends to be most commonly seen in children from 0 to 4 years of age. However, in areas where tuberculosis is less frequent, most meningitis cases are in adults.
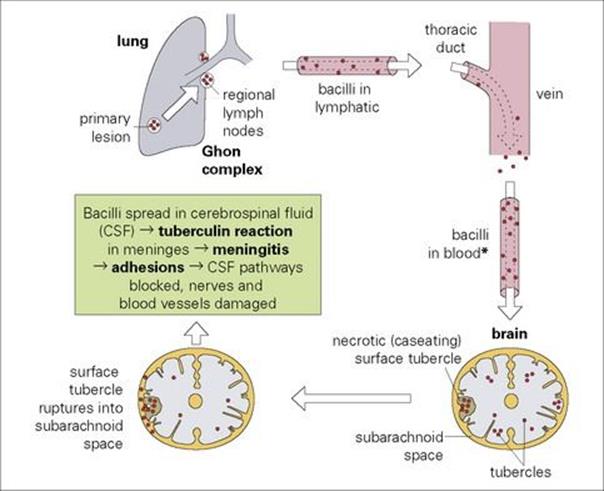
Figure 24.5 The association between acute miliary tuberculosis and meningitis. (* Leads to miliary tuberculosis (Latin: milium, millet seed – each tubercle resembles a millet seed). Miliary tuberculosis also occurs in the lungs and elsewhere.
Tuberculous meningitis usually presents with a gradual onset over a few weeks
There is a gradual onset of generalized illness beginning with malaise, apathy and anorexia and proceeding within a few weeks to photophobia, neck stiffness and impairment of consciousness. Occasionally, the onset is much more rapid and may be mistaken for a subarachnoid haemorrhage. The variability of presentation means that the clinician needs to maintain an awareness of possible tuberculous meningitis to make the diagnosis. A delay in making the diagnosis and in starting appropriate antimicrobial therapy (Table 24.2) results in serious complications and sequelae.
Spinal tuberculosis is uncommon now except in resource-poor countries; bacteria in the vertebrae destroy the intervertebral disks to form epidural abscesses. These compress the spinal cord and lead to paraplegia.
Fungal meningitis
Cryptococcus neoformans and Coccidioides immitis can invade the blood from a primary site of infection in the lungs and thence to the brain to cause meningitis. Cryptococcus has a marked tropism for the CNS and is the major cause of fungal meningitis. C. neoformans occurs as two varieties, each with two serotypes.
Cryptococcus neoformans meningitis is seen in patients with depressed cell-mediated immunity
It therefore occurs in individuals with AIDS and other immunosuppressive conditions. The onset is usually slow, over days or weeks. The capsulate yeasts can be seen in Indian ink-stained preparations of cerebrospinal fluid (CSF) (Fig. 24.6) and can be cultured (see Ch. 32). Antigen detection is also a useful diagnostic tool and evidence of a decline in antigen and an increase in antibody levels in the CSF can be used as a measure of successful therapy. Treatment with the antifungal drugs amphotericin B and flucytosine in combination is recommended, the former penetrating poorly into CSF.
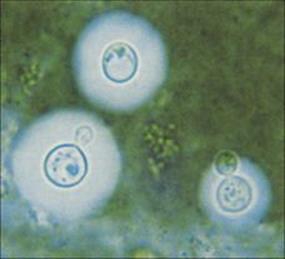
Figure 24.6 Cryptococcus neoformans in Indian ink-stained preparation of cerebrospinal fluid sediment.
(Courtesy of A.E. Prevost.)
Coccidioides immitis infection is common in particular geographic locations
These locations are notably southwest USA, Mexico and South America. CNS infection occurs in < 1% of infected individuals, but is fatal unless treated. It may be part of the generalized disease or may represent the only extrapulmonary site. The organisms are rarely visible in the CSF, and cultures are positive in < 50% of cases, but the diagnosis can be made by demonstrating complement-fixing antibodies in the serum. Treatment with amphotericin B, fluconazole or miconazole is recommended.
Protozoal meningitis
The free-living amoeba Naegleria can multiply in stagnant fresh water in warm countries, especially in the sludge at the bottom of lakes and swimming pools. If inhaled, they can reach the meninges via the olfactory tract and cribriform plate. Primary amoebic meningoencephalitis caused byNaegleria affects healthy individuals with no obvious defect in immunity. The disease shows a rapid onset, and the mortality rate is high.
Acanthamoeba spp. are widespread in the environment. They more commonly affect those who are already unwell or immunocompromised and are thought to enter via the skin or the respiratory tract. Acanthamoeba causes a chronic condition (granulomatous amoebic encephalitis).
Balamuthia mandrillaris is found in soil or stagnant water and humans become infected by inhalation of cysts or direct contamination of skin. Cases have been reported in patients with a variety of underlying medical conditions but also in immunocompetent individuals. Infection of the CNS produces encephalitis with raised protein, lymphocytic CSF and normal or low CSF glucose.
Under the microscope, Naegleria appear as slowly motile amoebae on careful examination of a fresh wet sample of CSF. Acanthamoeba are rarely seen in the CSF but can be visualized in brain biopsies. They also grow well in cultures prepared from tissue biopsies. Diagnosis of Balamuthiainfection is by histopathology of biopsy samples, serology and PCR on brain tissue or CSF. Treatment is not fully satisfactory. Amphotericin B, with miconazole and rifampin, has been used for Naegleria; a variety of drugs have been used for Acanthamoeba. Two survivors of Balamuthiainfection received a combination of albendazole and itraconazole.
Viral meningitis
Viral meningitis is the most common type of meningitis
It is a milder disease than bacterial meningitis, with headache, fever and photophobia, but less neck stiffness. The CSF is clear in the absence of bacteria, and the cells are mainly lymphocytes, although polymorphonuclear leukocytes may be present in the early stages (Table 24.1). The causes of viral meningitis are listed in Table 24.7 and before the advent of molecular-based methods of detection, viruses were isolated from the CSF in < 50% of cases.
