5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 4 Review the Knowledge You Need to Score High
9 Solids, Liquids, and Intermolecular Forces
IN THIS CHAPTER
In this chapter, the following AP topics are covered:
2.2 Intramolecular Force and Potential Energy
2.3 Structure of Ionic Solids
3.1 Intermolecular Forces
3.2 Properties of Solids
Summary: In the chapter on gases, we discuss the gaseous state. In this chapter, we will discuss the liquid and solid states and the forces that exist between the particles—the intermolecular forces. A substance’s state of matter depends on two factors: the average kinetic energy of the particles and the intermolecular forces between the particles. The kinetic energy tends to move the particles away from each other. The temperature of the substance is a measure of the average kinetic energy of the molecules. As the temperature increases, the average kinetic energy increases, and the particles tend to move farther apart. This is consistent with our experience of heating ice, for example, and watching it move from the solid state to the liquid state and finally to the gaseous state. For this to happen, the kinetic energy overcomes the forces between the particles, the intermolecular forces.
In the solid state, the kinetic energy of the particles cannot overcome the intermolecular forces; the particles are held close together by the intermolecular forces. As the temperature increases, the kinetic energy increases and begins to overcome the attractive intermolecular forces. The substance will eventually melt, going from the solid to the liquid state. As this melting takes place, the temperature remains constant even though energy is being added. The temperature at which the solid converts into the liquid state is called the melting point (m.p.) of the solid.
After all the solid has been converted into a liquid, the temperature again starts to rise as energy is added. The particles are still relatively close together and possess enough kinetic energy to move with respect to each other. Finally, if enough energy is added, the particles start to break free of the intermolecular forces, keeping them relatively close together and they escape the liquid as essentially independent gas particles. This process of going from the liquid state to the gaseous state is called boiling, and the temperature at which this occurs is called the boiling point (b.p.) of the liquid. Sometimes, however, a solid can go directly from the solid state to the gaseous state without ever having become a liquid. This process is called sublimation. Dry ice, solid carbon dioxide, readily sublimes.
These changes of state, also called phase changes, are related to temperature, but pressure changes will also influence the changes in state. We will see how these relationships can be diagrammed later in this chapter.
Remember that these phase changes are due to changes in the average kinetic energy of the particles. The intermolecular forces remain the same.

Keywords and Equations
No specific keywords or equations are listed on the AP Exam for this topic.
Intramolecular and Intermolecular Forces
Intermolecular forces are attractive or repulsive forces between particles caused by partial charges. These attractive forces are the ones that work to overcome the randomizing forces of kinetic energy. The structure and type of bonding of a specific substance have quite a bit to do with the type of interaction and the strength of that interaction. Before we start examining the different types of intermolecular forces, recall from Chapter 8, Bonding, that those molecules that have polar covalent bonding (unequal sharing of the bonding electron pair) may possess dipoles (having positive and negative ends due to charge separation within the molecule). Dipoles are often involved in intermolecular forces. Collectively, all of these are often referred to as van der Waals forces. Do not use this term to refer to any individual force. For large molecules, often biomolecules, the intermolecular forces may be between different regions of the same molecule.
Figure 9.1 illustrates a simplified view of a small group of water molecules. Each water molecule is enclosed within a dotted circle. Within each dotted circle are the intRAmolecular forces (covalent bonds in this case), and between each dotted circle are the intERmolecular forces (primarily hydrogen bonding here) (note the spelling differences).
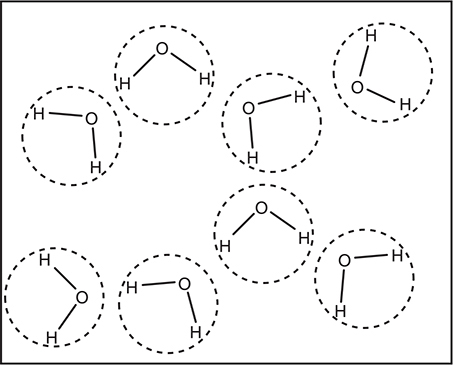
Figure 9.1 A collection of a few water molecules.
The following discussion introduces the different intermolecular forces, and with one exception, it is in order of decreasing strength.
Ionic, Covalent, and Metallic Bonds
These bonds were introduced in Chapter 8, and while they are not true intermolecular forces, there are many situations where the intermolecular forces discussed in the section must compete with these bonds. For example, when an ionic solid dissolves in water, it is necessary to relate the ionic bonds in the solid with the ion—dipole forces that form. Therefore, a few observations concerning these bonds need to be made.
When dealing with ionic bonds, the magnitude of the charges and the sizes of the ions are important. Coulomb’s law is important and is the structure of the solid. The greater the charge, the stronger the ionic bond. The smaller the size of the ion, the stronger the ionic bond. The effect of structure is normally small. For example, there are two forms of ZnS, with one being 0.2% more stable than the other, even though the charges and sizes are identical.
For covalent bonds, both the strength and the lengths of the bonds depend upon the atoms involved and the bond order. Smaller atoms tend to form stronger and shorter bonds than larger atoms do, but comparisons must deal with comparing bonds with the same bond order. For the representative elements, the bonds may be single, double, or triple (bond orders 1, 2, and 3, respectively). Other possibilities are known for the transition metals; however, these will not appear on the AP Exam. The higher the bond order, the stronger and shorter the bond.
Predictions involving metallic bonds are beyond the AP Exam.
