Medical Microbiology
Section 4 Clinical manifestation and diagnosis of infections by body system
28 Multisystem zoonoses
Introduction
Some multisystem infections in humans are animal diseases (i.e. zoonoses)
In these infections, a non-human vertebrate host is the reservoir of infection and humans are involved only incidentally. The human infection follows contact with or ingestion of infective material passed by an infected host, but is not essential for the microbe’s life cycle, or for its maintenance in nature. One striking feature of zoonotic infections, and of the arthropod-borne infections described in Chapter 27, is that few are transmitted effectively from human to human.
Sometimes, however, the zoonotic origin of these infections is less clear. For example, tularaemia can be acquired either by direct contact with the reservoir host or from an arthropod vector, and is included in this chapter. Plague is included because it is transmitted from infected rats via the rat flea, although it is also transmissible directly from human to human.
Other zoonoses are dealt with in their relevant chapters (e.g. toxoplasmosis in Chs 23–25, rabies in Ch. 24, salmonellosis in Ch. 22).
Arenavirus infections
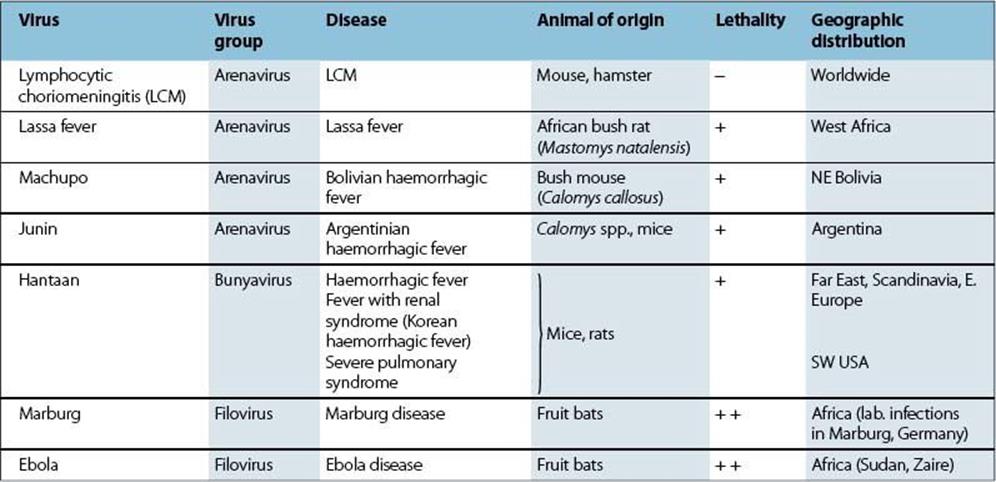
Arenaviruses are transmitted to humans in rodent excreta
Many zoonoses are caused by enveloped single-stranded RNA viruses with a genome consisting of two RNA segments called arenaviruses. On electron microscopy (Fig. 28.1) these pleomorphic virus particles with a diameter of 50–300 nm can be seen to contain ribosomes that have a sand-like granular appearance, giving rise to the name arena (Latin: arena, sand). Arenaviruses are carried by various species of rodent in which they cause a harmless lifelong infection with continuous excretion of virus in urine and faeces of apparently healthy infected animals. Humans may become infected via direct contact with infected rodents, inhalation of infectious excreta, working in agricultural environments or trekking in areas where the rodents exist, and may develop severe and often lethal disease involving extensive haemorrhaging and multiorgan involvement. A selection of arenaviruses and the diseases they cause are included in Table 28.1. Since 2007, nine new arenaviruses have been identified, some as a result of recombination events within one segment. They are divided into the Old and New World groups, of which the Old World viruses, Lassa fever and lymphocytic choriomeningitis virus (LCMV), are associated with the most common human infections involving this family. The distribution of the host is concordant with the distribution of the virus. LCMV is the only arenavirus with a worldwide distribution, the rest being seen in Africa or the New World. Of the New World Tacaribe serocomplex viruses, serious illness is associated with the Junin and Machupo viruses that cause Argentine and Bolivian haemorrhagic fevers, respectively. LCMV can cause acute central nervous system disease. As with most zoonoses, infection is not transmitted, or is transmitted with low efficiency, from human to human. However, healthcare workers have been infected by direct contact with blood or secretions from patients infected with Lassa fever virus, but this can be prevented by using barrier nursing techniques.
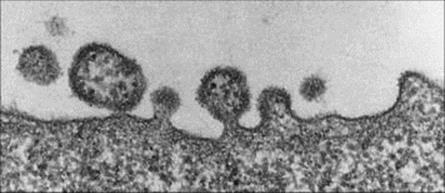
Figure 28.1 Electron micrograph of lymphocytic choriomeningitis virus budding from the surface of an infected cell. The sand-like granules in the virus particles are characteristic of arenaviruses.
(Courtesy of K. Mannweiler and F. Lehmann-Grübe.)
Table 28.1 Viral fevers and haemorrhagic diseases acquired from vertebrates or from unknown sources
Arenavirus infection is diagnosed by viral genome detection, serology or virus isolation
Diagnosis by testing for viral genome or specific antibodies, or by isolating viruses, can be carried out in special centres.
Prevention of infection by reducing exposure to the virus concerned was dramatically illustrated when rodent trapping terminated outbreaks of Bolivian haemorrhagic fever (Box 28.1). Treatment with the antiviral agent ribavirin has been successful if used early in Lassa fever infection. Post-exposure prophylaxis with oral ribavirin has been used. There are no World Health Organization-approved vaccines against arenaviruses. However, a live attenuated Junin virus vaccine was licensed in 2006 for use only in Argentina.
![]()
Box 28.1  Lessons in microbiology
Lessons in microbiology
Bolivian haemorrhagic fever: a lesson in ecology
In 1962, there was an outbreak of a severe and often lethal infectious disease in the small town of San Joachim, Bolivia. Patients developed fever, myalgia and an enanthem (internal rash), followed by capillary leakage, haemorrhage, shock and a neurologic illness. This disease was termed ‘Bolivian haemorrhagic fever’ and had a mortality rate of 15%. Extensive investigations failed to incriminate an arthropod vector, but the evidence pointed to a role for mice in the epidemic. Acting on this possibility, hundreds of mouse traps were airlifted to the beleaguered town, and it was soon shown that trapping mice had a dramatic effect on the incidence of the disease. The epidemic was completely halted. Quite separately, a virus was isolated from the tissues of a trapped local bush mouse (Calomys callosus). The virus was shown to cause a harmless lifelong infection in this animal, with continued excretion of virus in urine and faeces. The virus (given the name ‘Machupo’) was an arenavirus, a group that includes lymphocytic choriomeningitis (LCM) virus (infecting mice and hamsters) and Lassa fever virus (infecting an African bush rat). These viruses cause a harmless, persistent infection in the natural rodent host, but an often severe disease in humans exposed to infected animals.