Table 24.7 Causes of viral meningitis
|
Virus |
Virus group |
Comments |
|
Herpes simplex virus |
Alpha herpesvirus |
May follow genital infection with HSV-2 |
|
Mumps |
Paramyxovirus |
A quite common complication |
|
Lymphocytic choriomeningitis |
Arenavirus |
Uncommon infection from urine of mice, hamsters carrying the virus |
|
Enteroviruses including coxsackievirus, echovirus and poliovirus |
Picornaviruses (enterovirus group) |
Commonly seen, especially due to echoviruses |
|
Japanese encephalitis |
Togavirus |
India, South East Asia, Japan |
|
Eastern and Western equine encephalitis |
Togavirus |
East and West USA |
|
Louping Ill |
Togavirus |
Scotland |
|
HIV |
Retrovirus |
May occur early after infection |
There are five groups of human enteroviruses which include the echoviruses, coxsackie group A and B viruses, and the three types of polioviruses. Enteroviruses are common causes of seasonal aseptic meningitis. In contrast to bacterial meningitis, viral meningitis usually has a benign course, and complete recovery is the rule.
Encephalitis
Encephalitis is usually caused by viruses, but there are many cases where the infectious aetiology is not identified
The causes and pathogenesis of viral encephalitis are shown in Table 24.8 and Figure 24.7. Approximately 700 cases of viral encephalitis occur annually in England with a 7% mortality rate and substantial morbidity including physical, cognitive and behavioural difficulties. It is thought that the annual costs of illness caused by encephalitis to the United States health service amounts to around US$630 million.
Table 24.8 Infectious causes of encephalitis
|
Cause |
Infectious agent |
Comments |
|
Viruses (sporadic occurrence) |
Herpes simplex virus |
Infant and adult forms distinguished |
|
Mumps |
Much less common than meningitis |
|
|
Varicella-zoster virus |
A rare complication of ophthalmic zoster |
|
|
Cytomegalovirus |
In utero and in immunosuppressed (e.g. AIDS) |
|
|
Rabies |
55 000 deaths/year, in Asia and Africa |
|
|
Louping ill |
One of tick-borne encephalitis virus complex (others cause Russian spring–summer encephalitis etc.) |
|
|
HIV |
Subacute encephalitis (often together with other central nervous system infections) |
|
|
Viruses (may be outbreaks) |
Polio and other enteroviruses |
Uncommon; may be spastic paralysis |
|
Eastern and western equine encephalitis |
|
|
|
St Louis encephalitis virus |
||
|
Japanese encephalitis virus |
||
|
Californian encephalitis virus |
Mosquito-borne bunyavirus |
|
|
Slow viruses |
Rubella |
Infection in utero (microcephaly etc.) or subacute sclerosing panencephalitis (SSPE)-type disease |
|
Measles |
SSPE following uncomplicated measles after interval of up to 10 years |
|
|
JC virus (progressive multifocal leukoencephalopathy) |
Usually in immunocompromised, especially in AIDS |
|
|
Atypical agents of scrapie group (non-viral) |
Prions |
Creutzfeldt–Jakob disease (CJD) and kuru in humans; incubation period up to 20 years; ‘spongiform’ encephalopathy |
|
Postvaccinal or postinfectious |
? |
Occurs as a rare complication 2–3 weeks after exposure to certain viruses (e.g. measles) or vaccines; strong autoimmune component |
|
Protozoa and fungi |
Toxoplasma gondii |
Encephalitis a rare complication |
|
Cryptococcus neoformans |
Meningoencephalitis |
|
|
Plasmodium falciparum |
Cerebral malaria |
|
|
Trypanosoma spp. |
Sleeping sickness in Africa |
|
|
Bacteria |
Treponema pallidum |
Rare |
|
Mycoplasma pneumoniae |
Rare |
|
|
Borrelia burgdorferi |
Uncommon |
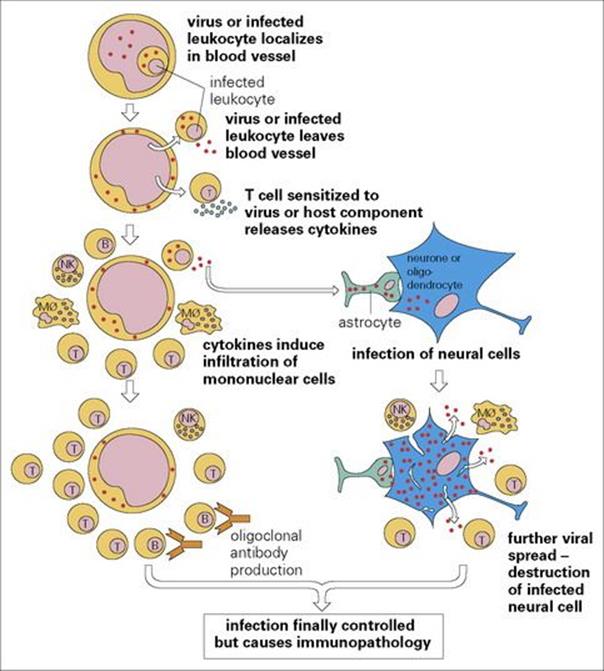
Figure 24.7 The pathogenesis of viral encephalomyelitis. Mφ, macrophage; NK, natural killer cell.
Characteristically, there are signs of cerebral dysfunction, as the substance of the brain is affected, unlike meningitis where the lining of the brain is inflamed. Someone with an encephalitic illness will present with abnormal behaviour, seizures and altered consciousness, often with nausea, vomiting and fever.