Intermolecular Forces
Intermolecular forces are a group of attractive interactions occurring between separate molecules or ions. These are not “normal” bonds (ionic, covalent, or metallic). In general, these interactions are relatively weak. The interactions holding molecules, ions, or metal atoms together are a separate class referred to as bonds. Bonds are intramolecular forces (note the spelling difference).
Ion—Dipole Intermolecular Forces
These forces are due to the attraction of an ion and one end of a polar molecule (dipole). This type of attraction is especially important in aqueous salt solutions, where the ion attracts water molecules and may form a hydrated ion, such as Al(H2O)63+. This is one of the strongest of the intermolecular forces.
It is also important to realize that this intermolecular force requires two different species—an ion and a polar molecule. This type of intermolecular force is especially important in aqueous solutions containing an electrolyte. When a water-soluble ionic compound, such as sodium chloride, dissolves in water, the sodium ions and the chloride ions dissociate from each other. This dissociation requires energy. Compensation for the energy required comes from the formation of ion—dipole forces. Figure 9.2 illustrates, in a simplistic way, some of these ion—dipole forces. The two ion—dipole forces formed in the figure do not release sufficient energy to compensate for all the energy required to dissociate NaCl; however, in reality, each ion has a large number (typically four or more) water molecules crowded around it, which allows the formation of many more ion—dipole attractions (far more than shown in the figure). The energy produced through the formation of all these attractions provides the energy required for the dissociation of the NaCl. Indeed, this argument may be used for all water-soluble ionic substances. When this compensation does not occur, the compound tends to be insoluble in water. (Note that the formation of ion—dipole forces is not the only factor governing the solubility of a substance.) Note also that ion—dipole forces are much stronger than dipole—dipole forces, including hydrogen bonds, because the charge on the ion is a “full” charge, which is much greater than the partial charges involved in polar covalent bonds. Do not forget that ion—dipole forces require two different species—an ion and a polar molecule.
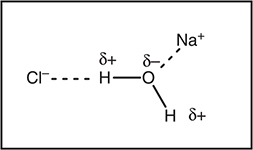
Figure 9.2 Two of the possible ion—dipole forces (dotted lines) that form when sodium chloride, NaCl, dissolves in water.
Dipole—Dipole Intermolecular Forces
These forces result from the attraction of the positive end of one dipole to the negative end of another dipole. For example, in gaseous hydrogen chloride, HCl(g), the hydrogen end has a partial positive charge, and the chlorine end has a partial negative charge, due to chlorine’s higher electronegativity. Dipole—dipole attractions are especially important in polar liquids. They tend to be a rather strong force, although not as strong as ion—dipole attractions. These forces may be present in a single substance or in a solution.
Dipole—dipole forces are always present in diatomic molecules containing atoms with differing electronegativities. If a molecule contains more than two atoms, it is necessary to consider the molecular geometry in addition to the electronegativity. Figure 9.3 illustrates the dipole—dipole attraction (dotted line) between a hydrogen bromide molecule and a water molecule. Both molecules contain polar covalent bonds. The dipole—dipole force is present due to the opposite partial charges (δ+ and δ—) between the H and Br atoms. Note that if the bromine were replaced with fluorine, this would be a much stronger hydrogen bond.
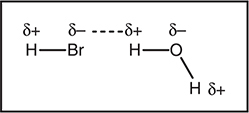
Figure 9.3 This illustrates the dipole—dipole attraction (dotted line) between a hydrogen bromide molecule and a water molecule.
Hydrogen Bond Intermolecular Forces
Hydrogen bonding is a special type of dipole—dipole attraction in which a hydrogen atom is polar-covalently bonded to one of the following extremely electronegative elements: N, O, or F. These hydrogen bonds are extremely polar bonds by nature, so there is a great degree of charge separation within the molecule. Therefore, the attraction of the positively charged hydrogen of one molecule and the negatively charged N, O, or F of another molecule is extremely strong. These hydrogen bonds are, in general, stronger than the typical dipole—dipole interaction. This is the only intermolecular force in this discussion that is out of order, as it is stronger than the preceding intermolecular force.
An individual molecule of water can potentially form four hydrogen bonds, as illustrated in Figure 9.4. Both hydrogen—oxygen bonds in each water molecule are polar covalent bonds. The four hydrogen bonds, in reality, have a three-dimensional arrangement about the central water molecule instead of the two-dimensional arrangement shown in the figure. As illustrated, the hydrogen bonds form between the partial charges on the hydrogen atoms (δ+) and the oxygen atoms (δ—). These are not normal dipole—dipole forces because the partial charge on the hydrogen atoms is much greater than the partial positive charges present in most other polar molecules.
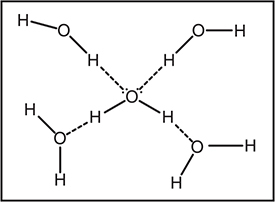
Figure 9.4 An individual molecule of water has the potential to form four hydrogen bonds (dotted lines).
Hydrogen bonding explains why HF(aq) is a weak acid, while HCl(aq), HBr(aq), and HI(aq) are strong acids. The hydrogen bond between the hydrogen of one HF molecule and the fluorine of another “traps” the hydrogen, so it is much harder to break its bonds and free the hydrogen to be donated as an H+. Hydrogen bonding also explains why water has such unusual properties—for example, it has an unusually high boiling point and its solid phase is less dense than its liquid phase. The hydrogen bonds tend to stabilize the water molecules and keep them from readily escaping into the gas phase. When water freezes, the hydrogen bonds are stabilized and lock the water molecules into a framework with a lot of open space. Therefore, ice floats in liquid water. Hydrogen bonding also holds the strands of DNA together.
