Medical Microbiology
Section 5 Diagnosis and control
36 Hospital infection, sterilization and disinfection
Introduction
Infections associated with healthcare settings are an increasingly complex issue
Amassing sick people together under one roof has many advantages, but some disadvantages, notably the easier transmission of infection from one person to another. In the past, the major environment for this interaction has been the hospital, which led to the term nosocomial infection (i.e. any infection acquired while in hospital). Increasing numbers of individuals in skilled nursing and homecare settings have prompted the more recent use of the term healthcare-associated infections (HAI). Nevertheless, hospitals remain the major environment associated with HAI. Hospital infection is generally defined as any infection acquired while in hospital (e.g. occurring 48 h or more after admission and up to 48 h after discharge). Most of these infections become obvious while the patient is in hospital, but some (e.g. postoperative wound infections) may not be recognized until after the patient has been discharged. Earlier discharges, encouraged to reduce costs, contribute to these unrecognized infections, although a shorter preoperative stay reduces the chance of acquiring hospital pathogens (see below).
Healthcare-associated infection may be acquired from:
• an exogenous source (e.g. from another patient – cross-infection – or from the environment)
• an endogenous source (i.e. another site within the patient – self- or auto-infection) (Fig. 36.1).
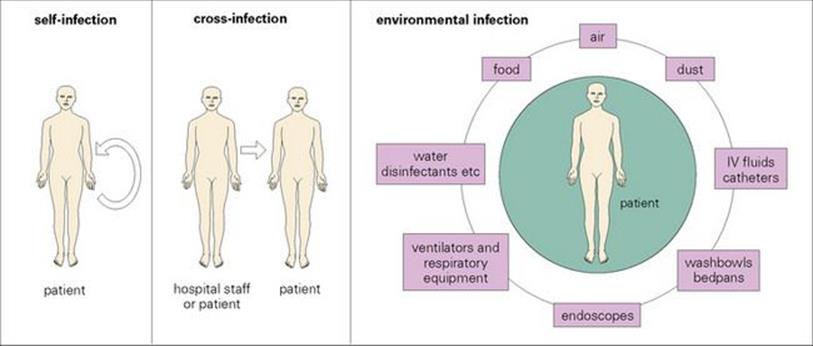
Figure 36.1 Hospital-acquired infection can be endogenous (i.e. self-infection from another site in the body) or exogenous (i.e. from another person or from an environmental source). The sorts of organisms acquired from environmental sources depend upon the nature of the source, e.g. moist areas tend to be colonized with Gram-negative rods (e.g. E. coli, Klebsiella, Pseudomonas) whereas air- and dust-borne organisms are those that can withstand drying (e.g. streptococci, staphylococci, mycobacteria and Acinetobacter). I, intravenous.
An infection that is incubating in a patient when he or she is admitted into hospital is not a hospital infection. However, community-acquired infections brought into hospital by the patient may subsequently become hospital infections for other patients and hospital staff.
Many hospital infections are preventable
In 1850, Semmelweiss demonstrated that many hospital infections are preventable when he made the unpopular suggestion that puerperal fever (an infection in women who have just given birth, see Ch. 23) was carried on the hands of physicians who came directly from attending an autopsy to the delivery ward, without washing. A death rate of 8.3% was reduced to 2.3% by introducing the simple measure of handwashing before and after any clinical examination. Recent studies in the USA suggest that about one-third of all infections acquired in hospital can be prevented. Current US estimates place HAI costs associated with hospital infection at approximately 2 million infections leading to nearly 100 000 deaths at a cost of US$4–5 billion annually.
Common hospital infections
Urinary tract infections are the most common hospital infections in adults
The infections most commonly acquired in hospitals are:
• surgical wound infection
• respiratory tract infection
• urinary tract infection (UTI)
• bacteraemia.
The relative frequencies of these infections are illustrated in Figure 36.2. Each may be acquired from an exogenous or endogenous source, and even the ‘self-source’ may be derived from outside by the patient who becomes colonized with pathogens during his or her stay in hospital. Bacteraemia may arise from a variety of sources and may be:
• primary – due to the direct introduction of organisms into the blood from, for example, contaminated intravenous fluids
• secondary to a focus of infection already present in the body (e.g. UTI).
Other infections that may cause outbreaks in the hospital setting include gastroenteritis and hepatitis.
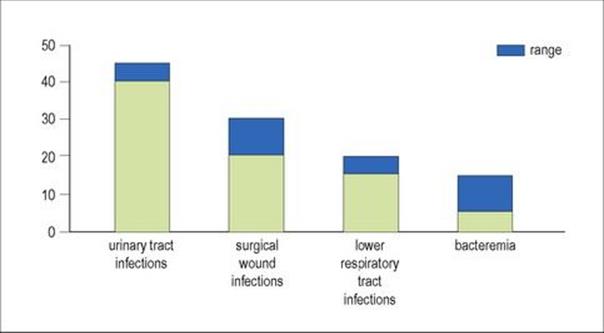
Figure 36.2 The relative frequencies of different kinds of hospital infection vary in different patient groups, but urinary tract infections are the most common hospital-acquired infections.
Important causes of hospital infection
Staphylococci and Escherichia coli are the most important Gram-positive and Gram-negative causes of infection, respectively, in hospitals
Almost any microbe can cause a hospital infection, though protozoal infections are rare. The pattern of hospital infection has changed over the years, reflecting advances in medicine and the development of antimicrobial agents. In the pre-antibiotic era, the majority of infections were caused by Gram-positive organisms, particularly Streptococcus pyogenes and Staphylococcus aureus. With the advent of penicillin and other antibiotics active against staphylococci, Gram-negative organisms such as Escherichia coli and Pseudomonas aeruginosa emerged as important pathogens. More recently, the development of more potent and broad-spectrum antimicrobials and the increase in invasive medical techniques has been accompanied by an increase in the incidence of:
• antibiotic-resistant Gram-positive organisms such as coagulase-negative staphylococci, enterococci (especially those resistant to vancomycin; VRE) and methicillin-resistant Staph. aureus (MRSA)
• multidrug-resistant Gram-negative organisms including those producing expanded-spectrum beta-lactamases (ESBLs, see Ch. 33)
• Candida.
Many of these organisms are considered as ‘opportunists’ – microbes that are unable to cause disease in healthy people with intact defence mechanisms, but that can cause infection in compromised patients or when introduced during the course of invasive procedures. Currently, coagulase-negative staphylococci, Staphylococcus aureus, and enterococci account overall for most healthcare-associated infections (Box 36.1).
![]()
Box 36.1  Order of Pathogen Importance
Order of Pathogen Importance
The general rank order of pathogen importance is listed for the different infection categories. Although a few species are the most important in all kinds of hospital infection, predominant pathogens vary in different infections. Staphylococcus aureus is very important in surgical wound infections and bacteraemia, but much less important in urinary tract infections. The importance of Gram-negative rods has increased since the advent of broad-spectrum antibiotics because these organisms often carry multiple antibiotic resistances.
Urinary tract infections
• E. coli
• Candida
• Enterococci
• other Gram-negatives (e.g., P. aeruginosa, K. pneumoniae, Enterobacter spp.)
Surgical wound infections
• Staphylococci (Staph. aureus and coagulase-negative)
• Enterococci
• E. coli, P. aeruginosa (other Gram-negatives to a lesser extent)
Lower respiratory tract infections
• Staph. aureus
• P. aeruginosa (other Gram-negatives to a lesser extent)
Bacteraemia
• Staphylococci (Staph. aureus and coagulase-negative)
• Enterococci
• Candida
• K. pneumoniae (other Gram-negatives to a lesser extent)
![]()
Some infections historically associated with hospitals are now increasingly seen outside of the healthcare setting
Recent reports in numerous countries have documented the emergence of virulent MRSA strains causing infection in individuals outside of the healthcare system. These community-associated MRSA (CA-MRSA) can be transported into the healthcare environment, thus blurring the distinction between community-associated and healthcare-associated infection. This has prompted guidelines for differentiating the increasing number of CA-MRSA infections from those associated with healthcare, summarized in Box 36.2.
![]()
Box 36.2  Criteria for Distinguishing Community-Associated MRSA (CA-MRSA) From Healthcare (Including Hospital)-Associated MRSA (HA-MRSA)
Criteria for Distinguishing Community-Associated MRSA (CA-MRSA) From Healthcare (Including Hospital)-Associated MRSA (HA-MRSA)
Individuals with MRSA infections that meet all of the following criteria likely have CA-MRSA infections:
• Diagnosis of MRSA was made in the outpatient setting or by a culture positive for MRSA within 48 h after admission to the hospital
• No medical history of MRSA infection or colonization
• No medical history in the past year of:
• Hospitalization
• Admission to a nursing home, skilled nursing facility, or hospice
• Dialysis
• Surgery
• No permanent indwelling catheters or medical devices that pass through the skin
![]()
Viral infections probably account for more hospital infections than previously realized
These affect both patients and healthcare workers and include:
• viruses acquired by the respiratory route, especially influenza, respiratory syncytial virus (RSV), parainfluenza, varicella-zoster virus (VZV); this may also include some of the viral causes of gastroenteritis
• viruses acquired by contact with vesicular lesions such as VZV and herpes simplex virus (HSV)
• viruses acquired by contact with contaminated fomites such as noroviruses and rotavirus
• viruses acquired by contact with blood-contaminated fomites, needlestick injury or splash on mucous membranes, such as hepatitis B virus (HBV), hepatitis C virus (HCV), HIV and human T-cell lymphotropic virus (HTLV). These may also be acquired in countries where blood and blood products are not screened or in the rare instance where the blood donor was in the early incubation period of infection, thereby escaping detection by the screening assay. The latter is referred to as the window period and may be missed even if a viral genome detection method is used.