This outbreak of Bolivian haemorrhagic fever provided an important lesson in ecology. Because of the high incidence of malaria in the San Joachim area, extensive DDT spraying had been carried out to control mosquitoes. As a result, geckos (small lizards that eat insects) accumulated DDT in their tissues and the local cats that preyed on geckos began to die with lethal concentrations of DDT in their livers. The shortage of cats, in turn, allowed the bush mice to invade human dwellings. The close vicinity of infected mice to humans and human food led to the epidemic (Fig. 28.2).
![]()
Lassa fever virus is an arenavirus that occurs naturally in bush rats in parts of West Africa
Infection arising from human exposure to infected rats, Mastomys natalensis, or their urine results in a febrile disease, which is generally not very severe. Viral entry into host cells is directed by a fusion glycoprotein sited in the viral outer lipid envelope. The cellular receptor for Lassa fever and certain other arenaviruses is α-dystroglycan, a membrane protein found in the mast cells, that anchors the cytoskeleton and the extracellular matrix. There are about 300 000 cases with 5000 deaths/year, and Lassa fever is the commonest febrile illness in hospitals in parts of Sierra Leone. Transfer of virus from hospital patient to healthcare worker via blood or tissue fluids can result in a more severe illness with high mortality. This involves haemorrhage, capillary damage, haemoconcentration and collapse, and was seen when the disease was first recognized in Americans in the village of Lassa in 1969. However, person-to-person transmission via droplet spread is thought to be rare. The usual incubation period is 5–10 days.
Outbreaks have been reported in Central Africa, Liberia, Nigeria and Sierra Leone. An outbreak in Sierra Leone, from January 1996 to April 1997, involved 823 cases with a mortality rate of 19%. The incubation period would allow an infected individual to carry the disease anywhere in the world and, indeed, there have been cases imported into Europe and the USA. Therefore, Lassa fever must be considered in travellers from these endemic areas with fevers of unknown origin.
Lymphocytic choriomeningitis virus occurs worldwide
Lymphocytic choriomeningitis (LCM) has caused sporadic infection in people living in mouse-infested dwellings, and has been reported in children possessing apparently healthy, but infected hamsters. There is generally a non-specific febrile illness, but occasionally an aseptic lymphocytic meningitis occurs, with recovery.
Haemorrhagic fever with renal syndrome (HFRS)
The Hantaan and Seoul viruses infect rodents and cause HFRS in Asia
The Hantaan and Seoul viruses are bunyaviruses that causes a harmless persistent infection in various species of mice and rats. They differ from other bunyaviruses as the latter are transmitted by arthropod vectors. After exposure to the urine of infected animals, there is a febrile illness, often with hypotension, haemorrhage and a renal syndrome. Many American soldiers suffered severe infections in Korea, and a milder disease is seen in Eastern Europe and Scandinavia. Related viruses are present in mice and rats in the USA, and outbreaks in the southwestern USA caused 26 deaths with severe pulmonary disease. The latter is called hantavirus cardiopulmonary syndrome and has been reported in the Americas as a result of Sin Nombre virus infection. In Europe, Puumala virus causes a mild form of HFRS known as nephropathia epidemica. Laboratory diagnosis is by molecular and serological methods detecting viral RNA or specific IgM or IgG antibody, respectively.
Marburg and ebola haemorrhagic fevers
Fruit bats are the reservoir for Marburg and Ebola viruses
Marburg and Ebola haemorrhagic fevers occur in central and east Africa and are caused by filoviruses, long filamentous single-stranded RNA viruses. Patients develop fever, haemorrhage, rash and disseminated intravascular coagulation (see Ch. 17). There is no specific treatment and no vaccine for either virus. The reservoir of origin and natural cycle of maintenance for Marburg virus was not known until Marburg virus RNA was detected in cave-dwelling fruit bats after a small outbreak of Marburg haemorrhagic fever was seen in some miners in Uganda in 2007. A fruit bat reservoir was also found for the Zaire Ebola virus, one of five Ebola virus species.
Infection with Marburg virus was first recognized in 1967 in Marburg, Germany, after exposure of laboratory workers to infected African green monkeys from Uganda. However, these monkeys are not the natural hosts. Mortality was about 20% and, as with Ebola virus infection, it was noted that the virus could be detected in semen for months after clinical recovery; one patient transmitted the infection to his wife by this route.
Outbreaks of a similar disease occurred in 1976 in southern Sudan and in the region of the Ebola River in Zaire (now Democratic Republic of the Congo). Overall, there were 602 cases and 397 deaths. Person-to-person transmission took place in local hospitals via contaminated syringes and needles, burial preparations and, rarely, sexual contact. The virus enters through mucous membranes or abraded skin. Infection does not occur through aerosol transmission. In 1989, monkeys infected with a similar virus were inadvertently imported into the USA from the Philippines. A number of the monkeys died but, although at least four people were infected, none developed disease. In 2004–2005, there was a large outbreak in Angola with a high mortality rate.
A large epidemic was seen in Kikwit, Zaire, in 1995, with 315 cases and 244 deaths. Gabon had three epidemics between 1994 and 1997 and the disease appeared in northern Uganda in 2000. A major outbreak in Congo-Brazzaville in 2003 claimed more than 100 lives, also killing many gorillas and chimpanzees.
Ecological niche modelling models have been used to predict where one might expect to find these filovirus infections. Interestingly, Ebola mapped to the broadleaf tropical rainforest and humid areas in equatorial Central Africa and parts of West Africa (although Angola did not fit this model). Marburg, however, mapped to the opposite, drier, more open areas away from the equator. In these models, bats were thought to be the potential reservoir hosts. Subsequently, tropical rain forest fruit bats were identified as the Ebola virus reservoir.
There is no treatment or post-exposure prophylaxis options for Ebola or Marburg virus infections.