Molecules such as CH3OH dissolve in H2O not because the molecules have hydrogen bonds but because H2O and CH3OH can form hydrogen bonds with each other.

There can be no hydrogen bonds if a molecule has no H (contrary to some answers on the AP Exam). In addition, the presence of H does not automatically mean that hydrogen bonds may form.
Ion-Induced Dipole and Dipole-Induced Dipole Intermolecular Forces
These types of attraction occur when the charge on an ion or a dipole distorts the electron cloud of a nonpolar molecule and induces a temporary dipole in the nonpolar molecule. Like ion—dipole intermolecular forces, these also require two different species, which means they are important in solutions. They are relatively weak interactions. Increasing the charge on an ion or the polarity of a dipole will increase the strength of these types of interactions.
London (Dispersion) Intermolecular Force
This intermolecular attraction occurs in all substances; however, London dispersion forces are only significant when the other types of intermolecular forces are absent. It arises from a momentary distortion of the electron cloud, with the creation of a very weak dipole. The weak dipole induces a dipole in another nonpolar molecule. This is an extremely weak interaction, but it is strong enough to allow us to liquefy nonpolar gases such as hydrogen, H2, and nitrogen, N2, and the noble gases like helium, He. If there were no intermolecular forces attracting these molecules, it would be impossible to liquefy them. The nitrogen molecule has more electrons than either the hydrogen molecule or the helium atom, and consequently N2 has the highest boiling point of the three. In general, the greater the number of electrons, the greater the London dispersion force (polarizability of the electrons), and for very large molecules the combination may result in a total attraction greater than from the other intermolecular forces. In addition, the presence of π bonds increases the polarizability of the electron cloud. An increase in the contact area between the molecules enhances the strength of London dispersion forces.
Let’s consider the formation of a London dispersion force between two helium atoms. The London dispersion force is due to the presence of temporary dipoles. The helium atom on the left in Figure 9.5 shows the more stable distribution of electrons (small black circles), which is as far from each other as possible. However, since electrons are continually moving, the arrangement in the helium atom on the right will occur from time to time. The arrangement on the right creates a dipole about this helium atom. Since electrons are continually moving, this dipole only lasts for an instance, hence the description as an instantaneous dipole. The dotted line in the figure represents how the instantaneous dipole attracts one of the electrons on the helium atom on the left. The more electrons present, the greater the number of possible instantaneous dipoles. In the case of very large molecules, instantaneous dipoles about interior atoms may be considered intramolecular forces instead of the intermolecular forces formed by atoms on the exterior.
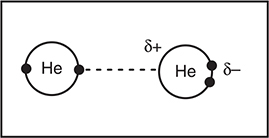
Figure 9.5 The London dispersion force between two helium atoms. See text for an explanation.

London dispersion forces and the other intermolecular forces (including hydrogen bonds) are NOT bonds.
While, in general, the more electrons, the greater the London dispersion forces, simply counting electrons is not the complete answer. This is illustrated by comparing the boiling points of helium, He, and deuterium, 2H2 or D2. Both substances contain two electrons and have approximately equal masses (He = 4.00 g mol—1 and D2 = 4.028 g mol—1); therefore, their London forces would be expected to be the same, leading to nearly identical boiling points. However, the boiling point of He is 4.3 K, and the boiling point of D2 is more than 5 times this at 23.7 K. Obviously, the presence of more than one atom makes a difference.
The Liquid State
At the microscopic level, liquid particles are in constant flux. They may exhibit short-range areas of order, but these do not last very long. Clumps of particles may form and then break apart. At the macroscopic level, a liquid has a specific volume but no fixed shape. Three other macroscopic properties deserve discussion: surface tension, viscosity, and capillary action. In the body of a liquid, the molecules are pulled in all different ways by the intermolecular forces between them. At the surface of the liquid, the molecules are only being pulled into the body of the liquid from the sides and below, not from above. The effect of this unequal attraction is that the liquid tries to minimize its surface area by forming a sphere. In a large pool of liquid, where this is not possible, the surface behaves as if it had a thin “skin” over it. It requires force to break the attractive forces at the surface. The amount of force required to break through this molecular layer at the surface is called the liquid’s surface tension. The greater the intermolecular forces, the greater the surface tension. Polar liquids, especially those that contain hydrogen bonds, have a much higher surface tension than nonpolar liquids.
Viscosity, the resistance of liquids to flow, is affected by intermolecular forces, temperature, and molecular shape. Liquids with strong intermolecular forces tend to have a higher viscosity than those with weak intermolecular forces. Again, polar liquids tend to have a higher viscosity than nonpolar liquids. As the temperature increases, the kinetic energy of the particles becomes greater, overcoming the intermolecular attractive forces. This causes a lower viscosity. Finally, the longer and more complex the molecules, the more contact the particles will have as they slip by each other, increasing the viscosity. The change in viscosity with a change in temperature is illustrated by the difference in behavior of cold honey versus warm honey.