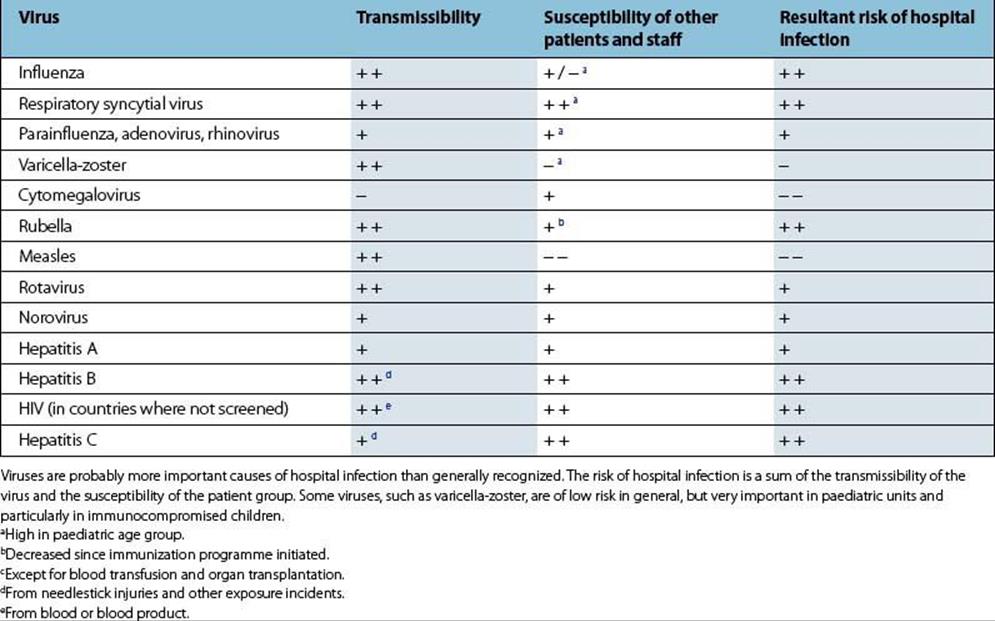
The risks of viral infections in hospital are summarized in Table 36.1.
Table 36.1 Examples of viruses causing hospital-acquired infection
Sources and routes of spread of hospital infection
Sources of hospital infection are people and contaminated objects
As stated above, the source of infection may be:
• human from other patients or hospital staff, and occasionally visitors
• environmental from contaminated objects (‘fomites’), food, water or air (see Fig. 36.1).
The source may become contaminated from an environmental reservoir of organisms, for example, contaminated antiseptic solution distributed for use into sterile containers (Fig. 36.3). Eradication of the source will also require eradication of the reservoir.
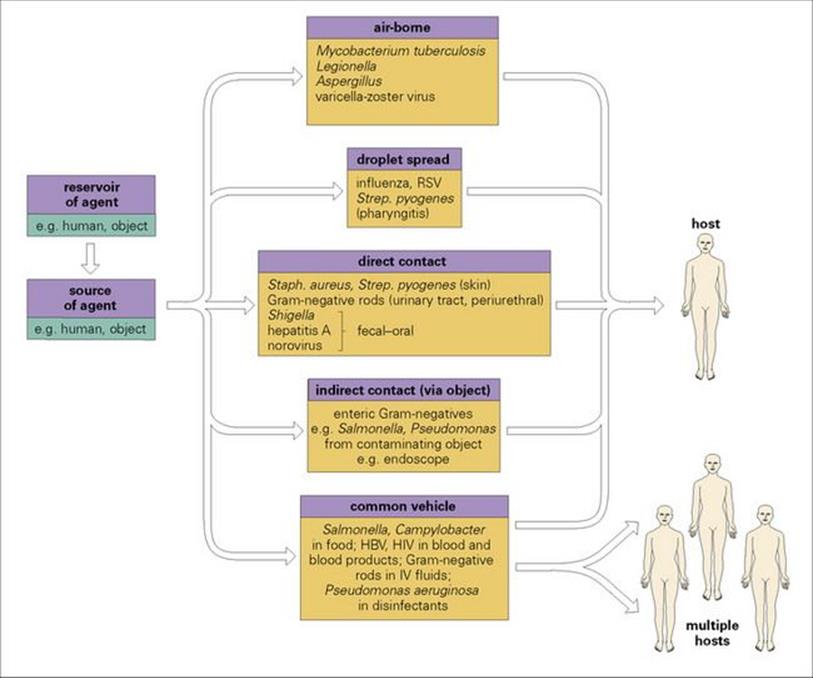
Figure 36.3 Hospital infections are spread by the same routes as infections spread in the community. The reservoir and the source of infection may be human or inanimate and may be one and the same (e.g. a nurse with an infected skin lesion). If the reservoir and source are distinct (e.g. contaminated distilled water supply used to prepare a variety of pharmaceuticals), both must be eliminated if the spread of infection is to be halted, otherwise the reservoir may continue to contaminate new sources. HBV, hepatitis B virus; IV, intravenous; RSV, respiratory syncytial virus.
Human sources may be:
• people who are themselves infected
• people who are incubating an infection
• healthy carriers.
The time period for which a human source is infectious varies with the disease (see Ch. 31). For example, some infections can be spread during their incubation period, others in the early stages of clinical disease, while others are characterized by a prolonged carrier state even after clinical cure (e.g. typhoid fever) (Fig. 36.4). Carriers of virulent strains of, e.g. Staph. aureus or Strep. pyogenes, may act as sources of hospital infection, although they themselves do not develop clinical disease. The carrier state may persist for a long time and go unnoticed unless there is an outbreak or, depending on the significance of the organism, a single case of infection that is traced to the carrier, e.g. a healthcare worker with chronic hepatitis B.
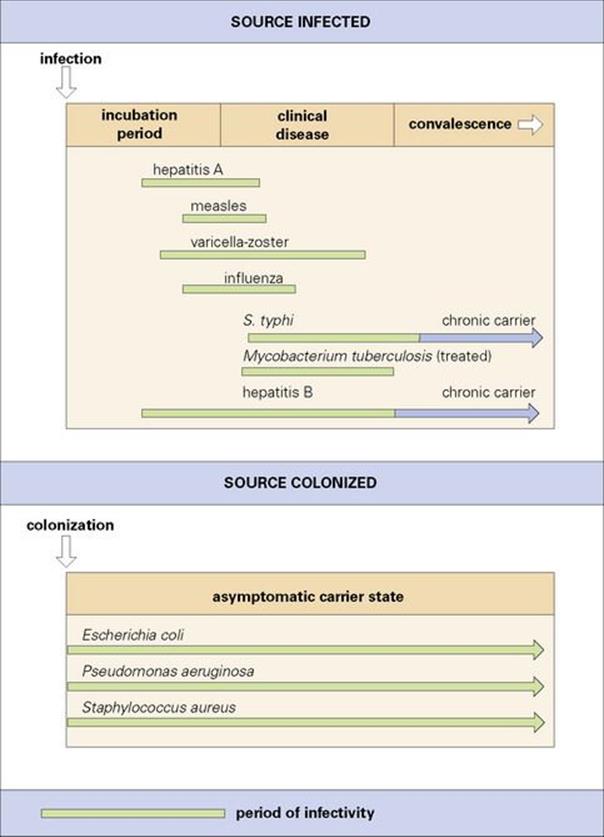
Figure 36.4 Pathogens differ in the time periods for which they can be disseminated from an infected person. For some, it is during the incubation period when infected people may not realize they are ill and infectious. Some people continue to carry organisms such as Salmonella typhi and hepatitis B virus long after they have recovered from the clinical disease. Opportunist pathogens are often members of the normal flora and may therefore be carried for long periods without the host experiencing any adverse effects.
Hospital infections are spread in the air and by contact and common vehicle
The important routes of spread of infection in hospitals are those common to all infections: airborne, contact and common vehicle. Examples of organisms spread by these routes in hospitals are illustrated in Figure 36.3. Although theoretically possible, vector-borne spread is very unusual in the hospital setting, as is sexually transmitted infection. It is important to remember that the same organism may be spread by more than one route. For example, Strep. pyogenes can be spread from patient to patient by the airborne route in droplets or dust, but is also transmitted by contact with infected lesions, for example on a nurse’s hand. In addition, a patient or healthcare worker with shingles can transmit VZV to a susceptible person having direct contact with rash blisters.
Host factors and hospital infection
Underlying disease, certain treatments and invasive procedures reduce host defences
Host factors play a fundamental role in the infection equation, particularly in hospitals because of the high proportion of hospital patients with compromised natural defences against infection. The spread of an infectious agent to a new host can result in a spectrum of responses: from colonization, through subclinical infection, to clinically apparent disease, which may be fatal. The degree of host response differs in different people depending upon their degree of compromise. The very young are particularly susceptible because of the immaturity of their immune system. Likewise, the elderly suffer a greater risk of infection because of predisposing underlying disease, impaired blood supply and immobility, which contribute to stasis and therefore to infection in, for example, the lungs. In all age groups, underlying disease and the treatment of that disease (e.g. cytotoxic drugs, steroids) may predispose to infection (Fig. 36.5), while invasive procedures allow organisms easier access to previously protected tissues (Fig. 36.6). The important host factors to be considered in hospital infection are summarized in Table 36.2. Infections in the compromised host are discussed in more detail in Chapter 30.
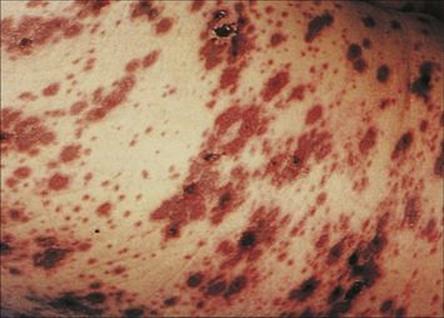
Figure 36.5 Varicella in a patient with chronic myeloid leukaemia resulting in purpuric confluent lesions on the trunk.
(Courtesy of G.D.W. McKendrick.)
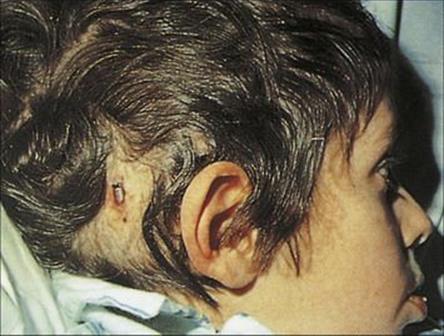
Figure 36.6 Child with infected Spitz–Holter valve used to relieve hydrocephalus.