Crimean–congo haemorrhagic fever, a tick-borne virus
Crimean–Congo haemorrhagic fever (CCHF), a severe haemorrhagic fever, with shock and disseminated intravascular coagulation, was described clinically during a large outbreak in the Crimea, part of the former Soviet Union, in 1944. The CCHF virus of the Bunyaviridae family,Nairovirus genus, was identified in 1967 and has a wide geographic range, including Africa, Asia, Central and Eastern Europe and the Middle East. It is transmitted by the bite of Ixodid ticks (both reservoir and vector), by contact with infected animals or person to person by exposure to infected body fluids including blood. A number of nosocomial outbreaks have been reported around the world. Although mortality rates of up to 80% have been reported, supportive management and the use of ribavirin have been shown to be effective.
Q Fever
Coxiella burnetii is the rickettsial cause of Q fever
The disease Q fever was first recognized in Australia in 1935, but the cause was unknown for several years – hence Q (‘query’) fever. The causative rickettsia, Coxiella burnetii, differs from other rickettsiae (see Ch. 27) in the following ways:
• It is not transmitted to humans by arthropods.
• It is relatively resistant to desiccation, heat and sunlight, and is therefore stable enough to be acquired from infected material by inhalation.
• Its main site of action is the lung rather than vascular endothelium elsewhere in the body, so that there is usually no rash.
C. burnetii is transmitted to humans by inhalation
C. burnetii can infect many species of wild and domestic animals. In many countries (e.g. USA) infection of livestock is quite common, but there are few human cases (132 reported in 2008 in the USA). Large seasonal Q fever outbreaks occurred in the Netherlands between 2007–2009. Infected dairy goat farms were the source of infection. More than 3500 human infections were notified over that time period. The southern part of the Netherlands was most affected, with >12% of the population found to have C. burnetii antibodies. People who come into contact with infected animals, especially their placentas (e.g. veterinarians, farmers, abattoir workers) are at risk from aerosolized organisms. Unpasteurized milk, tissue fluids and dust from infected stock can also transmit the disease.
After inhalation, the microbe multiplies in the terminal airways of the lung, and about 3 weeks later the patient develops fever, severe headache, and often respiratory symptoms and an atypical pneumonia. The rickettsia can also spread to the liver, commonly causing hepatitis. Recovery is usually complete in 2 weeks, but the disease can become chronic. The heart is then sometimes involved (endocarditis), with thrombocytopenia and purpura in some patients, and this condition is fatal if untreated.
Q fever is diagnosed serologically and treated with antibiotics
Polymerase chain reaction (PCR) can be used to determine whether a patient has Q fever; however, the sensitivity of this approach decreases after the first week of illness. C. burnetti cannot be detected in blood cultures and cannot be isolated by culture except in specialized laboratories. Thus, serological diagnosis is important. A fourfold or greater rise in complement fixing antibody titre is significant. There are two antigenic forms of the rickettsial lipopolysaccharide (LPS): phase 1 and phase 2. Increased antibody to phase 2 compared to phase 1 is seen in acute Q fever, while the reverse (higher antibody titres to phase 1 than phase 2) is seen in chronic disease. Definitive serological confirmation of acute Q fever is demonstrated by a fourfold increase in antibody titres measured by indirect immunofluorescence assay (IFA). The Weil–Felix test (see Ch. 27) is not used.
Acute infection is treated with oral tetracyclines; chronic infections may require drug combinations such as rifampin and doxycycline or trimethoprim-sulphamethoxazole. A killed vaccine is available for those at risk. The rickettsiae are destroyed when milk is pasteurized.
Anthrax
Anthrax is caused by Bacillus anthracis and is primarily a disease of herbivores
Bacillus anthracis is a large Gram-positive rod and is aerobic and non-motile. Most members of the genus Bacillus are harmless saprophytes, present in soil, water, air and vegetation. Bacillus cereus is a cause of food poisoning, but B. anthracis is the principal pathogen and is unique in having an antiphagocytic capsule made of D-glutamic acid. It forms spores, which survive for years in soil.
Anthrax is a disease of herbivores such as sheep, goats, cattle and horses, and bacilli are excreted in faeces, urine and saliva. Humans are relatively resistant, infection occurring following direct contact with infected animals, or by contact with spores present in animal products. The spores can enter the body via the skin and mucous membranes or, less commonly, via the respiratory tract. In resource-rich countries, where animal infection is now uncommon, human infection is rare and has been due to exposure to contaminated imported goods such as hides, skin, wool, goat hair and bristles, bones and bone-meal in fertilizers. Spores have also been used in bioterrorism.
Anthrax is characterized by a black eschar, and the disease can be fatal if untreated
B. anthracis spores germinate in tissues at the site of entry. The bacteria then multiply and produce the anthrax toxin, which consists of a protective antigen, an oedema factor (an adenylate cyclase) and a lethal factor; all are plasmid-coded. Toxic activity requires the protective antigen and at least one of the other two. Host defences are inhibited by the antiphagocytic capsule surrounding the bacillus (see Ch. 14).
The skin is the usual site of entry. As the toxic material accumulates, there is oedema and congestion, and a papule develops within 12–36 h. The papule ulcerates, the centre becoming black and necrotic to form an eschar or ‘malignant pustule’ (although there is no pus) which is painless and is often surrounded by a ring of vesicles (Fig. 28.3). The bacilli spread to the lymphatics and in about 10% of cases reach the blood to cause septicaemia. Continued multiplication and production of the toxin causes generalized toxic effects, oedema and death.
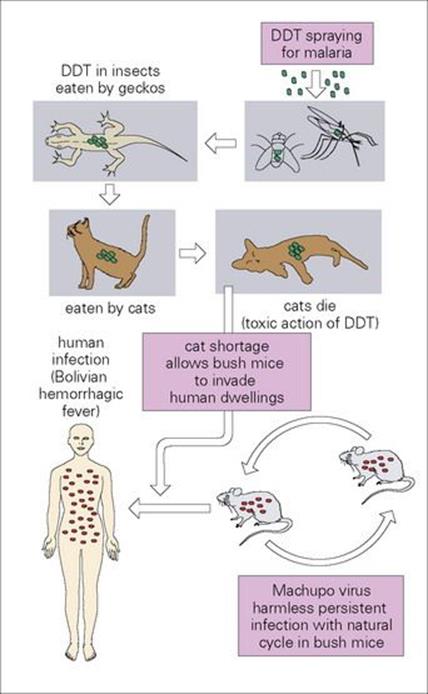
Figure 28.2 Bolivian haemorrhagic fever – a lesson in ecology. DDT, dichlorodiphenyltrichloroethane.