Up to 85% of individuals diagnosed with encephalitis globally are of unknown aetiology. Emerging viruses that can cause encephalitis include the Nipah virus, bat lyssaviruses, and avian influenza A H5N1 virus infections. Immune-mediated forms of encephalitis, including voltage-gated potassium channel and N-methyl-D-aspartate (NMDA) receptor antibody-associated encephalitis, must be considered in the differential diagnosis as they have a similar presentation as infectious causes.
Preventative measures include measles, mumps and rubella immunization. In addition, antiviral drugs are used to treat herpes simplex and varicella-zoster virus encephalitis and immunomodulation for the immune-mediated encephalitides including acute disseminated encephalomyelitis (ADEM) or other immune-mediated encephalitides.
Toxoplasma gondii and C. neoformans can also cause life-threatening encephalitis or meningoencephalitis. This is particularly likely in those with defective cell-mediated immunity, and cerebral malaria as a complication of Plasmodium falciparum infection is frequently fatal. Encephalitis may occur in Lyme disease (Borrelia burgdorferi) and Legionnaires’ disease (Legionella pneumophila), but the relative importance of bacterial invasion, bacterial toxins and immunopathology is unknown.
HSV encephalitis (HSE) is the most common form of severe sporadic acute focal encephalitis
It is thought that the incidence of HSE in the USA is about 1/250 000 to 500 000 population per year. A distinction is made between HSV infections of the CNS during the neonatal period and those in older children and adults. Neonates may acquire a primary and disseminated infection with a diffuse encephalitis after vaginal delivery from a mother shedding HSV-2 in the genital tract. Most HSE seen in older children and adults is due to HSV-1, of which most are due to virus reactivation in the trigeminal ganglia (see Ch. 15), the infection then passing back to the temporal lobe of the brain, and the minority are due to a primary infection. About 30% of HSE is seen in people < 20 years old, and 50% in the over-50 age range.
Herpetic skin or mucosal lesions may be present. The diagnosis is indicated by finding temporal lobe enhancement using CT and MRI scans (Fig. 24.8). HSV DNA detection should be carried out on a CSF sample using PCR. An electroencephalogram (EEG) may also be helpful. The 70% mortality rate in untreated patients is greatly reduced by early and prolonged treatment with intravenous aciclovir. The 21-day treatment course is important, as relapse can occur.
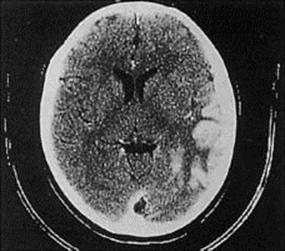
Figure 24.8 Herpes simplex encephalitis. Computerized tomographic scan showing enhancement of gyral structures in the left temporal lobe and associated cerebral oedema.
(Courtesy of J. Curé.)
Other herpesviruses less commonly cause encephalitis
With VZV, encephalitis generally occurs as a sequel to reactivation, and with cytomegalovirus (CMV) either during primary infection in utero (see Ch. 21) or reactivation as a complication of immunodeficiency, for example, in AIDS. HHV-6 encephalitis has also been reported in immunosuppressed patients. Finally, B virus is a Cercopithecine herpesvirus of macaque monkeys that does not really affect the animal but can cause a severe and fatal encephalitis in humans when bitten or scratched by an infected monkey. The wound should be cleaned immediately and antiviral prophylaxis is recommended.
Enteroviral infections
Poliovirus used to be a common cause of encephalitis
In the great 1916 polio epidemic in New York City, 9000 cases of paralysis were reported, nearly all in children < 5 years of age. CNS disease occurs in < 1% of those infected. After an initial 1–4 days of fever, sore throat and malaise, meningeal signs and symptoms appear, followed by involvement of motor neurones and paralysis (see Fig. 24.2).
There are successful vaccines; the structure (Fig. 24.9) and replication of the virus are better understood, and efforts to eradicate the disease by 2002 had driven the incidence of polio to its lowest point in history. The disease is completely preventable by vaccination (see Ch. 31) and has been disappearing in resource-rich countries since vaccination programmes were first carried out in the 1950s (Fig. 24.10). The Global Polio Eradication Initiative reduced the number of polio-endemic countries from 20 to 10 between 2000 and 2001. By 2010, a 95% and 94% fall in the number of children paralysed by polio in Nigeria and India were reported, respectively, together with a 92% decline in polio cases due to type 3 wild poliovirus (WPV3) globally. Imported wild poliovirus transmission was also interrupted in all countries that reported reinfections in 2009.The fall in PV transmission in these countries and WPV3 worldwide was due to a new bivalent oral polio vaccine and new ways of delivering the vaccine. However, an outbreak of paralytic polio occurred in Tajikistan and the Democratic Republic of Congo in 2010 due to importation of wild PV.
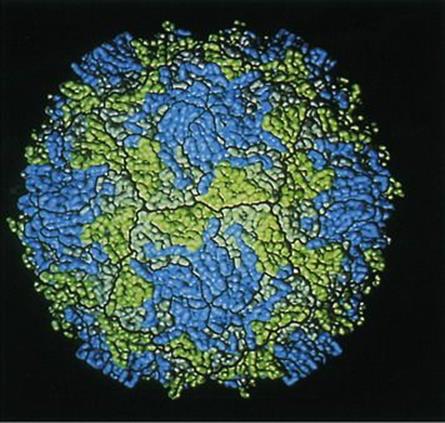
Figure 24.9 Computer graphic model of the surface of a poliovirus based on X-ray diffraction studies. The capsid protein subunits visible on the surface of the virus particle are viral protein 1 (VP1) in blue, VP2 in green and VP3 in grey.
(Courtesy of A.J. Olson, Research Institute of Scripps Clinic, La Jolla, California.)