(Courtesy of J.A. Innes.)
Table 36.2 Factors which predispose patients to hospital infection
|
Age |
Patients at extremes of age are particularly susceptible |
|
Specific immunity |
Patient may lack protective antibodies to, e.g. measles, chickenpox, whooping cough |
|
Underlying disease |
Other (non-infectious) diseases tend to lead to enhanced susceptibility to infection, e.g. hepatic disease, diabetes, cancer, skin disorders, renal failure, neutropenia (either as a result of disease or of treatment) |
|
Other infections |
HIV and other immunosuppressing virus infections; patients with influenza prone to secondary bacterial pneumonia; herpes virus lesions may become secondarily infected with staphylococci |
|
Specific medicaments |
Cytotoxic drugs (including post-transplant immunosuppression) and steroids both lower host defences; antibiotics disturb normal flora and predispose to invasion by resistant hospital pathogens |
|
Trauma |
Burns, stab or gunshot wounds, road traffic accidents |
Hospital patients are not all at equal risk of infection. Some factors that predispose to infection can be influenced by, e.g. treating underlying disease, improving specific immunity and avoiding inappropriate use of antibiotics. Other factors such as age are unalterable.
A variety of factors predispose to wound infection
Wound infection or wound sepsis is characterized by the presence of inflammation, pus and discharge in addition to the isolation of organisms such as Staph. aureus. Extensive studies of postoperative wound infection have identified a number of predisposing factors:
• Prolonged preoperative stay increases the opportunity for the patient to become colonized with antibiotic-resistant hospital pathogens.
• The nature and length of the operation also have an effect (Table 36.3 and Fig. 36.7; see also Ch. 26).
• Wet or open wounds are more liable to secondary infection.
From these studies, it has been possible to identify the patients and operations with greatest risk and apply preventive measures such as prophylactic antibiotic regimens and ultra-clean air in orthopaedic operating theatres (see below).
Table 36.3 Risk factors for postoperative infections
|
Length of preoperative stay |
Longer stay – more likely to become colonized with virulent and antibiotic-resistant hospital bacteria and fungi |
|
Presence of intercurrent infection |
Operating on an already infected site more likely to cause disseminated infection |
|
Length of operation |
Longer – greater risk of tissues becoming seeded with organisms from air, staff, other sites in patient |
|
Nature of operation |
Any operation which results in faecal soiling of tissues has higher risk of infection (e.g. postoperative gangrene), ‘adventurous’ surgery tends to carry greater risks |
|
Presence of foreign bodies |
For example, shunts, prostheses, impairs host defences |
|
State of tissues |
Poor blood supply encourages growth of anaerobes; inadequate drainage or presence of necrotic tissue predisposes to infection |
The risks of infection after surgery have been studied in considerable detail, and surgeons are consequently much more aware of the problems. However, ‘high-tech’ surgery is often long and difficult and predisposes the patient to postoperative infection.
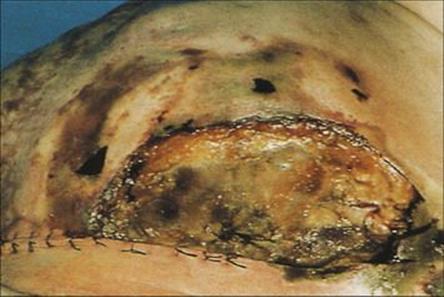
Figure 36.7 Postoperative gangrenous cellulitis. There is a huge area of ulceration filled with gangrenous skin, with sloughing adjacent to the wound and surrounding cellulitis.
(Courtesy of M.J. Wood.)
Consequences of hospital infection
Hospital infections affect both the patient and the community
Hospital infection may result in:
• serious illness or death
• prolonged hospital stay, which costs money and results in a loss of earnings and hardship for the patient and his or her family
• a need for additional antimicrobial therapy, which is costly, exposes the patient to additional risks of toxicity, and increases selective pressure for resistance to emerge among hospital pathogens
• the infected patient becoming a source from which others may become infected, in hospital and in the community.
Prevention of hospital infection
There are three main strategies for preventing hospital infection
For the reasons outlined above, the prevention of hospital infection deserves a very high priority, and the three main strategies are:
• excluding sources of infection from the hospital environment
• interrupting the transmission of infection from source to susceptible host (breaking the chain of infection)
• enhancing the host’s ability to resist infection.
Exclusion of sources of infection
Exclusion of inanimate sources of infection is achievable, but it can be difficult to avoid contamination by humans
Exclusion of inanimate sources of infection is both desirable and, to a large extent, achievable. For example, the provision of sterile instruments and dressings, sterile medicaments and intravenous fluids, clean linen and uncontaminated food, and the use of blood and blood products screened for infectious agents. However, many of the sources of infection are human or are objects that become contaminated by humans, in which case exclusion is more difficult. Hospitals must attempt to prevent patient contact with staff who are carriers of pathogens. The problem is the identification of staff who are carriers of pathogens and their relocation to less hazardous positions.
Staff must undergo health screening before employment and should have regular health checks (Box 36.3). For example, in the UK all new healthcare workers (HCWs) are offered testing for HIV and hepatitis C. Hepatitis B immunization is offered and HCWs must know their post-immunization status (surface antigen negative or, if positive, e-antigen negative with a viral load of 103 genome equivalents/mL or less) before carrying out exposure-prone procedures (EPPs). It is critical that those carrying out EPPs who either do not know their post-immunization status or have not responded to the hepatitis B vaccine are checked to ensure that they do not have a current HBV infection or have a protective level of hepatitis B surface antibody. This is because HBV could be transmitted to the patients if the HCW carrying out EPPs is a hepatitis B carrier and also because the unprotected HCW is at risk of infection from a hepatitis B carrier patient. New HCWs carrying out EPPs must also be non-infectious for HIV (antibody negative) and hepatitis C (antibody negative or, if positive, negative for hepatitis C RNA).
![]()
Box 36.3  Examples of Infectious Diseases where Staff Contact with Patients Should be Avoided
Examples of Infectious Diseases where Staff Contact with Patients Should be Avoided
Recommended work restrictions for staff with infectious diseases. In the event of a member of staff becoming infected, either in the hospital or outside, he or she should be relieved from direct contact with patients. Kitchen staff should also be relieved from duty if they are suffering from diarrhea or hepatitis A, or have infected lesions on their hands.
• Diarrhea
• Hepatitis A
• Herpes simplex on hands (herpetic whitlow)
• Streptococcus pyogenes infections
• Staphylococcus aureus skin lesions
• Measles
• Mumps
• Whooping cough
• Rubella
• Varicella-zoster infections
• Upper respiratory tract infections (high-risk patients)
![]()
Hospitals have blood-borne virus exposure policies for the management of healthcare workers and others who may have been exposed to viruses, including HBV, HCV, and HIV, having sustained a needlestick injury or mucous membrane splash from a potentially infected source. Prophylaxis includes active and/or passive immunization against hepatitis B and a short course of antiretroviral therapy for HIV exposure (see later). The risk of transmission is highest, ca. 33%, for HBV in unimmunized recipients, ca. 0.32% for HIV after a single needle stick injury, and HCV is thought to be between 1% and 3%. However, as reporting of exposure incidents and follow-up of the recipient improves, the better our understanding of the outcomes of the incident itself. Fortunately, most HCWs reported have recovered spontaneously or after having had ribavirin and pegylated interferon treatment.
In general, staff should be encouraged to report any incidences of infection (e.g. an infected cut or a bout of diarrhea). Appropriate immunizations should be offered and in some instances made mandatory. Work restrictions for personnel with selected infectious diseases are summarized inBox 36.3. However, healthy carriers of, for example, virulent staphylococci are difficult to identify unless bacteriologic screening is undertaken, which is not feasible on a routine basis. In addition, staff are sources of opportunist organisms such as coagulase-negative staphylococci or enterobacteria, which are part of their normal flora and cannot be excluded.
Breaking the chain of infection
There are two elements to be considered in breaking the chain of infection: the structural and the human. The structure of the hospital and its equipment can play a role in preventing airborne spread of infection and in facilitating aseptic practices by the staff, but this is of no avail if staff do not use the facilities correctly and do not themselves act positively to prevent the spread of infection.
Control of airborne transmission of infection
Ventilation systems and air flow can play an important role in the dissemination of organisms by the airborne route
Wards comprising separate rooms have been shown to afford some protection against airborne spread, and rooms with controlled ventilation are even better. However, neither prevents the carriage of organisms into the room on staff and their clothing, and some studies suggest that this is a more important route of infection than airborne spread. However, Legionella infection is acquired by the airborne route, and air-conditioning systems throughout the hospital should be maintained so as to prevent the multiplication of these organisms (see Ch. 19). Aspergillus infection in hospitals has been attributed to dissemination of the spores in hospital air, especially when building work is ongoing in the locality.
Ventilation systems in operating theatres must be properly installed and maintained to prevent the ingress of contaminated air and to minimize air currents carrying organisms from the staff in the operating room to the operation site. ‘Ultra-clean’ air is air passed through high-efficiency filters to remove bacteria and other particles and has been shown to contribute positively to a reduction in the number of postoperative wound infections developing after long orthopaedic operations.
Airborne transmission of infection can be reduced significantly by isolating patients
Patient isolation may be carried out:
• to protect a particularly susceptible patient from exposure to pathogens (i.e. protective isolation)
• to prevent the spread of pathogens from an infected patient to others on the ward (i.e. source isolation).
Isolation also helps to prevent the transmission of infection by other routes by limiting access to the patient and reminding staff of the importance of contact in the spread of infection.
Protective isolation can be provided by a single room on a ward or by enclosing the patient in a plastic isolator. With appropriate positive-pressure ventilation, air should flow from the ‘clean’ patient area out of the room or isolator. Staff entering the room or in contact with the patient should wear sterile gowns, gloves and masks to prevent organisms they are carrying or have picked up from other patients from coming in contact with the patient.