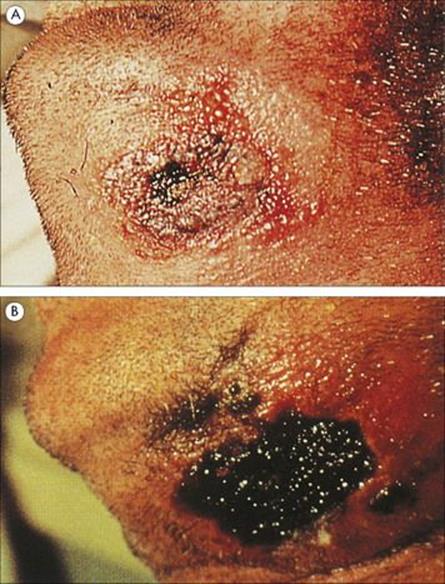
Figure 28.3 Anthrax. (A) Characteristic black eschar surrounded by a ring of vesiculation. (B) Some 8 days later the eschar has enlarged to cover the previously vesicular area, and the surrounding oedema has diminished.
(Courtesy of F.J. Nye.)
When the spores are inhaled and enter alveolar macrophages, bacterial growth in the lung leads to pulmonary oedema and mediastinal haemorrhage, with spread to the blood and death. Pulmonary anthrax is now very rare in most resource-rich countries, where it was referred to as ‘woolsorter’s disease’.
Anthrax is diagnosed by culture and treated with penicillin
Films from skin lesions show Gram-positive bacilli, but diagnosis can be confirmed and the organism distinguished from non-pathogenic bacilli by culture on blood agar or by PCR assay. Antibodies to toxin antigens indicate presence of the bacillus.
Anthrax is successfully treated by ciprofloxacin. Cutaneous anthrax is fatal in 20% of cases when untreated.
Anthrax, as a natural infection, is now mainly confined to resource-poor countries. Vaccines are available. Bioterrorism is an important threat
The disease is largely confined to resource-poor countries (parts of Asia, Africa, Middle East).
Animals can be protected by vaccination with live avirulent bacteria. Infected animals are isolated, killed and buried or cremated without autopsy. A vaccine consisting of purified protective antigen is available for humans at high risk. Human infection is reduced by rigidly controlled disinfection of imported animal products such as hides, hair and wool.
Anthrax is one of the three bacteria categorized by CDC as high-priority bioterrorism threats. Spores sent by mail infected 22 people in the USA in 2001 and this generated renewed interest in antimicrobial post-exposure control (e.g., fluoroquinolone plus doxycycline).
Plague
The plague is caused by Yersinia pestis, which infects rodents and is spread from them by fleas to humans
Yersinia pestis is a small Gram-negative rod with a surrounding antiphagocytic capsule that is associated with virulence. The sylvatic reservoirs are rodents such as rats, squirrels, gerbils and field mice, in which the infection is generally mild, the bacteria being spread between animals and to humans by fleas (Fig. 28.4). Infections in urban rats have been the most important sources of plague in humans, and the disease has at times decimated populations and influenced the course of history. In the fourteenth century, about 25% of the population of Europe died in plague epidemics (Box 28.2). Early in the twentieth century, the disease arrived in North America and is at present endemic in wild rodents in western USA. Plague in humans is now extremely rare in Europe and uncommon in the USA.

Figure 28.4 The epidemiology of plague.
![]()
Box 28.2  Lessons in Microbiology
Lessons in Microbiology
The Black Death in fourteenth-century England
For thousands of years, Yersinia pestis has been endemic in rodents in the Far East, with occasional epidemic spread into Europe and elsewhere. In January 1348, three galleys laden with spices from the East brought the plague to the port of Genoa, Italy. The disease, for reasons that are not clear, became known as ‘The Black Death’ and soon spread to the rest of Europe, arriving in London in December 1348. To the medieval mind, the speed and violence with which the illness passed from person to person (in the pneumonic form in the winter) was its most terrifying feature. The bubonic form was also important, especially in the warmer summer months, there being at least one family of black rats per household and three fleas to a rat.
The disease was attributed to earthquakes, to the movement of the planets, to a Jewish or Arab plot (350 massacres of Jews took place during the Black Death in Europe), and most commonly to God’s punishment for human wickedness. One could become infected without touching a plague victim, and to many it seemed that there was something – a miasma or a poison – in the air. Physicians wore strange masks, and infected houses were labelled and boarded up, together with the inhabitants. But it was impossible to isolate all those who were sick. Rich and poor perished.
The population of England was about 4 million, and over a period of 2.5 years, approximately 35% (more than 1 million) died. The clergy, for unknown reasons, suffered an even greater mortality of nearly 50%. Altogether in Europe, at least 25 million people died. The Black Death was a major human disaster, with lasting effects on economic and social structure. There were a further five, less severe, outbreaks in England in the fourteenth century. The epidemic in 1665, the year before the Great Fire of London, was graphically described by Daniel Defoe (who was only 5 years old at the time) in his Journal of a Plague Year in London. The last pandemic arose in China and reached Hong Kong in 1894, where Yersin and (independently) Kitasato described the causative bacillus.
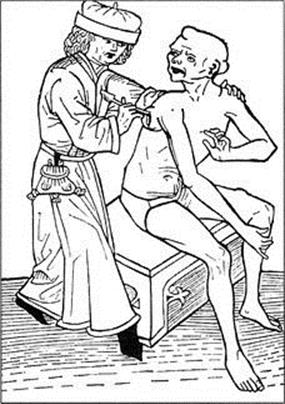
Figure 28.5 Fifteenth-century German woodcut showing incision of a bubo.
(Courtesy of the World Health Organization.)
![]()
The rat flea (Xenopsylla cheopis) carries infection from rat to rat and from rat to human. Y. pestis causes blood to clot in the gut of the flea, multiplies profusely in the clot and eventually blocks the gut lumen, so that the flea regurgitates infected material as it attempts to feed. As infected rats sicken, their fleas leave and may bite humans, thus transmitting ‘bubonic’ plague. This disease is not generally transmitted from person to person. However, when there is extensive replication of bacteria in the lung, with bronchopneumonia and large numbers of bacteria in the sputum, the infection can spread from person to person by droplets, causing ‘pneumonic’ plague, with extremely rapid onset.
Rodent infection is endemic in India, SE Asia, central and southern Africa, South America, Mexico and the western states of the USA. Sporadic plague continues to occur in these parts of the world, for instance, in over an 8-week period in 2010, 31 cases of plague were reported in Peru leading to three deaths in a province containing important export harbours. In 2009, a plague outbreak in a farming area of northwestern China resulted in three deaths.