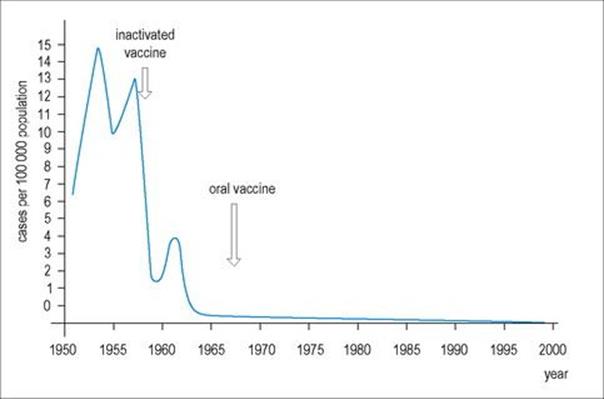
Figure 24.10 The incidence of paralytic poliomyelitis in the USA from 1951 to 2000.
There are three serologic (antigenic) types of poliovirus, with little cross-reaction between them, so that antibody to each type is necessary for protection. At least 75% of paralytic cases are due to type 1 polioviruses.
Enterovirus-71-associated hand, foot and mouth epidemic resulted in a high rate of neurologic complications
Other enteroviruses such as coxsackieviruses and echoviruses occasionally cause meningoencephalitis. However, in 1998, there was a large outbreak of enterovirus 71 (EV71) hand, foot and mouth (HFMD) infection in Taiwan, in which most of the 405 patients were children under 5 years of age, with a mortality rate of 19%. The most severely affected children had brainstem involvement, and many were left with permanent neurological sequelae. Treatment is supportive and there is no vaccine. Since then, EV71, having been identified in Guangdong province in the People’s Republic of China and having caused epidemics in south China, several epidemics have been reported in the middle or north of China, such as in Anhui, Shandong, Shanghai, and Beijing. Overall, 488 955 HFMD cases were reported nationwide, including 126 fatal cases in 2008.
Paramyxoviral infections
Mumps virus is a common cause of mild encephalitis
Asymptomatic CNS invasion may be common because there are increased numbers of cells in the CSF in about 50% of patients with parotitis. However, meningitis and encephalitis are often seen without parotitis.
Nipah virus encephalitis, a zoonotic paramyxovirus infection
In 1998, an outbreak of encephalitis with a high mortality rate was reported among pig farm workers in Malaysia. In total, there were 105 deaths among 265 patients with Nipah virus encephalitis. At first attributed to Japanese encephalitis, the clinical, epidemiologic and virologic characteristics showed that the virus was a paramyxovirus which was transmitted to humans by close contact with infected pigs, probably by aerosol. The outbreak was ended by culling more than 1 million infected or exposed pigs in the local and surrounding regions in Malaysia. The island flying fox, Pteropus hypomelanus, a fruit bat, is the natural reservoir for the virus and the virus can be found in the urine and saliva of infected bats. The pigs were infected having eaten food contaminated by fruit bat secretions. Human-to-human transmission can also play an important role in Nipah virus transmission.
Rabies encephalitis
More than 55 000 people die of rabies worldwide each year
• Rabies occurs in more than 150 countries and territories.
• Wound cleansing and immunization within a few hours after contact with a suspect rabid animal can prevent the onset of rabies and death.
• Every year, more than 15 million people worldwide receive a post-exposure preventive regimen to avert the disease – this is estimated to prevent 327 000 rabies deaths annually.
The causative agent of rabies is a rhabdovirus, a bullet-shaped single-stranded RNA virus. The Lyssavirus genus sits within the Rhabdoviridae family and there are seven genotypes: genotype 1 occurs worldwide and is the classic rabies virus, genotypes 2, 3 and 4 are the African Lagos, Mokola and Duvenhage bat viruses, respectively, genotypes 5 and 6 are the European bat Lyssaviruses (EBLV) 1 and 2, respectively, and genotype 7 is the Australian bat Lyssavirus.
The virus is excreted in the saliva of infected dogs, foxes, jackals, wolves, skunks, raccoons and vampire and other bats, and transmission to humans follows a bite or salivary contamination of other types of skin abrasions or wounds. The infection is eventually fatal, although the course of the disease varies considerably between species. If an apparently healthy dog is still healthy 15 days after biting a human, rabies is extremely unlikely. However, the virus may be excreted in the dog’s saliva before the animal shows any clinical signs of disease.
The virus can infect all warm-blooded animals. Rabies from vampire bats causes more than 1 million deaths per year in cattle in Central and South America. Dogs are the source of more than 99% of human rabies deaths and are involved in most of the 55 000 cases of human rabies that occur in the world each year. In all the mainland masses, the infection maintains itself in non-human mammalian hosts; islands such as Australia, Great Britain, Japan, Hawaii, most of the Caribbean islands and also Scandinavia, are free of rabies because of strict controls over the importation of animals such as dogs and cats, although this is changing. Since the development of the Channel Tunnel linking the UK and the rest of Europe, a number of rabies infections occurred associated with contact with bats. Thirty different bat species have been identified in Europe, a number of which carry EBVL 1 or 2. They are distinct from rabies genotype 1 infections in foxes, dogs and other terrestrial animals. In the USA, the incidence of human rabies has been falling since the 1940s and 1950s, when most cases followed exposure to infected dogs. Since then, the source has more often been non-domesticated animals such as skunks, raccoons and bats, or exposure to dogs in other countries.
Raccoon rabies spread slowly northwards from Florida in the 1950s, and in the 1980s caused an explosive epidemic in Virginia, Maryland and the District of Columbia. This outbreak was due to the importation of raccoons from infected areas for sporting purposes.
The incubation period in humans is generally 4–13 weeks, although it may occasionally be as long as 6 months, possibly due to a delay in virus entry into peripheral nerves. The virus travels up peripheral nerves and, in general, the further the bite is from the CNS, the longer the incubation period. For instance, a bite on the foot leads to a longer incubation period than a bite on the face.