While source isolation in the past typically involved patient accommodation in an isolation unit in a separate building (e.g, the tuberculosis sanatoria), hospital isolation is typically arranged in a separate ward or in side rooms off the main ward. To prevent airborne transmission of organisms from the patient’s room to the ward, air should flow from the ward to the isolation room. In practice, it is difficult to maintain the correct air flows without sophisticated designs, including double doors and air locks.
Facilitation of aseptic behaviour
A general state of cleanliness throughout the hospital is essential, and the design of hospital facilities affects the ease with which the environment can be kept clean and the staff can practise good techniques.
Bacteriologically effective handwashing is one of the most important ways of controlling hospital infection
The hands of staff convey organisms to patients from septic lesions and healthy carrier sites of other patients, from equipment contaminated by these sources and from carrier sites of the staff themselves (Table 36.4 and Fig. 36.8).
Table 36.4 Contact spread of opportunist pathogens
|
Patient |
Nursing activity |
Number of Klebsiellae recovered per handa |
|
A |
Physiotherapy |
10–100 |
|
Taking blood pressure and pulse |
100–1000 |
|
|
Washing patient |
10–100 |
|
|
Taking oral temperature |
100–1000 |
|
|
B |
Taking radial pulse |
100–1000 |
|
Touching shoulder |
1000 |
|
|
Touching groin |
100–1000 |
|
|
C |
Touching hand |
10–100 |
|
D |
Extubation |
100–1000 |
|
Touching tracheostomy |
1000 |
Nursing procedures involving skin contact resulting in contamination of staff hands. These data are derived from experiments performed during an outbreak of Klebsiella infection among urology patients.
a Control hand washings taken prior to procedure yielded no Klebsiellae.
(Data from Casewell and Phillips.)
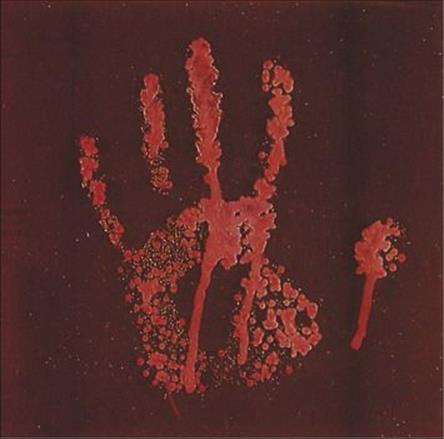
Figure 36.8 Gram-negative rods are not usually part of the resident skin flora except in moist environments, but are readily carried on hands and can be transferred from a source to a susceptible patient. This picture shows an impression of a hand that was inoculated with approximately 1000Klebsiella aerogenes.
Staff should therefore wash their hands:
• before any procedure for which gloves or forceps are necessary
• after contact with an infected patient or one who is colonized with multiply-resistant bacteria
• after touching infective material.
While soap and water are adequate in many circumstances, emphasis is shifting to the use of fast-drying alcohol-based gels and solutions which are easier to use and appear to have a more antibacterial result. A mandate from the US Centers for Disease Control, for example, has put this approach into practice in US hospitals. Drying hands after any washing procedure is important. A more prolonged and thorough hand decontamination is required before commencing surgery.
The design of taps, soap dispensers and other washing facilities, including bedpan washers, has reached a high degree of sophistication. However, human behaviour can be influenced by architectural design only to a limited degree, and there is often a disappointingly low compliance with the simple technique of handwashing. Therefore, training and regular reinforcement in appropriate behaviour is essential.
Enhancing the host’s ability to resist infection
Host resistance can be enhanced by boosting immunity and reducing risk factors
Although attempts can and should be made to control and prevent hospital infection by removing sources of infection and preventing transmission from sources to susceptible hosts, neither of these strategies is fail-safe. In addition, they do not protect the host from endogenous infection. A way of tipping the balance in favour of the host is to enhance his or her ability to resist infection, both by boosting specific immunity and by reducing personal risk factors. The following aspects should be considered:
• boosting specific immunity by active or passive immunization
• the appropriate use of prophylactic antibiotics
• care of invasive devices that breach the natural defences (e.g. urinary catheters, intravenous lines)
• attention to the risks predisposing to postoperative infection.
Boosting specific immunity
Passive immunization provides short-term protection
Boosting specific immunity by immunization has been discussed in Chapters 34 and 35. The problem for the immunocompromised patient is that they may not be able to mount an antibody response. Passive immunization can afford short-term protection, for example, with chickenpox exposure in susceptible patients who are neutropenic as a result of cytotoxic therapy and whose white cell count should recover after successful treatment. Active immunization with hepatitis B vaccine is recommended for all susceptible patients attending dialysis units. Other immunizations to protect hospital patients are summarized in Table 36.5.
Table 36.5 Boosting specific immunity of patients
|
Patient group |
Immunization |
|
|
Active |
Passive |
|
|
Elderly (especially those with multisystem disease) |
Influenza vaccine |
|
|
Pre-splenectomy |
|
|
|
Haemodialysis patients |
Pneumococcal vaccine |
|
|
Infants born to HBsAg positive mothers |
Hepatitis B vaccine |
Hepatitis B immunoglobulin (especially if mother HBeAg positive) |
|
Immunocompromised: exposed to varicella-zoster virus (VZV) |
Live attenuated VZV vaccine |
Zoster immune globulin as soon as possible after exposure (and within 4 days) |
|
Exposed to measles |
measles containing vaccine |
Normal human immune globulin within 5 days |
Many patients will have been protected against some infections by routine immunization during childhood, but sometimes it is helpful to boost specific immunity by immunization of patients at particular risk of infection.
Appropriate use of prophylactic antibiotics
There are well-documented uses for prophylaxis, but antibiotics tend to be misused
This is discussed in Chapter 33. There are several well-documented uses for prophylactic antibiotics in ‘dirty’ surgery and when the consequences of infection would be disastrous (e.g. in cardiac, neuro- and transplant surgery). However, there is a tendency to misuse antibiotics:
• first, by using them too often or for too long, thereby increasing the selection pressure for the emergence of resistant organisms
• second, by choosing inappropriate agents.
Treatment (as opposed to prophylaxis) of patients and staff who are carriers of pathogens such as Staph. aureus or Strep. pyogenes has been used successfully to prevent endogenous infection and to control outbreaks of infection with these organisms. Topical preparations of antibiotics such as pseudomonic acid (mupirocin), a fermentation product of Pseudomonas fluorescens, have been shown to be efficacious. However, resistance (both low and high level) to the drug has occurred.
Gut decontamination regimens and selective bowel contamination aim to reduce the reservoir of potential pathogens in the gut
Gut decontamination regimens to reduce the aerobic Gram-negative flora of neutropenic patients has been practised for some time. With some patients (e.g. liver transplant) in intensive care units (ICU), selective bowel decontamination (SBD) has been employed. The aim is to reduce the reservoir of potential pathogens in the gut by oral administration (or via a nasogastric tube) of a high concentration of a mixture of antibiotics. At the present time, there is still controversy about the efficacy and safety of SBD.
Care of invasive devices
Care of invasive devices is essential to reduce the risk of endogenous infection
It is essential to take care of intravascular devices to reduce the risk of endogenous infection from skin organisms, and of catheters to reduce the risk that the periurethral flora will cause endogenous infection of the bladder in catheterized patients. Guidelines for the care of urinary catheters are discussed in Chapter 20.
The majority of hospital-associated bacteraemias and candidaemias are infusion-related
These infusion-related bacteraemias and candidaemias derive mainly from vascular catheters. Most bacteraemias associated with invasive devices are caused by the patient’s own skin flora, although this may be a more resistant flora acquired during the patient’s stay in hospital replacing susceptible resident bacteria. Coagulase-negative staphylococci are the most common aetiologic agents, but enterococci, Candida, and various Gram-negative rods are also implicated. These infections are largely preventable if appropriate steps are taken. The sources of infection and measures for prevention are shown in Figure 36.9.
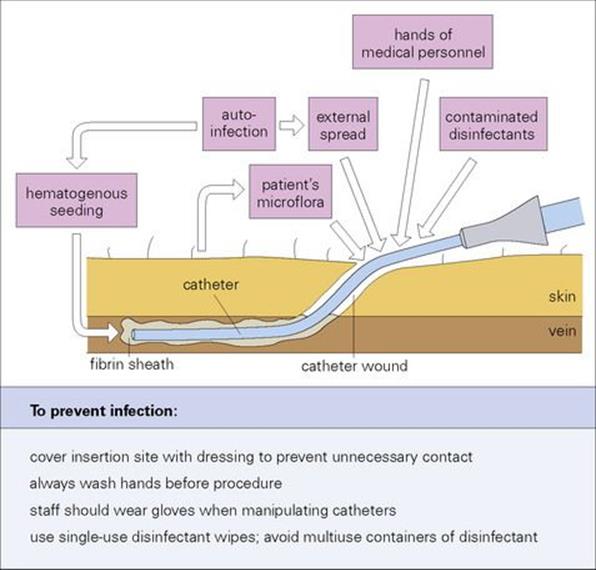
Figure 36.9 Sources of intravascular device-related infection and opportunities for the prevention of infection.
Reducing the risks of postoperative infection
Prevention of postoperative infection involves minimizing the risks
Reducing the risks of postoperative infection involves an understanding of the risks and the ways in which they can be circumvented. For example:
• The preoperative length of stay in hospital should be kept to a minimum.
• Intercurrent infections should be treated appropriately before surgery whenever possible (e.g. treatment of UTI before resection of the prostate).
• Operations should be kept to the minimum duration consistent with good operating technique.
• Adequate debridement of dead and necrotic tissue is essential, together with adequate drainage and maintenance or re-establishment of a good blood supply to provide the body’s natural defences with optimum working conditions.
• Prevention of pressure sores and stasis by good nursing techniques and active physiotherapy minimizes the risks of developing respiratory tract infection or UTI.