Clinical features of plague include buboes, pneumonia and a high death rate
The infecting bacteria multiply at the site of entry in the skin, and spread via the lymphatics to local and regional lymph nodes. They produce a number of virulence factors, including an antiphagocytic capsular antigen (fraction 1, coded by a plasmid), endotoxin and various other protein toxins. Lymph nodes in the armpit or groin become very tender and enlarge to form ‘buboes’ with haemorrhagic inflammation 2–6 days after the flea bite. The patient develops fever. In mild forms, the infection is arrested at this stage, but spread to the blood often occurs, with septicaemia, haemorrhagic illness and multisystem involvement (spleen, liver, lungs, CNS).
Common complications are disseminated intravascular coagulation, pneumonia and meningitis. The death rate is about 50% in untreated bubonic plague, and nearly 100% in pneumonic plague. On recovery, there is solid immunity, and bacteria are eliminated from the body.
Plague is diagnosed microscopically and treated with antibiotics
Organisms can be recovered in fluid aspirated from lymph nodes, or from sputum in pneumonic plague and stained with Giemsa, Gram or fluorescent antibody (the staining is bipolar); they can also be cultivated. Streptomycin is the standard treatment; doxycycline or ciprofloxacin are also used.
Plague has been prevented by the following measures:
• classically, by quarantine measures in ports and on ships
• by rodent control, especially of rats at the site of entry of ships and aircraft into plague-free countries
• by strict isolation of patients with plague
• by chemoprophylaxis (doxycycline) during an epidemic or visit to an affected area
• by vaccination of military personnel and of certain workers in endemic areas.
An older vaccine formulation consisting of formalin-killed bacteria has been replaced by efforts to develop a more effective (recombinant) formulation.
Yersinia enterocolitica infection
Yersinia enterocolitica is a cause of diarrheal disease (see Ch. 22) and is mentioned here because it has a reservoir in rodents, rabbits, pigs and other livestock.
Tularaemia
Tularaemia is caused by Francisella tularensis and is spread by arthropods from infected animals
Tularaemia is caused by the small Gram-negative rod Francisella tularensis, first isolated from rodents in Tulare County, California, in 1912 and later shown by Edward Francis to cause human disease. It is present in rodents and in a wide variety of other wild animals in many countries in the northern hemisphere, including the USA (especially Arkansas and Missouri), Russia, Scandinavia and Spain, and can occur in contaminated water. The variety found in North America causes a more severe disease than that found in Europe and Asia. In the infected animal, it causes a plague-like disease and is spread via ticks, mites, lice and biting flies. In Dermacentor ticks, the bacteria are transmitted vertically by infected female ticks to her offspring via the ovum. Human infection is sporadic, the normal means of infection being contact with the carcass of an infected animal (e.g. skinning of hares, rabbits, muskrats) or the bite of an arthropod vector. There is no spread from person to person.
Clinical features of tularaemia include painful swollen lymph nodes
F. tularensis parasitizes the reticuloendothelial system and lives intracellularly in macrophages, inhibiting phagosome–lysosome fusion. It spreads at the site of entry, aided by an antiphagocytic capsule, and after 3–5 days forms a skin ulcer. There is a febrile illness, and lymphatic spread results in swollen painful regional lymph nodes. Blood invasion and involvement of lungs, gastrointestinal tract and liver is not uncommon, with the formation of granulomatous nodules around infected reticuloendothelial cells. There may be a rash. Mortality in untreated patients is 5–15%. The conjunctiva or oral mucosa can be infected via contaminated fingers, resulting in ocular or oral manifestations. Infection by inhalation is less common and gives a febrile illness with respiratory symptoms.
Tularaemia is diagnosed clinically and serologically. Streptomycin is the drug of choice, although other antimicrobials have been used (doxycycline and gentamicin)
Infected tissues can be examined by fluorescent antibody staining, but isolation of bacteria is not often attempted, because of the high risk of laboratory infection. Antibody tests are more commonly used in diagnosis.
Streptomycin is an effective treatment. A live attenuated bacterial vaccine is available for people with an occupational risk (e.g. fur trappers) but has issues of toxicity and incomplete protection, prompting efforts to develop a more effective preparation. Handling animals with gloves, particularly when skinning or eviscerating, gives protection, and contact with ticks should be avoided.
Pasteurella multocida infection
Pasteurella multocida is part of the normal flora of cats and dogs and is transmitted to humans by an animal bite or scratch
Pasteurella multocida is an encapsulated Gram-negative rod and is distributed worldwide. A number of capsular types exist. It is part of the normal oral flora in cats, dogs and other domestic and wild animals, in which it can also cause pneumonia and septicaemia. It is transmitted to humans by animal bites (especially cat bites) or scratches.
P. multocida infection causes cellulitis, is diagnosed by microscopy and treated with amoxicillin/clavulanate
Local multiplication of bacteria leads within a day or two to cellulitis and lymphadenitis; other types of bacteria including anaerobes are often present in the lesion. Infection can become systemic in patients with compromised immune systems. Virulence factors include endotoxin and the capsule.
P. multocida can be cultivated and identified in material from the wound.
Amoxicillin/clavulanate is an effective treatment, and has also been used in prophylaxis after cat or dog bites. Bite wounds should be cleansed and debrided.
Leptospirosis
Leptospirosis is caused by the spirochete Leptospira interrogans, which infects mammals such as rats
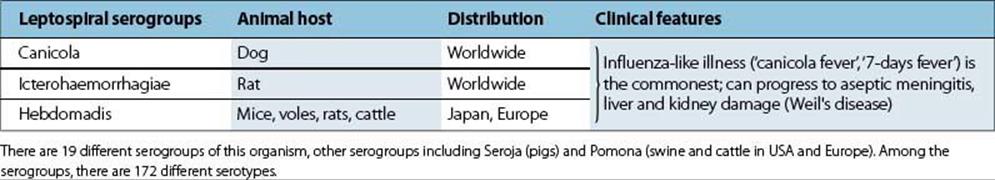
Leptospires are tightly coiled spirochetes 5–15 μm long. They show active rotational movement and have two flagella, originating at each end but located within the cell as in Borrelia. Their delicate outline is best seen by dark field microscopy because they are not very well stained by dyes. There are many species, each with several serotypes. The biflexa complex is free-living, the interrogans complex is pathogenic. The ends of L. interrogans are bent into a question-mark shape, hence the specific name. This species infects many domestic and wild mammals in various parts of the world (Table 28.2), dogs and rats being important sources of infection. Infected animals develop a chronic kidney infection with excretion of large numbers of bacteria in urine. The spirochetes are soon killed on drying, heating and exposure to detergents or disinfectants, but they remain viable for several weeks in stagnant alkaline water or wet soil. Humans are infected by ingestion of, or exposure to, contaminated water or food. The bacteria, aided by their motility, enter through breaks in skin or mucosae, so infection can be acquired by swimming, working or playing in contaminated water. Therefore, miners, farmers, sewage workers, and water sports enthusiasts are especially at risk. There are about 50 cases/year in England and Wales, and about 100/year are reported in the USA. Bacteria are excreted in human urine, but person-to-person transmission is rare. Immunity is serotype specific.