While the virus is travelling up the axons of motor or sensory neurones, there is no detectable antibody or cell-mediated immune response, possibly because antigen remains sequestered in infected muscle cells. Hence, passively administered rabies immunoglobulin may be given during the incubation period.
Once in the brain, the virus spreads from cell to cell until a large proportion of neurones are infected, but there is little cytopathic effect, even when viewed by electron microscopy, and almost no cellular infiltration. The striking symptoms of this disease are largely due to dysfunction rather than visible damage to infected cells. The change in behaviour of infected animals results from virus invasion of the limbic system.
Clinical features of rabies include muscle spasms, convulsions and hydrophobia
After developing a sore throat, headache, fever and discomfort at the site of the bite, the patient becomes excited, with muscle spasms and convulsions. Involvement of the muscles of swallowing when attempting to drink water gave the old name for rabies, hydrophobia, as the symptoms are sometimes precipitated by the mere sight of water.
Once rabies has developed it is fatal, death occurring following cardiac or respiratory arrest. Paralysis is often a major feature of the disease. One or two patients treated in intensive care units have recovered, but with serious neurologic sequelae.
Rabies can be diagnosed by detecting viral antigen or RNA
Laboratory diagnosis can be made by the detection of viral antigen by immunofluorescence or using PCR to detect rabies viral RNA in skin biopsies, corneal impression smears or brain biopsy. Characteristic intracytoplasmic inclusions called Negri bodies are seen in neurones (Fig. 24.11). There is no treatment except supportive care.
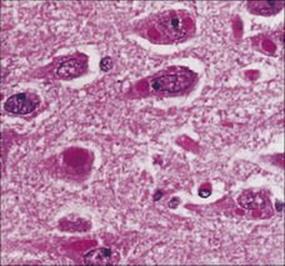
Figure 24.11 Multiple cytoplasmic Negri bodies in pyramidal neurones of the hippocampus in rabies.
(Courtesy of P. Garen.)
Many countries have developed vaccination programmes for domestic dogs, e.g. France, and in Canada and elsewhere, wild foxes have been vaccinated by dropping food baited with live virus vaccine from the air. For the rabies-free countries, constant vigilance at borders and strict quarantine regulations are necessary to prevent the introduction of infected animals. In 1886, there were 36 human rabies deaths in England, 11 of them in London. As recently as 1906, rabies was still endemic in England, and there were deaths due to rabies in the deer in Hampton Court Park, London.
After exposure to a possibly infected animal, immediate preventive action should be taken
This action includes:
• prompt cleaning of the wound (alcoholic iodine, debridement)
• confirmation of whether or not the animal is rabid (clinical observation of suspected dogs, histologic observation of the brain of other suspected species)
• administration of human rabies immunoglobulin (RIG) to ensure prompt passive immunization; RIG is infiltrated intramuscularly around the wound site
• active immunization with killed diploid cell-derived rabies virus (see Ch. 34). The chances of preventing the disease are greater when vaccination is started as early as possible after infection. Vaccine and RIG must never be administered at the same anatomical site.
Togavirus meningitis and encephalitis
Numerous arthropod-borne togaviruses can cause meningitis or encephalitis
These togaviruses are listed in Tables 24.7 and 24.8, and sometimes cause outbreaks of infection. In different parts of the world, different mammals, birds or even reptiles act as reservoirs and there are a variety of arthropod (mosquito and tick) vectors. Usually, < 1% of humans infected develop neurologic disease (see Ch. 27). There may be a febrile illness, but asymptomatic infection is common. In California, for instance, western equine encephalomyelitis (WEE) virus and St Louis encephalitis (SLE) virus are prevalent and transmitted by the mosquito Culex tarsalis; a WEE vaccine is available, but only for horses.
Japanese encephalitis virus infection is common in India and can result in a mortality of more than 50% in older age groups; a vaccine for humans has been developed.
West Nile virus, another emerging viral cause of encephalitis
In 1999, an epidemic of viral encephalitis was reported in New York City, leading to 62 patients with encephalitis, seven of whom died. Meningoencephalitis was rare in the younger age groups and more common in those over 50 years of age. Originally thought to be due to St Louis encephalitis (SLE), once again the clinical, epidemiologic and virologic characteristics resulted in the correct identification of West Nile virus infection, as there had been an epidemic of deaths among wild and other birds which are the avian reservoir of SLE but are not usually killed by the virus. West Nile virus belongs to the Japanese encephalitis serogroup of flaviviruses that includes SLE, had not been seen in the western hemisphere but was well recognized in Africa and the Middle East. West Nile virus is primarily an infection of birds and culicine mosquitoes, with humans and horses acting as incidental hosts. Since 1999, the virus has been successfully dispersed by migratory birds and has spread through most of the USA. Transmission has also been reported in four organ transplant recipients having received organs from one donor with West Nile viraemia antemortem.
The diagnosis can be made by detecting West Nile viral RNA or an IgM response in serum and/or CSF samples. Treatment is supportive, there is no vaccine, and prevention includes mosquito control programmes.