Investigating healthcare-associated infection
Many of the epidemiologic principles outlined in Chapter 31 apply to the investigation of healthcare-associated infection. Outbreaks within hospitals are epidemics – they are detected because the incidence of an infection is seen to be above normal levels for that institution. Investigation therefore must determine the extent of the problem, identify the source of infection and the way in which it is spread, identify those at risk, and propose effective methods for control. As with infectious diseases in general, the application of statistical techniques (e.g. calculation of risk ratios) and mathematical modelling has helped to provide an analytical and predictive framework for such infections, but day-to-day investigations still require the application of proven microbiologic approaches.
Hospital infections, like community infections, can involve all the major groups of pathogens, from viruses to arthropods. However, a particular problem with hospital infections, compared with those commonly occurring in the community, is the transmission of antibiotic-resistant bacteria, the emergence of which, and their spread, is favoured by the hospital environment. The recent surge in community-associated MRSA infections is an unfortunate exception to this trend. Epidemiologic investigations of infections place great importance on typing to identify the causative organism. Such molecular epidemiology can make a very important contribution to tracking and controlling infection.
In many hospitals, the responsibility for investigating hospital infection falls on the infection control committee, which includes an infection control officer (who may be a physician or microbiologist) and at least one nurse. The roles of the infection control committee include:
• the surveillance of hospital infection
• the establishment and monitoring of policies and procedures designed to prevent infection (e.g. catheter care policy, antibiotic policy, disinfectant policy, blood-borne virus exposure incidents, including needlesticks and blood splashes)
• the investigation of outbreaks – tracking the source and routes of transmission.
Surveillance
Surveillance allows early recognition of any change in the number or type of hospital infections
National and international surveys continue to highlight the prevalence and importance of hospital infection. By maintaining local surveillance, the infection control team can establish the normal trends in their hospital and proactively recognize any change in the number or type of infections. Sources of surveillance data are:
• Microbiology laboratory reports. These can be used for general surveillance, for example, monitoring haemodialysis patients regularly for hepatitis B surface antigen and HCV antibody, as outbreaks of HBV and HCV infection have been reported in renal units around the world, or for monitoring ‘sentinel’ or ‘alert’ organisms such as Staph. aureus, Strep. pyogenes, M. tuberculosis, Salmonellae and Shigellae.
• Ward rounds. New cases of infection can be identified by direct inspection, and previously identified cases of infection can be followed up. Surveys can also be carried out on the wards (e.g. of wound infections after different practices or procedures).
• Other sources include autopsy reports, staff health records and surveys of patients after discharge from hospital.
Investigation of outbreaks
When an outbreak (or epidemic) occurs or when routine surveillance highlights an increase in the incidence of infection, the infection control team should initiate an investigation. There is no universally applicable routine for finding the cause of an outbreak, but in principle each investigation has an epidemiologic element and a microbiologic element.
There must be a description of an outbreak in epidemiologic terms
This involves obtaining information about a number of relevant factors:
• How many people are infected?
• When were they admitted?
• When did they develop their infection?
• Are they all on the same ward?
• Are they all treated by the same medical or surgical team?
• Have they all been exposed to the same treatments?
The causative organism needs to be isolated and/or detected in all patients in the outbreak
It is the role of the microbiology laboratory to attempt to isolate the causative organism and to show that it occurs in all patients in the outbreak (i.e. they are all infected with organisms that are indistinguishable – see below). The identity of the infecting organism can provide clues as to the possible source:
• Respiratory and intestinal viruses implicate the source of infection as a patient or attending medical staff.
• Hepatitis indicates spread via contaminated blood products or hypodermics.
• An outbreak of wound infection with Staph. aureus is likely to be associated with contact spread from staff in theatre or on the ward.
• An outbreak of Salmonella gastroenteritis is more likely to originate in the kitchen.
• Infections with Legionella or Pseudomonas are likely to reflect environmental (especially water) contamination.
In addition, the location of the outbreak, whether in a general ward, a surgical ward, a paediatric unit or intensive care unit (once described as the epicentre of hospital infections) may also provide valuable clues.
Stages in tracking infection
Once the problem has been identified clinically, appropriate specimens should be collected from the patients and, if the indicators are that medical staff are involved, from hospital personnel (see Ch. 31). Likely sources of environmental contamination (surfaces, materials, equipment, water) should also be sampled. This is an important step, as data (using a non-infectious DNA marker as an experimental infectious organism) have shown that after release there is a rapid spread from hands of medical staff to almost all available surfaces (computers, charts, telephones, control knobs, door handles, heater controls, patient monitors). Once samples have been collected, the microbiology laboratory then has the task of identifying and typing the organisms concerned.
While the investigation is proceeding, steps should be taken to contain the outbreak and prevent spread to other patients. Infected patients must be isolated and treated appropriately. Staff who show a similar infection, or who are subsequently found to be carriers, must be suspended from duty until they have been treated. At the end of the investigation, the relevant procedures must be reviewed to try and prevent the reoccurrence of a similar outbreak.
Epidemiologic typing techniques
Bacteria are the commonest causes of nosocomial infections and of the greatest concern because of the prevalence of antibiotic resistance. For example, between January 2006 and October 2007 over 600 hospitals reported more than 28 000 nosocomial infections to the US National Health Safety Network. Of the pathogens involved, 13% were fungi but 87% were bacteria, most commonly coagulase-negative staphylococci, S. aureus (e.g., MRSA), Enterococcus species (e.g., VRE), Candida species, Escherichia coli, and Pseudomonas aeruginosa. Tracking infection is therefore disproportionately concerned with this group of pathogens, although molecular techniques are also applied to monitoring viral infections.
A variety of phenotypic and genotypic characters are used to ‘fingerprint’ bacteria for epidemiologic purposes
In epidemiologic studies of the spread of hospital infections, as in the investigation of outbreaks in the community, it is necessary to identify isolates of the infectious organisms to determine whether or not they are distinct (it may not be possible to say that two organisms are the same, only that they are indistinguishable). In the case of bacteria, if the species is a regular member of the normal human flora or is found frequently in the environment, it is necessary to distinguish the ‘outbreak’ strain from other strains of the same species not involved in the outbreak, but that may also be isolated during the course of the investigation. Essentially, typing is used to look for evidence of a clonal spread of a particular pathogen.
To be valuable in this context, good typing techniques must:
• be discriminatory (i.e. able to show differences between strains of the same species)
• be reproducible (i.e. the same strain gives the same result when tested on different occasions and in different places)
• have a high degree of typability (i.e. capable of assigning a type to all strains).
Antibiotic susceptibility patterns
Antibiotic susceptibility testing is readily performed in the diagnostic laboratory (see Ch. 31) and is useful as a preliminary clue as to whether two isolates are indistinguishable. However, discrimination is poor: many susceptibility patterns are common, and quite different strains may have the same pattern. Conversely, during an outbreak, strains may gain or lose plasmids carrying antibiotic resistance markers. More specialized typing techniques are commonly performed in reference laboratories. This has the advantage that quality assurance can be optimized, but also means that there is an inevitable delay in reporting the results and therefore in learning whether an outbreak of hospital infection is caused by a single strain.
Specialized typing techniques
Serotyping distinguishes between strains, using specific antisera
This classic technique distinguishes between strains by a difference in their antigenic structure, which is recognized by reaction with specific antisera. The ‘O’ somatic antigens and ‘H’ flagellar antigens are therefore used to divide salmonellae into types (sometimes referred to as species; seeCh. 22). Strep. pneumoniae, Neisseria meningitidis and Klebsiella aerogenes can be typed on the basis of their capsular (K) antigens, and Strep. pyogenes on the basis of their M- and T-cell wall proteins. However, serotyping requires the production and maintenance of appropriate banks of reagents (e.g., antisera), which is both time-consuming and costly. Therefore, this approach, when employed, is usually restricted to reference laboratories.
Bacteriophage (phage) typing has been used to type Staph. aureus, Staph. epidermidis and Salmonella typhi
This technique compares the pattern of lysis obtained when isolates (grown as lawns on agar plates) are exposed to a standard series of phage suspensions (Fig. 36.10). In the past, this method has been important for typing Staph. aureus, Staph. epidermidis and Salmonella typhi, but has also been applied to other species such as P. aeruginosa. However, as with serotyping, phage typing requires a reference laboratory for the production, maintenance and testing of the standard phage suspensions and has thus generally fallen out of favour. In the USA, the Centers for Disease Control has forgone the use of bacteriophage typing in favour of molecular techniques such as pulsed field gel electrophoresis (PFGE) (see below).
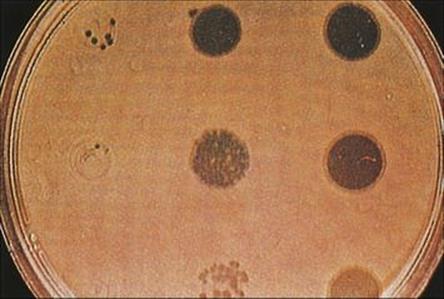
Figure 36.10 Bacteriophage (phage) typing of staphylococci. After seeding the surface of an agar plate with the organism to be typed, suspensions of different phages are dropped onto the surface and the plate incubated. Phages that are able to lyse the strain will produce zones of clearing of the bacterial lawn. The patterns of lysis obtained with the same set of bacteriophages on different isolates of Staph. aureus collected, for example, during an outbreak of wound infections, can be compared.
Molecular typing
Molecular typing techniques involve characterizing an organism’s DNA
The above methods have been of great use in the epidemiologic analysis of nosocomial pathogens but are all variations on the phenotypic characterization of isolates. Since the chromosome represents the most fundamental ‘molecule of identity’ in the cell, genotypic approaches are used for characterization, often referred to as ‘molecular epidemiology’.
Plasmid profiles are an example of ‘first-generation’ molecular epidemiology
Comparison of plasmid carriage in different isolates is only useful for species that carry a variety of plasmids and suffers from the drawback that what is actually being characterized is the plasmid and not the organism containing it. Different Gram-negative rods may acquire the same plasmids by conjugation between different species. However, this method has also been used to map the spread of antibiotic resistance plasmids among hospital pathogens (Fig. 36.11).