Capillary action is the spontaneous rising of a liquid through a narrow tube against the force of gravity. It is caused by competition between the intermolecular forces in the liquid and those attractive forces between the liquid and the tube wall. The stronger the attraction between the liquid and the walls, the higher the level will be. Liquids that have weak attractions to the walls, like mercury in a glass tube, have a low capillary action. Liquids like water in a glass tube have strong attractions to the walls and will have a high capillary action.
As we have noted before, water, because of its very strong intermolecular forces (hydrogen bonding) has some unusual properties. It will dissolve a great number of substances, both ionic and polar covalent, because of its polarity and ability to form hydrogen bonds. It is sometimes called the “universal solvent.” Water has a high heat capacity, the heat absorbed to cause the temperature to rise, and a high heat of vaporization, the heat needed to transform the liquid into a gas. These thermal properties are due to the strong hydrogen bonding between the water molecules. Water has a high surface tension for the same reason. The fact that the solid form of water (ice) is less dense than liquid water is because water molecules in ice are held in a rigid, open, crystalline framework by the hydrogen bonds. As the ice starts melting, the crystal structure breaks and water molecules fill the holes in the structure, increasing the density. The density reaches a maximum at around 4°C; then the increasing kinetic energy of the particles causes the density to begin to decrease.
The Solid State
At the macroscopic level, a solid is defined as a substance that has both a definite volume and a definite shape. At the microscopic level, solids may be one of two types—amorphous or crystalline. Amorphous solids lack extensive ordering of the particles. There is a lack of regularity of the structure. There may be small regions of order separated by large areas of disordered particles. They resemble liquids more than solids in this characteristic. Amorphous solids have no distinct melting point. They simply get softer and softer as the temperature rises, leading to a decrease in viscosity. Glass, rubber, and charcoal are examples of amorphous solids.
When a solid melts, energy must be added (this same energy would be released upon freezing). This energy is not as great as the energy required to boil a liquid, because, unlike melting, vaporization leaves the constituents no longer in contact. In general, sublimation is the sum of the energy required for melting and the energy required for vaporization. The magnitudes of these energies depend upon the strengths of the bonds and/or intermolecular forces in the substance.
Crystalline solids display a very regular ordering of the particles in a three-dimensional structure called the crystal lattice. In this crystal lattice there are repeating units called unit cells. Figure 9.6 shows the relationship of the unit cells to the crystal lattice.
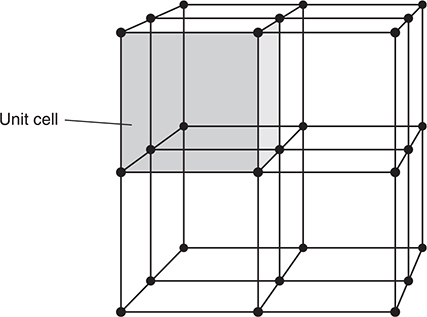
Figure 9.6 The crystal lattice for a simple cubic unit cell.
Several types of unit cells are found in solids. The cubic system is a common type. Three types of unit cells are found in the cubic system:
1. The simple cubic unit cell has particles located at the corners of a simple cube.
2. The body-centered unit cell has particles located at the corners of the cube and in the center of the cube.
3. The face-centered unit cell has particles at the corners and one in the center of each face of the cube, but not in the center of the cube itself.
Figure 9.7 shows three types of cubic unit cells.
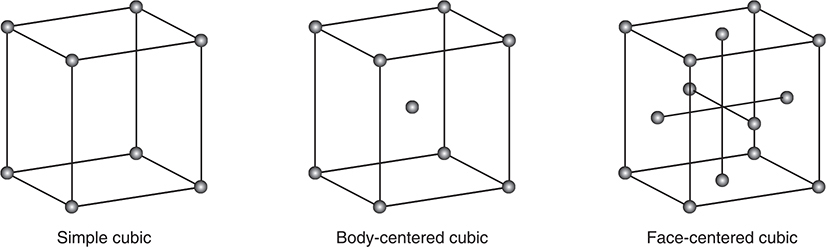
Figure 9.7 The three types of unit cell of the cubic lattice.
Five types of crystalline solid are known:
1. In atomic solids, individual atoms are held in place by London forces. The noble gases are the only substances known to form atomic solids; therefore, atomic solids are often grouped with molecular solids.
2. In molecular solids, lattices composed of separate molecules are held in place by London forces, dipole—dipole forces, and hydrogen bonding. Solid methane and water (ice) are examples of molecular solids. As the forces holding the molecules together are relatively weak, molecular solids tend to be soft nonconductors with low melting and boiling points. Some molecular solids may contain extremely large polymer molecules or biomolecules that have stronger interactions as a cumulative result of the London dispersion forces or other van der Waals forces.
3. In ionic solids, lattices composed of ions are held together by the attraction of the opposite charges of the ions (ionic bonding). These crystalline solids tend to be hard, with high melting points because of the strength of the intermolecular forces. NaCl and other salts are examples of ionic solids. Figure 9.8 shows the lattice structure of NaCl. Each sodium cation is surrounded by six chloride anions, and each chloride anion is surrounded by six sodium cations. The ions adjust to maximize the attraction between oppositely charged ions and to minimize the repulsion between ions with like charges.