HIV meningitis and encephalitis
HIV can cause subacute encephalitis, often with dementia
HIV (see Ch. 21) often invades the CNS shortly after initial infection, resulting in an increase in cells in the CSF and a mild meningitic illness. At a later stage, and quite independently of the disease picture that results from immune deficiency, a subacute encephalitis may develop, often with dementia. This is sometimes difficult to distinguish from the neurologic disease caused by microorganisms such as T. gondii, C. neoformans, cytomegalovirus and JC virus. JC virus, a polyomavirus, occasionally invades oligodendrocytes in immunodeficient people, particularly in AIDS, and eventually gives rise to progressive multifocal leukoencephalopathy (PML). In HIV-related dementia, the brain is shrunken, with enlarged ventricles and vacuolation of myelin tracts. HIV mainly infects macrophages and microglia in the CNS, and because the clinical disease is more severe than might be expected from the pathologic changes, additional pathogenic mechanisms have been proposed. For instance, there are amino acid sequence similarities between the HIV envelope protein gp120 and certain transmitter molecules, so that HIV-derived molecules could block the action of natural neurotransmitters. Highly active antiretroviral therapy has reduced the severity of HIV-associated neurocognitive disorders (HAND). However, their prevalence remains high. This will be improved by using blood–brain barrier-penetrating drugs, possibly at an earlier stage, treating other possible causes including other medical conditions that affect neural function as well as proinflammatory processes, free radical formation and apoptosis. HAND is correlated with long-term central nervous system inflammation.
Viral myelopathy
A number of viral infections can cause inflammation of the spinal cord, a myelitis. Acute myelitis may result in symmetrical symptoms if it transverses the spinal cord. These will include motor weakness and sensory loss, for example. The symptoms will be asymmetrical if only part of the spinal cord is involved. When the anterior horn cells of the cord are affected by polio, coxsackie, enterovirus 71 and West Nile virus infection, the symptoms are motor and result in acute flaccid paralysis. A number of herpesviruses including HSV, CMV, EBV and VZV have been associated with a myelitis. Post-infectious causes have also been reported.
Chronic myelopathy can be caused by HTLV-1 infection and patients present with tropical spastic paraparesis (TSP), also called HAM (HTLV-1-associated myopathy). HIV-1 infection is also part of the differential diagnosis.
Post-vaccinial and post-infectious encephalitis
Encephalitis following viral infection or vaccination possibly has an autoimmune basis
Encephalitis very occasionally occurs 1–2 weeks after an apparently normal measles virus infection, and even less commonly after varicella. It is also seen after Mycoplasma infection and various influenza-like illnesses. The infectious agent is generally not recoverable from the CNS, and the perivascular infiltration, sometimes with demyelination, suggests an autoimmune pathogenesis. A similar condition occurs after administration of brain-derived inactivated rabies vaccine, which is now obsolete, and after other immunizations with non-infectious materials. The clinical picture resembles experimental allergic encephalitis, and is probably due to autoimmune responses triggered by the infection or by the injected material.
An analogous inflammatory demyelinating condition of the peripheral nervous system called Guillain–Barré syndrome has been associated with a variety of viral infections, as well as with immunization with non-infectious material. In 1976, most adults in the USA were given inactivated influenza virus vaccine, which resulted in a small but highly significant number of cases of Guillain–Barré syndrome.
In addition, rubella or measles virus invades the CNS, but virus growth is slow, often incomplete, and partially controlled by host defences (see Ch. 11); clinical disease appears after an incubation period of up to 10 years. For instance:
• In otherwise uncomplicated measles, CNS invasion can take place and eventually result in SSPE.
• Rubella very occasionally causes a similar disease to SSPE but, more commonly, like CMV, it invades the brain of the fetus, interfering with development to cause mental retardation.
Neurologic diseases of possible viral Aetiology
It has often been suggested that certain neurologic diseases of unknown origin, including multiple sclerosis, amyotrophic lateral sclerosis, Parkinson’s disease, schizophrenia and senile dementia, have a viral origin. Although so far there is no definitive evidence for this, it is possible that viruses and other infectious agents may, at times, trigger dangerous autoimmune-type responses in the CNS.
Spongiform encephalopathies caused by scrapie-type agents
Scrapie-type agents are closely associated with host-coded prion protein
Scrapie-type agents infect a variety of mammals, including humans, and are transmissible to laboratory rodents or primates. They show a number of remarkable biological characteristics; their molecular biology is now well described and experiments in laboratory mice have revealed much about their interaction with host tissues (see Ch. 7). Disease is characterized by the appearance of a spongiform appearance of nervous tissues, caused by vacuolation and plaque formation. Infections in animals seem to have originated from sheep and goats with scrapie (see Fig. 7.2), which has been present in Europe for 200–300 years. Affected animals itch and scrape themselves against posts for relief.
CNS Disease caused by parasites
The CNS is an important target in toxoplasmosis
Although congenitally acquired Toxoplasma gondii is initially generalized, it may become localized in the CNS. Damage to the eye is the most common consequence (see Ch. 25), but the brain may also be affected, with hydrocephalus and intracerebral calcification. In the days before the advent of highly active antiretroviral therapy, toxoplasmosis was an important cause of death in AIDS patients, with encephalitis and toxoplasma abscess due to necrosis as contributory causes.
Cerebral malaria is a major killer
The life cycle of Plasmodium falciparum shows an unusual feature in that red blood cells containing the asexual stages (asexual stages are in humans; sexual stages are in mosquitoes, see Ch. 27) adhere to the walls of capillaries. When this occurs in the brain, cerebral malaria may result, and this is an important cause of mortality in African children. Fever is followed by a variety of symptoms, including convulsions and coma, and these lead rapidly to death if not treated. Artemisinin combination therapy is replacing quinine as the treatment of choice for severe malaria. Coma is reversible, mostly without residual neurologic deficit, when treatment is successful.
Toxocara infection can result in granuloma formation in the brain and retina
The cat and dog roundworms Toxocara cati and Toxocara canis infect humans, usually children, when Toxocara eggs derived from kitten or puppy faeces are ingested (see Ch. 6). After ingestion by humans, the eggs hatch and the larvae migrate from the gut to the liver, lung, eye (see Ch. 25), brain, kidney and muscles. However, as humans are dead-end hosts for these parasites, they cannot reach full maturity. Granulomas form around the larvae, which in the brain may cause convulsions, and in the eye a tumour-like mass can cause retinal detachment and eventually blindness. Peripheral blood eosinophilia is rarely seen in ocular toxocariasis.