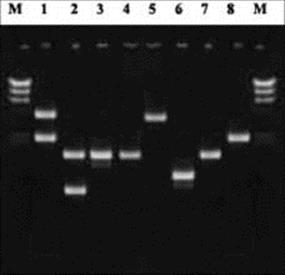
Figure 36.11 Analysis of plasmid carriage in eight bacterial clinical isolates using agarose-gel electrophoresis. Lanes labelled ‘M’ represent molecular size standards.
Restriction enzymes and probes represent ‘second-generation’ molecular epidemiology
Restriction-enzyme digestion of total cellular DNA from isolates results in a pattern of different-sized fragments which can be separated and compared by agarose gel electrophoresis-restriction-enzyme analysis (REA). All bacterial cells possess chromosomal DNA and can theoretically be analysed by this process. However, the DNA sequences recognized by most restriction enzymes, such as EcoRI, HindIII, etc. are present in hundreds of copies throughout a typical bacterial chromosome. Thus, the challenge is to compare accurately electrophoretic patterns comprising hundreds of restriction fragments which often co-migrate in clusters of similar size, and may include resident plasmid DNA.
The principle of complementary DNA sequences hybridizing with each other (e.g. Southern hybridization; named after its inventor, Ed Southern) has led to applications where specific DNA appropriately labelled ‘probes’, complementary to ‘target’ sequences found at various chromosomal locations, are hybridized against isolate REA patterns. Northern blotting is similar in principle but characterizes RNA sequences. Antibiotic resistance genes, and a variety of repeated sequences (e.g. transposons), have been especially useful targets in this context. The result is a pattern of hybridization with different restriction-fragments, commonly termed restriction-fragment length polymorphism (RFLP) analysis, corresponding to the chromosomal location of the probed sequences, which provides an indication of chromosomal relatedness between different isolates (Fig. 36.12A). For example, copies of the genes for ribosomal RNA (5 S, 16 S and 23 S rRNA) are found at different locations on the chromosome of many medically important bacteria. These highly conserved sequences (i.e. very similar sequences in different species) allow RFLP analysis using a common probe (i.e. ribotyping). However, discrimination between strains of the same species may be less because of the conserved nature of the target sequences. The greatest success with RFLP analysis has primarily involved probes for insertion sequences that provide sufficient coverage (i.e. in number and diversity of chromosomal location) to reflect epidemiologically relevant interrelationships. The use of IS6110 probes in the RFLP analysis of Mycobacterium tuberculosis isolates is an example of a successful use of this approach. While superior to REA alone, RFLP analysis remains only moderately discriminatory for epidemiologic analysis.
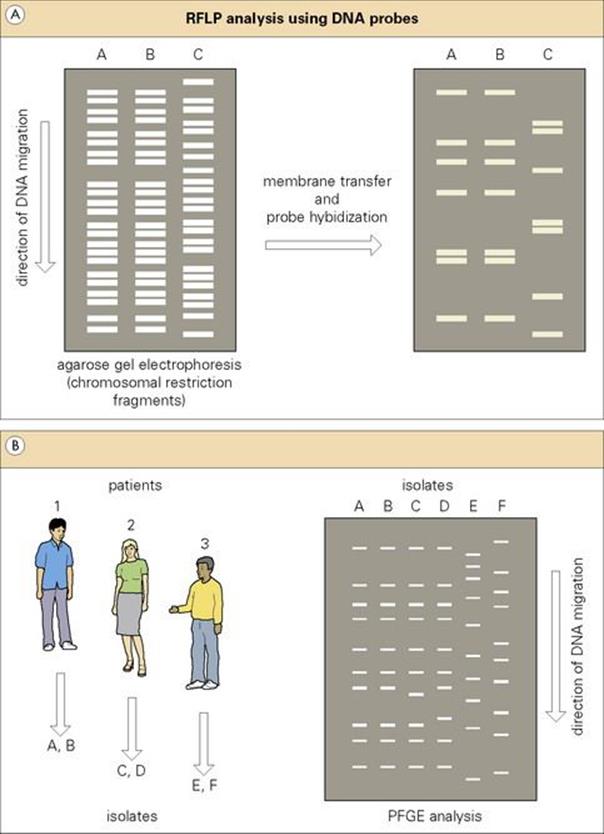
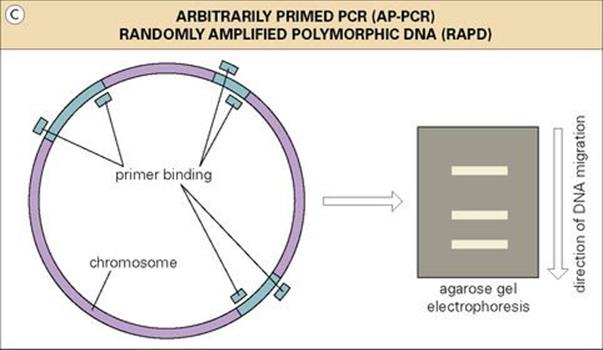
Figure 36.12 (A) Restriction-fragment length polymorphism (RFLP) analysis using DNA probes. An illustration of three nosocomial isolates (A and B epidemiologically related; C unrelated) analysed by restriction-enzyme analysis and subsequently by a specific DNA probe. (B) Pulsed field gel electrophoresis (PFGE) analysis of two bacterial isolates from each of three patients. Isolates in the first two patients are highly related (although slightly different in patient 2). Isolates from patient 3 are epidemiologically unrelated.(C) In the RAPD/AP-PCR approach to epidemiologic analysis, PCR products result from the random binding of PCR primers to chromosomal sequences, and the pattern is expected to be similar in epidemiologically related isolates. RAPD, randomly amplified polymorphic DNA; AP-PCR, arbitrarily primed polymerase chain reaction.
PFGE and PCR are ‘third-generation’ approaches to molecular epidemiology
Instead of using frequently ‘cutting’ restriction enzymes, chromosomal DNA may be digested using enzymes with rare recognition sites in bacterial chromosomes (e.g. NotI, SfiI, SpeI and XbaI in most Gram-negatives; AscI, RsrII, SgrAI and SmaI in most Gram-positives). The extremely large DNA fragments produced are too large to be separated by conventional agarose gel electrophoresis but may be resolved by electrophoretic current ‘pulsed’ in different directions for different lengths of time-pulsed field gel electrophoresis (PFGE). PFGE has proved to be a powerful epidemiologic tool. The macro-restriction patterns produced by PFGE provide a sense of ‘global’ chromosomal monitoring – genetic events that affect distances between rare restriction-site sequences can be inferred from changes in restriction-fragment size (Fig. 36.12B). To date, the major disadvantage to PFGE analysis has been extra time and effort involved in producing unbroken chromosomal molecules necessary for reproducible macro-restriction-fragment patterns. In general, the overall success with which PFGE analysis has been employed has made it the method of choice – the ‘gold standard’ – for the epidemiologic analysis of most pathogens of clinical concern.
Economy, speed and the relatively low level of technical expertise required by the polymerase chain reaction (PCR) (Ch. 31) have led to a wealth of amplification-based applications for epidemiologic analysis. One of the earliest and most common PCR-based approaches has been randomly amplified polymorphic DNA (RAPD), also called arbitrarily primed PCR (AP-PCR). The method is based on the use of relaxing conditions affecting the stringency (i.e. specificity), with which PCR primers bind to DNA templates. PCR primers are allowed to randomly bind to chromosomal sequences of varying homology, resulting in products which can be comparatively analysed by agarose gel electrophoresis. A group of clinical isolates representing inter-patient transfer of a single strain would thus be expected to exhibit the same degree of ‘randomness’, resulting in identical PCR products (Fig. 36.12C). However, several studies have shown that this method is especially prone to artefact and inter- and intra-laboratory variation. Nevertheless, the overall simplicity and utility of PCR continues to drive development and refinement of additional epidemiologic approaches which are beyond our ability to explore here.
‘Fourth-generation’ molecular epidemiology is based on DNA sequence analysis
Since the chromosome is the most fundamental molecule of identity in the cell, a comparison of actual chromosomal sequences would seem the most fundamental means of assessing potential interrelationships in nosocomial isolates. While a comparison of total chromosomal sequences is not a practical option, analysis of a subset of nucleotide sequences is the basis for what one could consider fourth-generation molecular epidemiology. Thus, recent years have seen a variety of sequence-based approaches to assessing microbial relatedness. These have included:
• microarrays, capable of comparing bacterial isolates for the presence or absence of specific genes (e.g., virulence, antibiotic resistance)
• a comparison of specific chromosomal regions in isolates looking for changes in DNA base (A, T, G, or C) coding, termed single nucleotide polymorphisms (SNPs)
• multi-locus sequence typing (MLST), where differences in the sequences of several (e.g., 6 or 7) essential ‘housekeeping’ genes serve as the basis for an assessment of isolate relatedness.
However, issues related to the choice of epidemiologically relevant sequences, method of analysis, data output, and interpretation continue to be explored and optimized, a process being greatly facilitated by current developments in microbial whole genome sequencing.
Molecular techniques for epidemiologic fingerprinting have many advantages
Although molecular techniques may require expertise and equipment, they have several advantages. They can be extremely precise, can be performed rapidly, do not involve handling infectious organisms and can be used to type all of the relevant isolates.
Investigation of viral infections
Nosocomial viral infections usually occur via the airborne route, contaminated fomites or blood-to-blood contact as outlined previously with, for example, RSV, noroviruses or hepatitis B, respectively. These are investigated mostly by detecting virus in samples from symptomatic patients and then, depending on the clinical setting, collecting samples from asymptomatic patients when deciding whom to include in a cohort from whom isolates can be obtained. In general, only identification of the microbe as a virus is required in outbreaks of viral gastroenteritis, as the management is the same for all the viral causes of gastroenteritis. However, in this setting it is important from an epidemiologic perspective to identify the cause of the outbreak. Surveillance is critical to monitor any changes in the virus as these alterations to parts of its genome may result in the virus evading detection as the primers used in the diagnostic test may no longer match the complementary sequence of the template. In addition, for those viruses for which we have a vaccine, it is important to know which strains are circulating currently to ensure a good antigenic match with the vaccine strains.
In an outbreak of respiratory infection, identification and typing of the virus is important not only for epidemiological purposes but also for issues of treatment and prophylaxis.