Table 28.2 Disease caused by the three main serogroups of the Leptospira interrogans complex
Clinical features of leptospirosis include kidney and liver failure
The bacteria reach the blood and, after an incubation period of 1–2 weeks, cause a febrile, influenza-like illness. In about 90% of cases, this resolves uneventfully, but multiplication can cause:
• hepatitis, jaundice and haemorrhage in the liver
• uraemia and bacteriuria in the kidney
• aseptic meningitis and conjunctival or scleral haemorrhage in the cerebrospinal fluid (CSF) and the aqueous humor (Fig. 28.6).
The main clinical signs result from damage to the endothelia of blood vessels, the clinical picture depending to some extent upon the particular type of leptospire involved. Weil’s disease, the severe form with haemorrhagic complications and kidney and liver failure, occurs in only 5–10% of patients with leptospirosis.
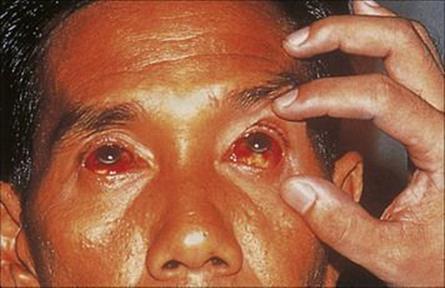
Figure 28.6 Conjunctival haemorrhages in a jaundiced patient with leptospirosis.
(Courtesy of D. Lewis.)
Leptospirosis is diagnosed by microscopy and serologic tests and treated with antibiotics
There is often a history of exposure. Bacteria can be isolated from blood, CSF and urine, and a rise in agglutinating serotype-specific antibody can be demonstrated.
Penicillin and doxycycline have been valuable in treatment when given within a day or two of the onset of illness, and doxycycline will prevent disease in those exposed to infection.
Measures for prevention include:
• rodent control
• protective clothing
• prophylactic penicillin after cuts and abrasions in those at risk.
Rat-bite fever
Rat-bite fever is caused by bacteria transmitted to humans by a rodent bite
This uncommon but worldwide condition is caused by one of two species: Spirillum minus, a Gram-negative spiral-shaped organism (spirillar fever), or Streptobacillus moniliformis, a Gram-negative filamentous bacillus (streptobacillary fever). These bacteria are found in the oropharyngeal flora of 50% of healthy wild and laboratory rats and also in other rodents. Transmission to humans is by biting.
Clinical features of rat-bite fever can include endocarditis and pneumonia
After an incubation period of 7–10 days there is an onset of fever, headache and myalgia. Bacteria multiply at the site of the bite, and in the case of S. moniliformis, cause an inflamed local lesion. Spread of infection to lymph nodes and the blood leads to lymphadenopathy, rash and arthralgia. Fever may be recurrent if untreated.
Complications include endocarditis and pneumonia, and there is a mortality of up to 10% in untreated patients.
Rat-bite fever is diagnosed by microscopy or culture and is treated with antibiotics
S. moniliformis can be cultured from the wound site, lymph nodes and blood, but Spirillum minus cannot be cultivated and must be demonstrated in tissues by dark field microscopy.
Penicillin and streptomycin are effective treatments.
Measures for prevention include:
• rodent control
• prevention of rat bites in laboratory workers.
Brucellosis
Brucellosis occurs worldwide and is caused by Brucella species
Brucellae are small Gram-negative non-motile coccobacilli, adapted to intracellular replication. Four ‘species’ cause disease in humans: Brucella abortus, B. melitensis, B. suis, B. canis, but, on the basis of DNA homology, these are all variants of B. melitensis. The first three share common A and M antigens (B. abortus primarily A and B. melitensis primarily M); B. canis is distinct.
Brucellae are primarily animal pathogens, infecting humans after contact with infected animals or their products (Fig. 28.7):
• B. abortus infects cows worldwide, but has been eliminated from several resource-rich countries. It causes mild disease in humans.
• B. melitensis infects goats and sheep and is common in Malta and other Mediterranean countries, Mexico and South America. It causes more severe disease in humans.
• B. suis infects pigs in the USA, South America, and SE Asia. It causes severe disease with destructive lesions in humans.
• B. canis infects dogs and is an uncommon cause of mild disease in humans.
In cows and goats, brucellae localize in the placenta, causing contagious abortion, and also in mammary glands, from where they are shed for long periods in milk. They are present in uterine discharges, faeces and urine.
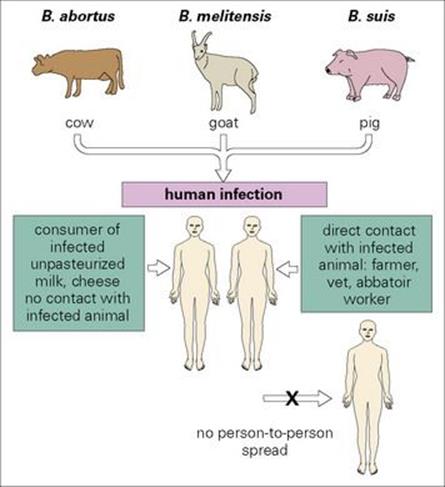
Figure 28.7 Transmission of brucellosis. Human infection follows contact with infected animals or consumption of infected animal products.
Human brucellosis (undulant fever, Malta fever) occurs when the bacteria enter the body via abrasions in the skin, via the alimentary tract or, most commonly, via the respiratory tract. Infection is therefore more common in farmers, veterinarians and abattoir workers. Unpasteurized cows’ milk (UK, USA), goats’ milk or cheese (Mediterranean countries) are less frequent sources of infection. There is no spread from person to person. Infection is common worldwide, but incidence is low in the resource-rich world.