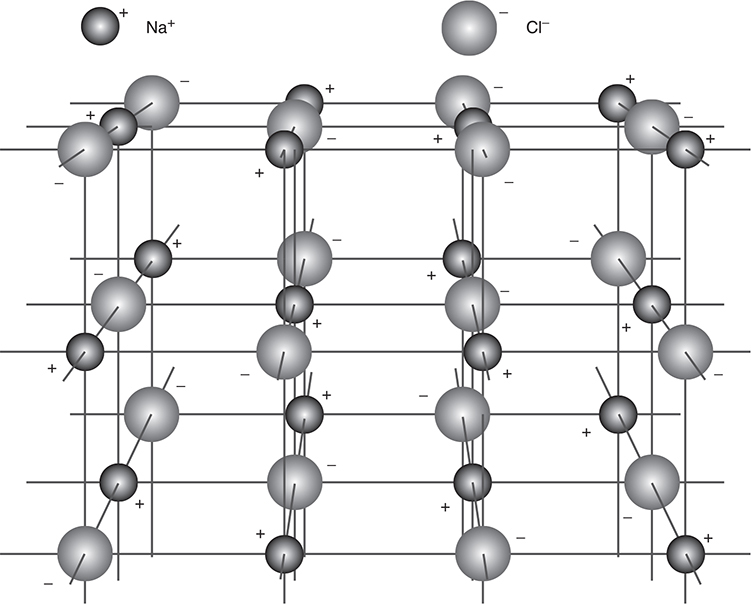
Figure 9.8 Sodium chloride crystal lattice.
The strong interactions in ionic solids lead to low vapor pressure, high melting points, and, indirectly, high boiling points. Even though ions are present, ionic solids will not conduct electricity unless the ions become mobile, which occurs in the molten state and in solution. Most ionic solids are relatively brittle.
4. In metallic solids, metal atoms occupying the crystal lattice are held together by metallic bonding. In metallic bonding, the electrons of the atoms are delocalized and are free to move throughout the entire solid. This explains electrical and thermal conductivity, as well as many other properties of metals. Compared to other types of bonds, metallic bonds can be easily deformed, which results in metals being malleable and ductile.
5. In covalent network solids, covalent bonds join atoms together in the crystal lattice, which is quite large. There are not too many examples in this category. Graphite, diamond, and silicon dioxide (SiO2) are examples of network solids. The crystal is one giant molecule. In general, covalent network solids are hard nonconductors with high melting and boiling points. In most cases, the covalent network is a three-dimensional one. Graphite is a well-known exception as the network is only two dimensional, it is soft, and it is a conductor of electricity.
Relationship of Intermolecular Forces to Phase Changes
The intermolecular forces can affect phase changes to a great degree. The stronger the intermolecular forces present in a liquid, the more kinetic energy must be added to convert it into a gas. Conversely, the stronger the intermolecular forces between the gas particles, the easier it will be to condense the gas into a liquid. In general, the weaker the intermolecular forces, the higher the vapor pressure. The same type of reasoning can be used about the other phase equilibria—in general, the stronger the intermolecular forces, the higher the heats of transition (heat of vaporization, etc.).
Example: Based on intermolecular forces, predict which will have the higher vapor pressure and higher boiling point: methyl alcohol, CH3—OH, or dimethyl ether, CH3—O—CH3.
Answer: Dimethyl ether will have the higher vapor pressure and the lower boiling point.
Explanation: Methyl alcohol is a polar substance with strong intermolecular hydrogen bonds. Dimethyl ether is a polar material with weaker intermolecular forces (dipole—dipole). It will take much more energy to vaporize water; thus, water has a lower vapor pressure and higher boiling point.
Note: Even though organic chemistry is not an AP Chemistry topic, questions containing organic compounds may still appear. In this example, the key is intermolecular force, not organic chemistry.
Potential Energy
One consequence of the interaction between two atoms is the “bond length” or most stable separation between two atoms. The complex interactions are illustrated in Figure 9.9.
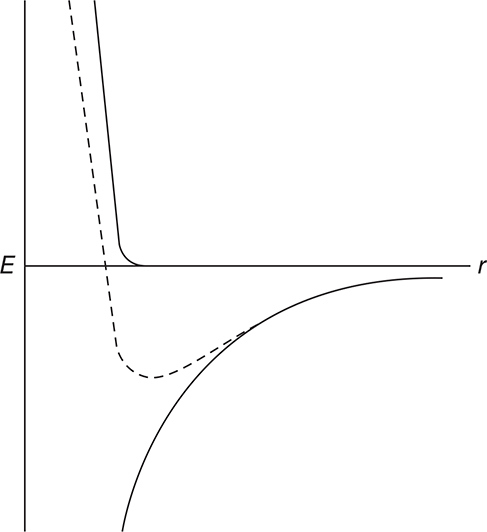
Figure 9.9 The potential energy, E, between two atoms as a function of the distance between the two atoms, r. See the text for an explanation.
In the graph, the lower solid line represents the attraction between the two atoms. According to Coulomb’s law, this attraction will increase as opposite charges get closer together. The upper solid line represents the repulsion resulting when the electron clouds of the two atoms begin to penetrate each other. This repulsion also follows Coulomb’s law; however, now it is the repulsion between like charges. The result of these two factors is the dotted line. The minimum in the dotted-line curve is the equilibrium “bond length.” This minimum also indicates the energy necessary to separate the atoms.
Experiments

The concept of intermolecular forces is important in the separation of the components of a mixture. For example, you are given a sample containing sand and salt and wish to determine the percentage of sand present.