Molecular detection and typing methodologies such as sequencing may be required, usually for epidemiologic purposes rather than direct management of patients. However, in a setting such as postoperative acute hepatitis B infection, an intensive investigation will be carried out covering the possible routes of transmission. This may include investigating blood products, healthcare workers who were involved in exposure-prone procedures, other patients on the operating list, sexual contacts, and other risk activities involving potentially blood-contaminated needles. Once the potential sources have been identified, serologic tests may be carried out to seek evidence of current, recent or past hepatitis B infection. Genome detection methods and sequencing of blood samples from the individual with acute hepatitis B, as well as the potential source or sources, will help to confirm the transmission event or events.
Corrective / Preventive measures
Once tracking is complete, corrective and preventive measures can be introduced
Typing of the aetiologic agent responsible for the outbreak and knowledge of its characteristics and mode of transmission allow preventive measures to be taken. What these include depends to a great extent on the pathogen involved, but all must aim to improve basic hygiene, from more effective handwashing and improved general cleaning to more effectively regulated sterilization of equipment. Hygiene is a crucial factor since agents of nosocomial infection can be spread between patients by hospital staff. With some organisms that are widely distributed in the environment (e.g. P. aeruginosa) or occur in water supplies (e.g. Legionella), corrective measures may involve radical improvements to facilities.
As noted earlier, awareness of the risks of being exposed to blood-borne virus infections in a hospital setting is important to prevent blood-borne virus exposure incidents. Important protective measures include immunization of HCWs, wearing appropriate personal protective equipment (PPE) for procedures that could result in a break in the skin or exposure of mucous membranes, and appropriate post-exposure steps in the case of an incident.
Nosocomial transmission of SARS (see Ch. 19) has shown how easily airborne infection can be transmitted in a hospital setting. The use of PPE that included an N95 respirator, eye protection, mask, gloves and gown was mandatory to reduce the chance of transmission. Disposable second layers of clothing were also used, for example outer gloves, a gown and hand and foot covering.
Sterilization and disinfection
It is clear that the prevention of hospital infection depends in part upon the availability of clean, and where necessary, sterile equipment, instruments and dressings, isolation facilities and the safe disposal of infected material. Sterilization and disinfection are often talked about by microbiologists in relation to the production of sterile culture media and other laboratory activities, but it must be stressed that the concept of sterility is central to almost all areas of medical practice. An understanding of the rationale of sterilization and disinfection will aid intelligent use of the range of sterile equipment (from needles to prostheses) and techniques (from surgery to handwashing) employed in medical practice.
Definitions
Sterilization is the process of killing or removing all viable organisms
An item that is sterile is free from all viable organisms – in this sense, viable means capable of reproducing. Sterilization is achieved by physical or chemical means, either by the removal of organisms from an object or by killing the organisms in situ, sometimes leaving toxic breakdown products (pyrogens) in the object.
Disinfection is a process of removing or killing most, but not all, viable organisms
Disinfection employs either:
• a chemical ‘disinfectant’, which kills pathogens but may not kill viruses or spores
• a physical process such as boiling water or low-pressure steam, which reduces the bioburden (i.e. the load of viable organisms).
Antiseptics are used to reduce the number of viable organisms on the skin
Antiseptics are a particular group of disinfectants. Some act differentially, destroying the transient flora but leaving untouched the normal skin flora deep in the skin pores and hair follicles (Fig. 36.13). It is impossible to sterilize the skin, but thorough washing with antiseptic soaps can reduce the numbers of organisms on the surface considerably and therefore reduce contact spread of infection (see above). However, the resident bacteria in the hair follicles and ducts of sweat glands can recolonize the skin surface within hours.
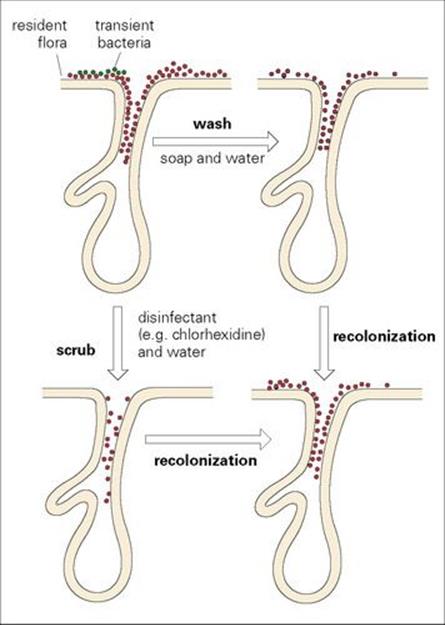
Figure 36.13 Normal skin is colonized with bacteria both on the surface and deep in the pores and ducts of the sweat and sebaceous glands. In addition, bacteria may be carried transiently on the skin surface and may be transmitted from a contaminated source to a susceptible patient. Careful handwashing with soap and water removes the transient flora and some of the superficial resident flora. Scrubbing the hands with disinfectants removes more of the resident flora, but the skin surface is recolonized within hours from the normal flora deep in the skin pores.
Pasteurization can be used to eliminate pathogens in heat-sensitive products
Pasteurization reduces the total numbers of viable microbes in bulk fluids such as milk and fruit juices without destroying flavour and palatability. It does not affect spores, but is effective against intracellular organisms such as Brucella and mycobacteria and many viruses.
Since the beginning of recorded history, various other techniques have been used to prevent the multiplication of microorganisms, such as drying and salting of food.
Deciding whether sterilization or disinfection should be used
Sterilization and disinfection processes are costly, and so it is important to choose the appropriate method and the one that causes the least damage to the material involved. A variety of considerations influence the choice of method. The detailed mechanisms of the death process of microorganisms may vary with the sterilizing technique used, but the net effect is similar in that essential cell constituents (nucleic acids or proteins) are inactivated.
It is easier to sterilize a clean object than a physically dirty one
This is because organic matter protects microbes and hinders penetration of heat or chemicals and may inactivate certain chemicals. In other words, a low bioburden is a prerequisite for cost-effective sterilization.
The rate of killing of microorganisms depends upon the concentration of the killing agent and time of exposure
The number of organisms surviving sterilization can be expressed by the equation: N is proportional to 1/CT, where N is the number of survivors, C is the concentration of agent and T is time of exposure to the agent. If a population of microbes is exposed to a sterilizing technique, and the number of survivors, expressed as a logarithm, is plotted against time, the slope of the graph defines the death rate (Fig. 36.14). These lines may be sigmoid or have shoulders, indicating that individual cells respond slightly differently, some being killed more easily than others. In the case of bacteria, the physiologic state of the organisms influences the shape of the killing curve; young, replicating cells are usually more vulnerable than stationary or decline-phase organisms or those that are sporing. Graphs like those shown in Figure 36.14 can be used to predict the conditions necessary to achieve sterility. However, these experimental data are usually based on pure cultures in the laboratory (bacterial spores are often used as model systems), whereas in real life, the bioburden is mixed. Therefore, predictions from such data may be inappropriate for mixed populations.

Figure 36.14 Theoretically, there is a straight-line relationship between the log viable count of a bacterial population and time when the population is exposed to a lethal temperature. In practice, these lines are usually sigmoid. The D value is the time required to reduce the population by 90% at a specified temperature. Bacillus stearothermophilus spores are used as biologic indicators of effective heat sterilization by including filter paper strips carrying a standard number of spores into the autoclave cycle. The strips are then incubated to attempt to recover viable organisms. The usual autoclave cycle of 121°C for 15 min is adequate to kill B. stearothermophilus with a margin of safety.
Techniques for sterilization
Sterilization may be achieved by:
• heat
• irradiation (gamma or ultraviolet)
• filtration
• chemicals in liquid or gaseous phase.
Other techniques of doubtful efficiency include freezing and thawing, lysis, desiccation, ultrasonication and the use of electrical discharges, but these are not applied in hospital practice.
Ultraviolet irradiation is inefficient as a sterilant, and its important uses in the hospital setting are in inhibiting growth of bacteria in water in complex apparatus such as auto-analysers and in air in safety hoods in virology laboratories. The potential for damage to the cornea and skin precludes wider use of ultraviolet irradiation. It should be remembered that the agents of Creutzfeldt–Jakob disease (CJD), bovine spongiform encephalopathy (BSE) and scrapie are highly resistant and are not completely inactivated by formalin, ultraviolet irradiation, ionizing radiation or regular autoclaving. Sterilization can be achieved by boiling in 1 N NaOH for 10 min at atmospheric pressure followed by autoclaving at a higher than normal temperature for a longer period than usual (134°C for 18 min), but obviously this technique cannot be applied to living tissues or materials that are damaged at high temperatures.
Heat
Heat, as a way of transferring energy, is the preferred choice for sterilization on the grounds of ease of use, controllability, cost and efficiency.
Dry heat sterilizes by oxidation of the cell components
Incineration and the use of the laboratory Bunsen burner are examples of sterilization by dry heat. Glassware can be sterilized in a hot air oven at 160–180°C for 1 h.
The most effective agent for sterilization is saturated steam (moist heat) under pressure
This can be achieved using an autoclave. Steam under pressure aids penetration of heat into the material to be sterilized (such as dressings), and there is a direct relationship between temperature and steam pressure. Steam under pressure has a temperature in excess of 100°C, which results in increased killing of microbes.
Sterilizing efficiency is improved by evacuating all of the air from the autoclave chamber. The subsequently introduced high-pressure steam rapidly penetrates to all parts of the chamber and its load, and results in predictable rises in temperature in the centre of articles to be sterilized. The length of an autoclave cycle is determined by the holding time plus a margin of safety, and is derived from the thermal death curves for heat-resistant pathogens such as clostridia. Therefore, the usual cycle of 121°C for 15 min is sufficient to kill the spores of Cl. botulinum with an adequate margin of safety. However, the spores of some bacterial species, especially soil organisms, are able to withstand this temperature. The safety margin is reduced in the presence of large numbers of organisms because there is a greater probability of more heat-resistant individuals existing in a large population; hence, the importance of cleaning instruments, whenever possible, before sterilization.