Clinical features of brucellosis are immune-mediated and include an undulant fever and chronicity
The infecting bacteria pass from the site of entry into local and regional lymph nodes, the thoracic duct and thus the blood (septicaemic phase). Reticuloendothelial cells are infected (liver, spleen, bone marrow, lymphoid tissues) and here the bacteria can survive for prolonged periods. The result is an inflammatory (granulomatous) reaction with epithelioid and giant cells, central necrosis and peripheral fibrosis.
Quite commonly, the infection is subclinical. The symptoms of acute brucellosis begin after an incubation period of 2–6 weeks with a gradual onset of malaise, fever, drenching sweats, aching and weakness. A rising and falling (undulant) fever is seen in a minority of patients. Enlarged lymph nodes and spleen may be detected and hepatitis can occur (Fig. 28.8). The bone marrow lesions may progress to osteomyelitis, and cholecystitis, endocarditis and meningitis are occasionally seen. Abortion occurs in infected cows, sows and goats, but not in humans, who lack the sugar compound erythritol, which stimulates bacterial growth in the placenta.
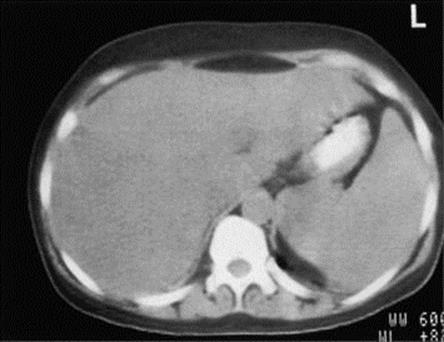
Figure 28.8 Computerized tomographic scan showing hepatosplenomegaly in Brucella melitensis infection.
(Courtesy of H. Tubbs.)
The patient generally recovers after a few weeks or months, but a chronic stage (more than 1 year’s illness) can develop with tiredness, aches and pains, anxiety, depression and occasional fever. Relapses and remissions may occur. Brucellae cannot be isolated at this stage, and chronic brucellosis is often a difficult diagnosis. Agglutinin titres are generally high, but antibodies are less relevant than cell-mediated immunity for this intracellular parasite.
Brucellosis is diagnosed by culture and by serologic tests and treated with antibiotics
Brucellae can be isolated in some cases from blood cultures (or from bone marrow or lymph nodes), and urine culture may be successful. This takes up to 4 weeks. IgM antibodies are present in acute brucellosis, IgG and IgA in chronic brucellosis. A rising titre suggests a current infection.
Brucellosis is typically susceptible to tetracycline and streptomycin; co-trimoxazole is also used. Because of the intracellular location of the bacteria, brucellosis is typically treated with combination therapy (e.g., doxycycline plus streptomycin) for a minimum of 6 weeks.
Brucellae in milk are destroyed by pasteurization. In the USA and UK, brucellosis has gradually declined (about 100 cases/year now reported in the USA) following eradication and control programmes. Protective clothing and goggles may be used by those in close contact with infected animals (farmers, veterinarians, abattoir workers). There is no satisfactory vaccine available for humans. Indeed, veterinarians may develop illness when accidentally infected with the live RB51 animal vaccine.
Helminth infections
Few helminth infections are true multisystem diseases
It is a somewhat arbitrary decision to include a particular helminth infection in a chapter on multisystem zoonotic infections. Many of the worm parasites that can be acquired from animals have stages that invade a number of the body systems. Others are primarily located in a particular organ, but cause pathologic changes that can be widespread in their effects. Conversely, although stages of certain worms may be widely distributed in the body, their pathologic effects are most commonly associated with a particular organ.
For example:
• The larvae of the pork tapeworm Taenia solium, which cause the disease cysticercosis, develop in a variety of tissues, including muscle. However, the most serious pathology is caused by larvae found in the CNS. Accordingly, this infection is discussed in Ch. 24.
• After infection with eggs of the dog nematode Toxocara canis, larvae migrate through the body, causing visceral larval migrans or ocular larva migrans. Again, the most serious effects are associated with larvae in the CNS (see Ch. 24) and the eye (see Ch. 25).
However, three helminths can be considered as genuinely multisystem in their effects. These are:
• the tapeworm Echinococcus granulosus
• the nematode Trichinella spiralis
• the nematode Strongyloides stercoralis.
Echinococcus
Echinococcus adults are tiny tapeworms in the small intestine of dogs or foxes, and their larvae cause hydatid cysts in humans. They cause two major types of echinococcosis, both of which result in significant human morbidity.
Echinococcus granulosus (cystic echinococcosis; cystic hydatid disease)
The adults of this species live as tiny (3 –5-mm long) tapeworms in the intestine of the dog. Eggs laid by the worm are passed in faeces, surviving for long periods. If swallowed (by sheep or accidentally by humans), the eggs hatch, releasing larvae which then penetrate the small intestinal mucosa to enter a blood vessel. Larvae then lodge in a capillary bed, most commonly in the liver, with the lung next most common, but any organ can potentially be affected. They then grow slowly into large, thick-walled, fluid-filled hydatid cysts, the resulting symptoms and signs being largely due to the mechanical pressure exerted by the cysts (Fig. 28.9).
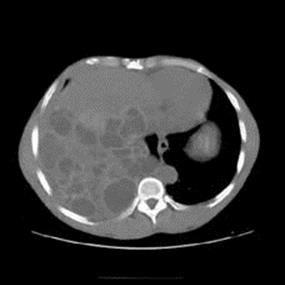
Figure 28.9 Extensive cystic hydatid disease of the liver
(courtesy of P. Chiodini).
Cystic hydatid disease is diagnosed by ultrasonography, CT or MRI scans and serologic tests assist diagnosis, but sensitivity and specificity are variable. Finding hooklets and protoscoleces in aspirated cyst fluid provides confirmation, but suspected hydatid cysts in the lung must never be aspirated. Treatment is according to WHO CE ultrasound classification (see bibliography). Depending on cyst type, therapy is with albendazole, plus praziquantel in some cases, with or without PAIR (Puncture, Aspiration, Injection, and Reaspiration) or open surgery. Dead cysts do not require treatment. Special care must be taken during aspiration or surgical removal to prevent leakage of fluid from the cysts. Not only may this trigger anaphylactic responses in sensitized individuals, but the numerous larvae present in the fluid (produced by asexual division) can cause local recurrence or metastatic infection in other sites.