Common Mistakes to Avoid

1. Don’t confuse the various types of intermolecular forces.
2. The melting point and the freezing point for a substance are identical.
3. Hydrogen bonding can occur only when a hydrogen atom is directly bonded to an N, O, or F atom.
4. In looking at crystal lattice diagrams, be sure to count all the particles, in all three dimensions, that surround another particle.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry Exam. Below are 21 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry Exam. This set of questions includes some questions to test your recollection of prior knowledge. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may use the periodic table and the equation sheet at the back of this book.
1. If you have a single water molecule, H2O, what is the strongest intermolecular force present?
(A) Dipole—dipole force
(B) Hydrogen bonding
(C) Covalent bonding
(D) There is no intermolecular force.
2. Which of the following best describes Fe(s)?
(A) Iron is composed of macromolecules held together by strong bonds.
(B) Iron is composed of atoms held together by delocalized electrons.
(C) Iron is composed of positive and negative ions held together by electrostatic attractions.
(D) Iron is composed of molecules held together by intermolecular dipole—dipole interactions.
3. The best description of the interactions in KNO3(s) is which of the following?
(A) KNO3 is composed of macromolecules held together by strong bonds.
(B) KNO3 is composed of atoms by delocalized electrons.
(C) KNO3 is composed of positive and negative ions held together by electrostatic attractions.
(D) KNO3 is composed of molecules held together by intermolecular dipole—dipole interactions.
4. Sand is primarily SiO2(s). Which of the following best describes the interactions inside a grain of sand?
(A) Sand is composed of macromolecules held together by strong bonds.
(B) Sand is composed of atoms held together by delocalized electrons.
(C) Sand is composed of positive and negative ions held together by electrostatic attractions.
(D) Sand is composed of molecules held together by intermolecular dipole—dipole interactions.
5. At sufficiently low temperatures, it is possible to form HCl(s). What best describes the interactions in this solid?
(A) HCl(s) is composed of macromolecules held together by strong bonds.
(B) HCl(s) is composed of atoms held together by delocalized electrons.
(C) HCl(s) is composed of positive and negative ions held together by electrostatic attractions.
(D) HCl(s) is composed of molecules held together by intermolecular dipole—dipole interactions.
6. Which of the following best describes diamond, C(s)?
(A) Diamond is an ionic solid.
(B) Diamond is a metallic solid.
(C) Diamond is a molecular solid containing polar molecules.
(D) Diamond is a covalent network solid.
7. What type of solid is solid sulfur dioxide, SO2(s)?
(A) Sulfur dioxide is an ionic solid.
(B) Sulfur dioxide is a metallic solid.
(C) Sulfur dioxide is a molecular solid containing polar molecules.
(D) Sulfur dioxide is a covalent network solid.
8. The approximate boiling points for hydrogen compounds of some elements in the nitrogen family are (SbH3 —15°C), (AsH3 —62°C), (PH3 —87°C), and (NH3 —33°C). Which of the following is the best explanation for the fact that NH3 does NOT follow the trend of the other hydrogen compounds?
(A) NH3 is the only one to exhibit hydrogen bonding.
(B) NH3 is the only one that is water soluble.
(C) NH3 is the only one that is nearly ideal in the gas phase.
(D) NH3 is the only one that is a base.
9. Why is it possible to solidify argon at a sufficiently low temperature?
(A) London dispersion forces are present.
(B) Covalent bonds form.
(C) Hydrogen bonds form.
(D) Metallic bonds form.
10. Which of the following best describes why diamond is so hard?
(A) London dispersion forces are present.
(B) Covalent bonds are present.
(C) Hydrogen bonds are present.
(D) Metallic bonds are present.
11. A sample of a pure liquid is placed in an open container and heated to the boiling point. Which of the following may increase the boiling point of the liquid?
(A) The moles of liquid are increased.
(B) The size of the container is increased.
(C) A vacuum is created over the liquid.
(D) The container is sealed.
12. The compounds 1-butanol, CH3CH2CH2CH2OH, and diethyl ether, CH3CH2OCH2CH3, are isomers (have the same chemical formula, C4H10O). However, the surface tension of 1-butanol is higher than that of diethyl ether. Which of the following best explains the higher surface tension of 1-butanol than that of diethyl ether?
(A) the higher density of 1-butanol
(B) the lower specific heat of 1-butanol
(C) the lack of hydrogen bonding in 1-butanol
(D) the presence of hydrogen bonding in 1-butanol
13. The compounds propanol, CH3CH2CH2OH, ethylene glycol, HOCH2CH2OH, and butane, CH3CH2CH2CH3, are all similar in size. Which of the following most likely represents the relative solubilities of these compounds in water?
(A) CH3CH2CH2OH < HOCH2CH2OH < CH3CH2CH2CH3
(B) CH3CH2CH2CH3 < HOCH2CH2OH < CH3CH2CH2OH
(C) CH3CH2CH2CH3 < CH3CH2CH2OH < HOCH2CH2OH
(D) CH3CH2CH2OH < CH3CH2CH2CH3 < HOCH2CH2OH
14. Each of the following compounds has a similar structure, and the interionic distances are about the same. Which compound is expected to have the highest lattice energy?
(A) LiCl
(B) MgO
(C) AlN
(D) NaF
15. The melting point of sodium fluoride, NaF, is 993°C, and the melting point of calcium fluoride, CaF2, is 1,423°C. Which of the following best explains why the melting point of calcium fluoride is higher?
(A) Sodium is a more reactive element than calcium is.
(B) The sodium ion is smaller than the calcium ion.
(C) The sodium ion has a lower charge than the calcium ion.