Serum can be tested by ELISA for antibodies to Toxocara excretory–secretory antigen, but may give false-negative results in ocular toxocariasis. Antibody detection in ocular vitreous fluid samples is more sensitive. The disease is prevented by de-worming puppies and kittens, and by reducing the contamination by dog excreta of children’s play areas. Anthelmintic therapy is not given in ocular toxocariasis. Corticosteroids and appropriate ophthalmic surgery are the mainstays of therapy.
Cystic hydatid disease is characterized by cyst formation, potentially in any organ but most commonly in the liver
Cystic hydatid disease is caused by the tapeworm Echinococcus granulosus (see Chs 6 and 22) which has a worldwide distribution, especially in sheep-rearing areas. When humans ingest eggs from infected dogs, the embryos emerge, migrate through the gut to the portal blood vessels, and subsequently develop into hydatid cysts. These may occur in any organ but are found especially in the liver and, less commonly, in the lungs, brain and kidney. Disease is caused by local pressure from the cyst, and sometimes hypersensitivity reactions to hydatid antigens. Neurologic symptoms include nausea and vomiting, seizures and altered mental status.
Hydatid disease is diagnosed by detecting serum antibody to hydatid antigens and, specifically for CNS involvement, by CT or MRI scanning, to demonstrate the presence of cysts (Fig. 24.12). Hydatid cysts in the CNS require surgical removal, with special care to avoid cyst rupture, plus adjunctive therapy with albendazole.
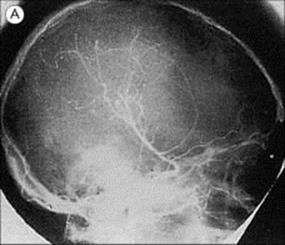
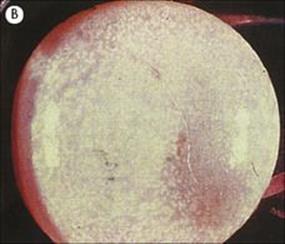
Figure 24.12 Echinococcosis. (A) Cerebral angiography showing displacement of vessels by a large frontal mass. (B) Cyst removed from patient in (A).
(Courtesy of H. Whitwell.)
The disease is prevented by interrupting the natural dog–sheep, dog–goat, or other carnivore–herbivore transmission cycle.
Cysticercosis is characterized by cyst formation in the brain and eye
Cysticercosis results from infection with the larval stage of Taenia solium, a human tapeworm. The eggs present in human faeces infect pigs, which develop cysts in muscle tissue (‘measly pork’) and are a source of further human infection. Humans ingest eggs in material contaminated with human faeces, often from another person rather than from a tapeworm infection of their own, which explains why vegetarians can contract cysticercosis. After passing through the gut wall, the parasite develops into cysts, usually in skeletal muscle, but also and more importantly in the brain (Fig. 24.13) or eye, causing convulsions or, if very heavily infected, cysticercotic encephalopathy. Diagnosis is by detecting specific antibody in serum or CSF (interestingly, there is a higher positivity rate in serum than in CSF), and visualizing cysts, preferably by MRI scan. Treatment is with albendazole or praziquantel under corticosteroid cover.
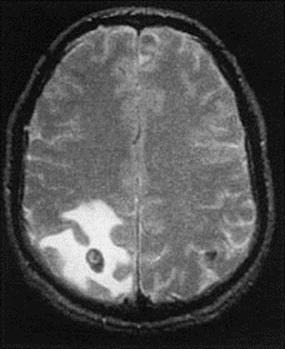
Figure 24.13 Cerebral cysticercosis. Magnetic resonance imaging scan showing a cyst containing a developing larva.
(Courtesy of J. Curé.)
Brain abscesses
Brain abscesses are usually associated with predisposing factors
Since the development of antibiotics, brain abscesses have become rare and usually follow surgery or trauma, chronic osteomyelitis of neighbouring bone, septic embolism or chronic cerebral anoxia. They are also seen in children with congenital cyanotic heart disease in whom the lungs fail to filter off circulating bacteria. Acute abscesses are caused by various bacteria, generally of oropharyngeal origin, including anaerobes. There is usually a mixed bacterial flora. Chronic abscesses may be due to Mycobacterium tuberculosis or C. neoformans. In immunosuppressed patients, opportunistic infection may occur with fungi and protozoan aetiologic agents.
Brain abscesses are diagnosed clinically and by CT and MRI brain scans. If an abscess is suspected, lumbar puncture is contraindicated but, if performed, generally shows raised CSF cells and proteins (see Table 24.1). Treatment is by surgical drainage if the abscess is well encapsulated, and antibiotics should be given for at least 1 month. Other infections that may manifest as chronic meningitis or brain abscess are summarized in Table 24.9.
Table 24.9 Infections causing chronic meningitis or brain abscess
|
Bacterial |
|
|
Tuberculosis |
Mycobacterium tuberculosis |
|
Syphilis |
Treponema pallidum |
|
Brucellosis |
Brucella abortus |
|
Lyme disease |
Borrelia burgdorferi |
|
Nocardiosisa |
Nocardia asteroides |
|
Actinomycosisa |
Actinomyces fumigatus |
|
Fungal |
|
|
Cryptococcosis |
Cryptococcus neoformans |
|
Coccidioidomycosis |
Coccidioides immitis |
|
Histoplasmosis |
Histoplasma capsulatum |
|
Candidiasis |
Candida albicans |
|
Blastomycosisa |
Blastomyces dermatitidis |
|
Parasitic |
|
|
Toxoplasmosisa |
Toxoplasma gondii |
|
Cysticercosisa |
Taenia solium |
a Disease manifest as brain abscess.