Moist heat in an autoclave is used to sterilize surgical instruments and dressings and heat-resistant pharmaceuticals. A method for the sterilization of heat-sensitive instruments such as endoscopes uses a combination of low-temperature (subatmospheric) steam and formaldehyde.
All of these processes need to be carried out in a suitable pressure vessel and are therefore usually available in the hospital central sterile supply department.
Immersion in boiling water for a few minutes can be used as a rapid emergency measure to disinfect instruments
Immersion in boiling water for a few minutes will kill vegetative bacteria and many, but not all, spores. The addition of 2% sodium carbonate to the water potentiates the sporicidal effect.
Pasteurization uses heat at 62.8–65.6°C for 30 min
This technique was devised by Pasteur to prevent the spoilage of wine by heating it to 50–60°C. It is now used for fluids such as milk to reduce the number of bacteria. This helps to eliminate pathogens present in small numbers and to improve the shelf-life of milk. The fluid is held at a temperature of 62.8–65.6°C for 30 min or may be ‘flash’ pasteurized at 71.7°C for 15 s. After either process, the fluid should be kept at a temperature below 10°C to minimize subsequent bacterial growth.
Irradiation
Gamma irradiation energy is used to sterilize large batches of small-volume items
The use of gamma irradiation energy for sterilization is an industrial process that works well with products such as needles, syringes, intravenous lines, catheters and gloves. It can also be used for vaccines and to prevent food spoilage. Although the capital cost of the equipment is high, the process is continuous and 100% efficient. Articles are sterilized while sealed in their original packaging, without any heat gain. The process must be conducted in a suitably constructed building, usually at a location distinct from the hospital and usually outside the hospital administration. However, irradiation can cause materials to deteriorate and is thus not suitable for resterilization of equipment. The killing mechanism involves the production of free radicals, which break the bonds in DNA. Irradiation kills spores, but at a higher dose than vegetative cells because of the relative lack of water in spores.
Sterilization using ultraviolet irradiation is discussed above.
Filtration
Filters are used to produce particle- and pyrogen-free fluid
Solutions that are heat-sterilized will contain pyrogens. These heat-stable breakdown products of microbes are capable of inducing fever and are therefore undesirable in products such as intravenous fluids. Filtration or separation of the product from the contamination has a long history in the clarification of water and wine. Modern filters composed of compounds such as nitrocellulose or mixed cellulose ester work by electrostatic attraction and physical pore size to retain organisms or other particles. The resulting fluid should be particle-free. Filtration is used in some parts of the world to purify drinking water.
Filtration techniques are also used to recover very small numbers of organisms from very large volumes of fluid (e.g. Legionella from cooling tower water) and can be used as a method for quantifying bacteria in fluids.
Chemical agents
The gases ethylene oxide and formaldehyde kill by damaging proteins and nucleic acids
The need for sterilization by gaseous chemicals has been greatly reduced by the success of gamma irradiation (see above), but two alkylating gases, ethylene oxide and formaldehyde, are still used:
• Ethylene oxide is used in some centres to sterilize single-use medical requisites such as heart valves. However, it is toxic and potentially explosive.
• Formaldehyde is not explosive, but has an extremely unpleasant odour and is an irritant to mucous membranes. It has been used as a disinfectant to decontaminate rooms (such as isolation rooms) and in the laboratory to disinfect exhaust-protective cabinets. A high relative humidity is essential for effective killing.
The liquid glutaraldehyde is used to disinfect heat-sensitive articles
Glutaraldehyde is less toxic than formaldehyde and can be stabilized in solution to remain active for up to several weeks at in-use concentration. It is used for the disinfection of, but does not sterilize, heat-sensitive articles such as endoscopes and for inanimate surfaces.
Many different antimicrobial chemicals are available, but few are sterilant
Some, like the derivatives of pine and turpentine, have been known since ancient times, and chloride of lime and coal tar fluids were in use before the germ theory of disease was established. Most fall into the category of disinfectant or antiseptic, but a few are capable of rendering articles sterile. Factors that affect their efficacy include:
• physical environment (e.g. porous or cracked surfaces)
• presence of moisture
• temperature and pH
• concentration of the agent
• hardness of water
• the bioburden on the object to be disinfected
• the nature and state of the microbes in the bioburden
• the ability of the microbes to inactivate the chemical agent.
It is obvious that the above factors are difficult to control in every circumstance. The main groups of chemical agents are shown in Table 36.6. They act by causing chemical damage to proteins, nucleic acids or cell membrane lipids. The activity of a given disinfectant may result from more than one pathway of damage.
Table 36.6 Examples of disinfectants for use in hospitals
|
Group |
Examples |
Advantages and disadvantages |
|
Phenolics |
Clear-soluble phenolic compounds, white fluids |
Good general-purpose disinfectants, not readily inactivated by organic matter, active against wide range of organisms including mycobacteria, not sporicidal |
|
Chloroxylenols |
Inactivated by hard water and organic matter, Pseudomonas grows readily in chloroxylenol solutions, limited activity against other Gram-negatives |
|
|
Halogens |
Hypochlorites (chloramine) |
Cheap, effective, act by release of free chlorine, active against viruses and therefore recommended for disinfection of equipment soiled with blood (because of hepatitis and HIV risk), inactivated by organic material, corrode metals |
|
Iodine and iodophors |
Useful skin disinfectants, sporicidal |
|
|
Quaternary ammonium compounds |
Benzalkonium chloride, cetyltrimethylammonium bromide |
Have detergent properties, activity against Gram-negative ← Gram-positive, improved by combination with biguanide, e.g. chlorhexidine, useful as skin disinfectants, inactivated by hard water and organic materials, contamination of stock solutions with Gram-negative rods can be a problem |
|
Diguanides |
Chlorhexidine |
Useful disinfectant for skin and mucous membranes, inactivated by many materials and too expensive for environmental use, alcoholic solutions are less easily contaminated, combinations of chlorhexidine and detergent highly effective for disinfection of hands |
|
Alcohols |
Ethyl alcohol, isopropyl alcohol |
Good choice for skin disinfection and for clean surfaces, sometimes used in combination with iodine or chlorhexidine (see above), water must be present for bacterial killing (i.e. 70% ethanol best), isopropyl preferred for skin and articles in contact with patient |
|
Aldehydes |
Formaldehyde/formalin |
Too irritant for use as general disinfectant |
|
Glutaraldehyde |
Kills vegetative organisms, including mycobacteria, slowly but effectively, more active, less toxic than formaldehyde, sporicidal (within 6 h when fresh), slightly irritant, used in alkaline solution which is stable for 1–2 weeks, expensive, limited use, e.g. disinfection of endoscopes |
|
|
Chlorinated bisphenols |
Hexachorophene |
Activity against Gram-positive → Gram-negative, used in soap or dusting powder as skin disinfectant (use restricted after potentially toxic blood levels found in infants who had hexachlorophene emulsion spread over whole body) |
|
Triclosan, a polychloro phenoxy phenol is used as substitute for hexachlorophene in personal products, antibacterial effect on repeated use |
Note that no one group of disinfectant has all the properties desirable for use both on skin and on inanimate surfaces.
Controlling sterilization and disinfection
In general, it is preferable to control the process rather than the product
This means that it is better to run checks on the technique while it is in operation rather than attempting to recognize process failure by isolating microorganisms from the product. Trying to discover whether one or a few viable organisms remain is analogous to trying to find a needle in a haystack. It is known that damaged bacteria can recover, given time and special nutrient recovery media, but it may not be feasible to hold back a batch of product for such tests. In addition, how many samples of the product should be tested? If too few are examined, the likelihood of missing a failed sample is high; if too many are examined, too much of the batch is used up in quality control to be economically sensible.
The usual process controls are either physical or chemical checks on the technique, for example, tests that show that the autoclave reached the desired temperature for the desired time. They do not show that there are no viable organisms remaining after the process, but this is assumed if the process satisfies the controls. However, the stringency of the controls can be altered intentionally or accidentally to give either an undersensitive or oversensitive test.
Disinfectants can be monitored by microbiologic ‘in-use’ tests
These tests involve challenging the solution with a bacterial suspension and withdrawing samples, which are then treated to prevent carryover of the disinfectant and cultured. However, these tests are rarely performed in the hospital setting, where the use of disinfectants is guided largely by the manufacturer’s recommendations.
![]()
 Key Facts
Key Facts
• Realization that infection can be associated with a variety of institutional settings has resulted in a preference for the term ‘healthcare-associated infection’ rather than hospital-acquired infection.
• Nosocomial infection refers to infection acquired in hospital.
• Hospital infections often have serious consequences for the individual, for the hospital community and for the community at large. They may be caused by almost any organism, but a few species cause the vast majority of infections.
• The hospital environment favours the survival of resistant strains and therefore infections are often caused by organisms with limited antibiotic susceptibility.
• MRSA, traditionally viewed as a problem in hospital infection, are increasingly seen in community-acquired infections in the absence of healthcare contact.
• Most common hospital infections are UTIs, respiratory tract infections, surgical wound infections and bacteraemia (septicaemia).
• The most important bacterial causes are Gram-positive cocci (staphylococci and streptococci) and Gram-negative rods (e.g. E. coli, Pseudomonas). Multiply-antibiotic-resistant organisms are common. Candida is the significant fungal cause, and viruses probably cause more hospital infections than previously recognized.
• Infecting organisms originate from the patient’s own flora (endogenous infection) or from other human or inanimate sources (exogenous or cross-infection). Airborne and contact spread are the most important routes of transmission.
• Host factors are of critical importance in determining susceptibility to infection.
• Surveillance should be an ongoing activity to facilitate early recognition of outbreaks of infection. Investigation of outbreaks involves both epidemiologic and microbiologic expertise. Molecular techniques to ‘fingerprint’ the causative organism are becoming increasingly sophisticated.
• Prevention of hospital infections by excluding sources, interrupting transmission and enhancing the patient’s resistance is fundamental to improving patient care and reducing costs.
• Sterilization and disinfection are key processes in the control and prevention of hospital infections as well as being central to many areas of medical practice.
![]()