(D) There are fewer fluoride ions in the formula of sodium fluoride.
16. Pure phosphoric acid, H3PO4, freezes at about 42°C. Which of the following best describes the interactions in solid phosphoric acid?
(A) The solid consists of a collection of molecules held together by London dispersion forces.
(B) The solid consists of a collection of atoms held together by delocalized electrons.
(C) The solid consists of positive and negative ions held together by ionic bonding.
(D) The solid consists of molecules held together by intermolecular dipole—dipole interactions.
17. Which of the following liquids will probably have the highest viscosity at a given temperature?
(A) CH3CH2CH2OH
(B) PCl3
(C) CCl4
(D) CH3CH2CH2CH3
18. Most molecular species vaporize as simple molecules. However, acetic acid, CH3COOH, is an exception. In the gaseous state, acetic acid molecules exist as pairs known as dimers. What is the most probable cause of this behavior?
(A) London dispersion forces
(B) covalent bonding
(C) hydrogen bonding
(D) metallic bonding
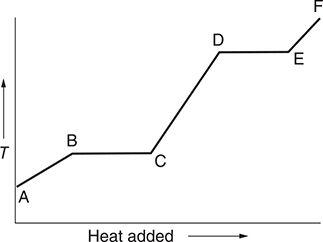
19. The above diagram represents the heating curve for a pure crystalline substance. Both solid and liquid are present in which region of the heating curve?
(A) between points A and B
(B) between points B and C
(C) between points C and D
(D) between points D and E
20. What is the strongest type of intermolar force present in an aqueous solution of sodium nitrate, NaNO3?
(A) Ionic bonding
(B) Ion-dipole forces
(C) Dipole-dipole forces
(D) Hydrogen bonding
21. Fish and many other life-forms living in water survive by “breathing” the oxygen dissolved in the water. What type of intermolecular forces are present in O2(aq) that are not present in the separated O2(g) and H2O(l)?
(A) Hydrogen bonding
(B) Covalent bonding
(C) Dipole-induced dipole forces
(D) Dipole-dipole forces
![]() Answers and Explanations
Answers and Explanations
1. D—Covalent bonding is not an intermolecular force; it is an intramolecular force (note the spelling difference). While both dipole—dipole forces and hydrogen bonding are intermolecular forces, they cannot be present in this case because all intermolecular forces are between molecules, and if there is only one water molecule, there is no other molecule for this one molecule to be attracted to. Unlike the covalent bonds present in water, molecules of water do not “have” hydrogen bonds; they can “form” hydrogen bonds.
2. B—Iron is a metal, and this answer describes a metallic solid.
3. C—Potassium nitrate is an ionic solid, and this answer describes an ionic solid.
4. A—Silicon dioxide is a covalent network solid, and this answer describes a covalent network solid.
5. D—Hydrogen chloride is a polar molecule, and this answer describes a solid consisting of discrete polar molecules. Even though HCl(aq) is a strong acid with ions in solution, there is no water here to lead to ionization.
6. D—Each of the carbon atoms is covalently bonded to four other carbon atoms.
7. C—Sulfur dioxide exists as molecules (molecular solid) that are polar.
8. A—The trend is the lighter the molecule, the lower the melting point, except for NH3. For NH3 to be an exception, its intermolecular forces must be different. Hydrogen bonding occurs when hydrogen is directly bonded to F, O, and in this case, N. The other three molecules have only dipole—dipole forces.
9. A—Argon is a noble gas; none of the other bonding choices is an option. Everything has London dispersion forces.
10. B—Diamond is a covalent network solid with many strong covalent bonds between the carbon atoms.
11. D—The size of the container and the number of moles are irrelevant, which eliminates answers A and B. Sealing the container will cause an increase in pressure that will increase the boiling point. A decrease in pressure will lower the boiling point, which eliminates answer C.
12. D—The compound with the higher surface tension is the one with the stronger intermolecular force. The —OH groups can form hydrogen bonds. The hydrogen bonding in 1-butanol is stronger than the dipole—dipole attractions in diethyl ether.
13. C—Butane is nonpolar, whereas the other two compounds can form hydrogen bonds through the —OH groups. This means that the butane should be listed first, which eliminates answers A and D. The more —OH groups, the more hydrogen bonding, and the more soluble in water (where hydrogen bonding also occurs). Ethylene glycol, with two —OH groups, should be more soluble (listed last) because propanol only has one —OH.
14. C—If the structures are similar and the interionic distances are about the same, then the only factor remaining that will influence the lattice energy is the magnitude of the charges. The ionic charges are Li+, Cl-, Mg2+, O2-, Al3+, N3-, Na+, and F-. The largest magnitude charges are in AlN, which will lead to the greatest attraction between the ions, which leads to the highest lattice energy.
15. C—For ionic compounds, the key factors are the sizes and the charges on the ions. Smaller ions lead to higher melting points. Higher charges lead to higher melting points. Answer B deals with size; however, if this were the key factor, the melting point of sodium fluoride would be higher, which is not the case. This leads to C being the correct answer. The higher charge on calcium (+2 versus +1) leads to a higher lattice energy, which results in a higher melting point.
16. D—This answer describes a solid consisting of discrete polar molecules. Answer A refers to a nonpolar molecule. Answer B refers to a metallic solid. Answer C might apply; however, even though phosphoric acid is an acid that forms ions in aqueous solution, there is no water present for ionization. The actual “strongest” interactions in solid H3PO4 are hydrogen bonds, which are a special type of dipole—dipole force.