Tetanus and botulism
Several bacteria release toxins that act on the nervous system (see Ch. 17), but do not themselves invade the CNS. In the case of Clostridium tetani and Clostridium botulinum, the major clinical impact is neurologic.
Tetanus
Cl. tetani toxin is carried to the CNS in peripheral nerve axons
Tetanus spores are widespread in soil and originate from the faeces of domestic animals. The spores enter a wound, and if necrotic tissue or the presence of a foreign body permits local and anaerobic growth of bacteria, the toxin tetanospasmin (see Ch. 17) is produced. All strains of Cl. tetaniproduce the same toxin. The wound can be anything from a small gardener’s scratch or cut to that seen in a large automobile or battlefield injury. However, in as many as 20% of cases, there is no history of injury. Infection of the umbilical stump can cause neonatal tetanus, which killed nearly 5000 infants in 2009 worldwide (compared with nearly 30 000 in 1989), especially in resource-poor countries (see Ch. 23).
The toxin is carried in peripheral nerve axons and probably in the blood to the CNS, where it binds to neurones and blocks the release of inhibitory mediators in spinal synapses, causing overactivity of motor neurones. It can also pass up sympathetic nerve axons and lead to overactivity of the sympathetic nervous system.
Clinical features of tetanus include muscle rigidity and spasms
After a period of 3–21 days, but sometimes longer, there are exaggerated reflexes, muscle rigidity and uncontrolled muscle spasms. Lockjaw (trismus) is due to contraction of jaw muscles. Dysphagia, risus sardonicus (a sneering appearance), neck stiffness and opisthotonos (especially in neonatal tetanus; see Ch. 23) are also seen. Muscle spasms may lead to injury and eventually there is respiratory failure. Tachycardia and sweating can result from effects on the sympathetic nervous system. Mortality is up to 50%, depending on the severity and quality of treatment.
The diagnosis is clinical. Organisms are rarely isolated from the wound, and only a small number of bacteria are needed to form enough toxin to cause disease.
Human antitetanus immunoglobulin should be given as soon as tetanus is suspected clinically
The wound should be excised if necessary and penicillin given to inhibit bacterial replication. Muscle relaxants are used and, if necessary, respiratory support in an intensive care unit.
Immunization with toxoid prevents tetanus, the effects of the vaccine lasting for 10 years after the last dose. Thus, tetanus represents a vaccine-preventable disease that is unique in not being communicable but, instead, acquired from the environment as a result of exposure to Cl. tetanispores. Wounds should be cleaned, necrotic tissue and foreign bodies removed, and a tetanus toxoid booster given. Those with badly contaminated wounds should also be given tetanus immunoglobulin and penicillin.
In resource-poor countries, routine immunization of women with tetanus toxoid and improved hygienic birth practices are having a significant impact in reducing the rates of neonatal tetanus.
Botulism
Spores of Cl. botulinum are widespread in soil and contaminate vegetables, meat and fish. When foods are canned or preserved without adequate sterilization (often at home), contaminating spores survive and can germinate in the anaerobic environment, leading to the formation of toxin.
Cl. botulinum toxin blocks acetylcholine release from peripheral nerves
Preformed botulinus toxin is ingested, then absorbed from the gut into the blood (see Ch. 22). It acts on peripheral nerve synapses by blocking the release of acetylcholine (see Ch. 17). It is therefore a type of food poisoning that affects the motor and autonomic nervous systems. Sometimes spores contaminate a wound and the toxin is then absorbed from this site. If the organism is ingested by infants, in the honey smeared on pacifiers, for instance, it can multiply in the gut and produce the toxin, causing infant botulism.
Clinical features of botulism include weakness and paralysis
After an incubation period of 2–72 h, there is descending weakness and paralysis, with dysphagia, diplopia, vomiting, vertigo and respiratory muscle failure. There is no abdominal pain, diarrhea or fever. Infants develop generalized weakness (‘floppy babies’), but usually recover.
Botulism is treated with antibodies and respiratory support
A diagnosis of botulism is mainly clinical. The toxin can be demonstrated in contaminated food and occasionally in the patient’s serum.
Since the specific Cl. botulinum strain(s) responsible are normally unknown, trivalent antitoxin (for type A, B and E toxins) must be given promptly together with respiratory support. The mortality is < 20%, depending upon the success of the respiratory support.
Prevention is by avoiding imperfectly sterilized canned or preserved food. Contaminated cans are often swollen due to the release of gas by clostridial enzymes. Home-preserved foods are often incriminated, but fruit, with its acidic pH, usually prevents the development of the spores. The toxin is heat labile and is destroyed by adequate cooking, for example, boiling for 10 min. The spores can, however, survive boiling for 3–5 h.
![]()
 Key Facts
Key Facts
• Microbial invasion of the CNS is uncommon, due to the presence of the blood–brain and blood–CSF barriers, which limit the spread of infection.
• Once infectious agents have traversed these barriers, they generally cause neurologic disease by involving the meninges (meningitis) or the brain substance (encephalitis).
• Viral aetiology of meningitis is most common, followed by bacterial meningitis, with cerebral abscesses and viral encephalitis as rarities. The spinal cord (in myelitis) or peripheral nerves (in neuritis) are occasionally affected.
• Disease results from interference with the function of infected nerve cells (e.g. rabies), from direct damage to infected nerve cells (e.g. poliomyelitis), or from the inflammatory sequel to CNS invasion (e.g. bacterial meningitis, viral encephalitis).
• Because the anatomically defined compartments of the nervous system are adjacent or interconnected, more than one of them can be involved in a given infectious disease.
• CNS disease is sometimes seen in the helminth infections toxocariasis, hydatid disease and cysticercosis.
• CNS disease can also result when bacterial neurotoxins reach the CNS either from extraneural sites of growth (tetanus) or from contaminated food (botulism).
![]()