Echinococcus multilocularis (alveolar echinococcosis; alveolar hydatid disease)
Echinococcus multilocularis results in the formation of a multilocular mass lesion consisting of hundreds of small vesicles. The parasite generally occurs as a fox–rodent cycle in China, North Europe, Siberia and parts of North America, and human infections occur via contamination by fox faeces. The macroscopic appearance is similar to that of a hepatic carcinoma. Almost all cases occur in the liver where the parasite leads to obstructive jaundice and weight loss. Treatment is with radical excision plus albendazole. Inoperable cases require life-long albendazole therapy. Liver transplantation is sometimes needed.
Trichinella
Trichinella spiralis is transmitted in undercooked pork and causes the disease trichinosis
T. spiralis is capable of infecting almost any warm-blooded animal. Its natural cycle involves predators (e.g. bears, seals) and their prey, or scavengers and the carrion they feed on, but a domestic cycle has become established in pigs and rats.
Humans are infected by eating undercooked meat (pork or wild animal) containing the encysted infected larval stages. These larvae mature rapidly into adults in the small intestine, their invasion of the mucosa causing an acute enteritis.
The clinical features of trichinosis are mainly immunopathologic in origin
Female worms release live larvae into the mucosa, which invade the blood vessels and become distributed around the body. The larvae attempt to invade the cells of many organs (including the heart and CNS), although they can mature only in striated muscles, where they form the characteristic cysts (Fig. 28.10). There is a wide spectrum of pathologic signs, such as fever, joint and muscle pains, eosinophilia, periorbital oedema, myositis, petechial haemorrhage; encephalitis and cardiac abnormalities may also occur. These signs are mainly caused by hypersensitivity and inflammatory responses.
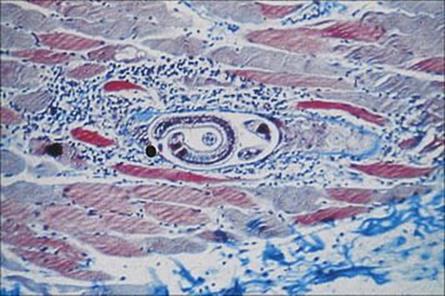
Figure 28.10 Inflammatory reaction around a nurse cell containing a coiled larva of Trichinella spiralis. Trichrome stain.
(Courtesy of I.G. Kagan.)
Trichinosis is diagnosed by microscopy and serologically and treated with anthelmintics and anti-inflammatories
Diagnosis of trichinosis is by muscle biopsy and demonstration of specific antibody by ELISA. Treatment is possible with benzimidazoles in the early stages of infection, but symptomatic treatment with anti-inflammatories is helpful during the muscle phase.
Strongyloides
Strongyloides infections are generally passed between humans, but can develop in animal hosts including dogs
Strongyloides infection is acquired by the penetration of infective larvae through the skin. The larvae migrate to the lung, enter the alveoli, pass up the bronchi and trachea, and are then swallowed. Only females develop parthenogenetically in the human host, and they lay strings of eggs into the intestinal mucosa (Fig. 28.11). The eggs hatch within the intestine to release larvae that pass out with the faeces and require warm moist soil to become infective. The geographic distribution of strongyloidiasis is similar to that of hookworm (tropical areas and in rural southern states of the USA).
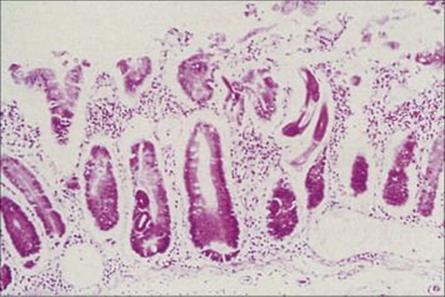
Figure 28.11 Strongyloides stercoralis. Adults and larvae in the mucosa of small intestine, showing disruption of the villous surface.
Infections are most often passed between humans, but the two species can also develop in animal hosts including dogs (S. stercoralis) and African primates (S. fulleborni). Faecal larval stages may develop directly into the infective stage and penetrate the mucosa or perianal skin to reinfect the host – the process of autoinfection.
Strongyloides infections are usually asymptomatic, but can cause disseminated disease in patients with immunodeficiency states or malnutrition
Many infected individuals are asymptomatic, though vomiting or diarrhea may occur. However, in immunodeficiency due to corticosteroid therapy, immunosuppression for transplantation, advanced malignancy, HTLV infection and malnutrition, autoinfection can lead to hyperinfection or disseminated strongyloidiasis, the larvae invading almost all organs and causing severe and sometimes fatal pathology. Infected patients may show vomiting, abdominal pain, diarrhea with malabsorption and dehydration, and pneumonitis. Eosinophilia is often absent in Strongyloideshyperinfection. Disseminated strongyloidiasis can arise long after initial infection. It has been firmly established that infections can persist for many years (> 30), being maintained by low-level autoinfection, then disseminate once the patient’s immune defences are reduced. HTLV-1 antibody testing should be advised in this setting, as the two infections are associated.
Strongyloides infection is diagnosed by microscopy and Strongyloides culture of faeces to detect larvae. Serology for IgG antibody to Strongyloides is helpful in migrants from endemic areas, but less sensitive in travellers. It may be negative in hyperinfestation.
Treatment of Strongyloides infection is with ivermectin. Thiabendazole is also effective but much less well tolerated by the patients.
![]()
 Key Facts
Key Facts
• The multisystem infections described in this chapter are zoonoses, being maintained naturally in a reservoir of non-human vertebrates.
• Humans are infected incidentally, generally from rodents (arenaviruses, hantaviruses, plague, tularaemia, leptospirosis) or from domestic animals (brucellosis, leptospirosis, trichinosis).
• There is generally no transmission from person to person (plague is an exception).
• The nature and the extent of human–animal contact are determining factors.
• Some of these infections are highly virulent.
• When the reservoir host is common in crowded human communities (e.g. plague), disease epidemics have been major events in history.
• When humans have less extensive contact with the reservoir host, the infection, even when virulent, has less impact (e.g. Lassa fever, Ebola fever).
• Most of these infections are now less frequent in resource-rich countries (e.g. anthrax, brucellosis, hydatid disease), but remain as frequent causes of disease in other parts of the world and thus may present in migrants from those regions.
• Anthrax is seen as a major bioterrorism threat.
• There are satisfactory antimicrobial agents for most of the non-viral infections, but effective vaccines are generally not available.
![]()