17. A—Compound A can form hydrogen bonds. Compound B has dipole—dipole interactions. Compounds C and D are both nonpolar; therefore, their strongest intermolecular force is London dispersion force. For relatively small molecules such as these, the stronger the intermolecular force, the higher the viscosity. Hydrogen bonding in the strongest of the three intermolecular forces present in these molecules.
18. C—The carbonyl, C=O, and —OH groups can participate in hydrogen bonds, as illustrated by the dotted lines in the following diagram:
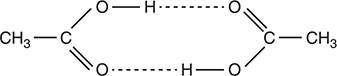
The hydrogen bonds hold the two molecules together as a dimer.
19. B—The solid begins to melt at B and finishes melting at C. Once the solid begins to melt, both solid and liquid are present.
20. B—Sodium nitrate is an ionic solid with ionic bonding; however, these ionic bonds are broken when the compound dissolves in water. The fact that this ionic compound dissolves to form an aqueous solution implies that it is a strong electrolyte. Water, the solvent, has hydrogen bonding, some of the hydrogen bonds are broken as the NaNO3 dissolves. New intermolecular forces form as the NaNO3 dissolves and forms Na+(aq) and NO3—(aq). The sodium and nitrate hydrated ions are held together by strong ion-dipole forces, which, in general are stronger than hydrogen bonding.
21. B—Oxygen gas exists as discrete nonpolar molecules, which means that isolated O2 molecules only have London dispersion forces. Water has hydrogen bonding (a type of dipole-dipole force). When O2 dissolves in water the nonpolar O2 interacts with polar H2O which leads to the H2O inducing a dipole in the O2. This interaction leads to the formation of dipole-induced dipole forces.
![]() Free-Response Question
Free-Response Question
You have 15 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
Write a brief explanation concerning each of the following observations.
(a) An aqueous solution in a glass buret has a meniscus that is concave, whereas in a plastic buret, the meniscus is convex.
(b) The surface tension of liquid CBr4 is greater than the surface tension of liquid CCl4.
(c) At the same temperature, liquid HF has a higher viscosity than liquid HCl.
(d) When heated, glass does not melt; it becomes soft.
![]() Answer and Explanation
Answer and Explanation
(a) The concave surface results from capillary action, and the convex surface results from surface tension. Surface tension is the result of strong intermolecular forces between the solution and the glass; these forces are greater than those within the solution, causing a convex surface. The convex surface results from a stronger interaction within the solution than between the solution and the plastic.
Give yourself 1 point for this answer.
(b) The greater surface tension results from stronger intermolecular forces in CBr4. Both CCl4 and CBr4 are nonpolar molecules; therefore, the intermolecular forces are London dispersion forces. For simple molecules, such as these, the one with more electrons, CBr4, will have the stronger London dispersion forces and hence the greater surface tension.
Give yourself 1 point for this answer.
(c) Viscosity, the resistance to flow, is related to the strength of attraction between the molecules. Both HF and HCl are polar molecules with dipole—dipole forces; however, HF exhibits an especially strong form of dipole—dipole force known as hydrogen bonding. Since hydrogen bonding is stronger than normal dipole—dipole forces, HF has a higher viscosity.
Give yourself 1 point for this answer.
(d) Glass is a liquid with very high viscosity (amorphous solid). Heating any liquid gives the molecules more kinetic energy, which lowers the viscosity. In the case of glass, the decrease in viscosity begins with the material becoming softer and softer until it flows freely.
Give yourself 1 point for this answer.
Total your points. There are 4 points possible.
![]() Rapid Review
Rapid Review
• The state of matter in which a substance exists depends on the competition between the kinetic energy of the particles (proportional to temperature) and the strength of the intermolecular forces between the particles.
• The melting point is the temperature at which a substance goes from the solid to the liquid state and is the same as the freezing point.
• The boiling point is the temperature at which a substance goes from the liquid to the gaseous state. This takes place within the body of the liquid, unlike evaporation, which takes place only at the surface of the liquid.
• Sublimation is the conversion of a solid to a gas without ever having become a liquid. Deposition is the reverse process.
• Phase changes are changes of state.
• Intermolecular forces are the attractive forces between atoms, molecules, or ions due to full or partial charges. Be careful not to confuse intermolecular forces with intramolecular forces, the forces within the molecule.
• Ion—dipole intermolecular forces occur between ions and polar molecules.
• Dipole—dipole intermolecular forces occur between polar molecules.
• Hydrogen bonds are intermolecular forces between dipoles in which there is a hydrogen atom attached directly to an N, O, or F atom.
• Ion-induced dipole intermolecular forces occur between an ion and a nonpolar molecule.
• London (dispersion) forces are intermolecular forces between nonpolar molecules.
• Liquids possess surface tension (a thin “skin” on their surface, due to unequal attraction of molecules at the surface of the liquid), viscosity (resistance to flow), and capillary action (flow up a small tube).
• Amorphous solids have very little structure, like liquids.
• Crystalline solids are defined by their well-ordered structure.
• The crystal lattice of a crystalline solid is the regular ordering of the unit cells.
• Know the five types of crystalline solid: atomic, molecular, ionic, metallic, and network.
• Phase changes can be related to the strength of intermolecular forces.
• In a reaction, the intermolecular forces may aid or influence the reaction, but they are not the reaction.