5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
STEP 2 Determine Your Test Readiness
3 Take a Diagnostic Exam
IN THIS CHAPTER
Summary: The AP Exam writers assume that you are bringing a certain level of knowledge to your AP Chemistry class. The AP Chemistry Exam does not test this “prior knowledge” directly, but it is still necessary to understand this material before taking the exam. For example, it is assumed that you already know that if you dissolve a strong electrolyte, such as solid NaNO3, in water, then you also know that the solution contains no NaNO3(aq) but Na+(aq) and NO3—(aq). The following sample problem is another example.
Sample Problem: Given the following chemical equation:
![]()
what is the Ag+(aq) concentration present after mixing 10.00 mL of a 0.1000 M AgNO3 solution with 5.00 mL of a 0.1000 M K3PO4 solution? The Ksp of Ag3PO4 is 1.4 × 10—16.
The prior knowledge needed here is that you need to balance the equation before solving the problem.
There is a Pre-AP Diagnostic Exam in the Appendix for your benefit. It will test your understanding of the knowledge you should bring to your AP class. This exam will let you know where you need to spend most of your study time preparing for each topic. This does not mean you can skip the review of other topics; you should always review all topics. The exam has only multiple-choice questions and not a mixture of multiple-choice and free-response questions like you will see on the AP Exam.
The Pre-AP Diagnostic Exam will give you an idea of where you are in terms of your preparation for your AP class. The questions have been written to approximate the coverage of material that you will see on the AP Exam. However, there will be a few questions on content that will not be directly tested on the AP Exam; these questions refer to basic chemistry knowledge that your teacher (and the exam writers) will expect you to know and that you will need to know before taking the AP Chemistry Exam. Once you are done with the exam, check your work against the given answers, which also indicate where you can find the corresponding material in the book.
This final Diagnostic Practice Exam below will be the final one that you will take a few days before taking the AP Exam. By this point you have reviewed the material and have taken two practice exams. This exam is your last opportunity to spot your weaknesses. Take it as if it were the “real” AP Chemistry Exam, allowing the same time, as directed in the instructions, and so on. After you take the exam, score it, and note your weaknesses. Review those topics and look over anything else about which you are uneasy. Then review the part of Chapter 1 on What Should I Bring to the Exam? Be sure to get a good night’s sleep and earn that 5!

Key Ideas
![]() Answer questions that approximate the coverage of topics on the AP Exam.
Answer questions that approximate the coverage of topics on the AP Exam.
![]() Check your work against the given answers.
Check your work against the given answers.
![]() Determine your areas of strength and weakness.
Determine your areas of strength and weakness.
![]() Highlight the topics to which you must give special attention.
Highlight the topics to which you must give special attention.
This practice exam is designed for you to take after you have completed your initial study for the AP Exam and before you begin your final study. Make sure you follow these guidelines as to when you will take this exam. The AP Exam is a timed exam; keep this in mind as you prepare. When taking the various tests presented in this book, you should follow the AP Exam rules as closely as possible. Anyone can improve his or her score by using notes, books, or unlimited time. You will have none of these on the AP Exam, so resist the temptation to use them on practice exams. Carefully time yourself, do not use other materials, and use a calculator only when expressly allowed to do so and after you have finished an exam, you may use other sources to go over questions you missed or skipped. We have seen many students get into trouble because the first time they attempted a test under “test conditions” was on the test itself.
Make notes concerning the questions you did not understand well (even if you got the correct answer). Use these notes to help you during your final review.
Getting Started: The Diagnostic Exam
The following questions refer to different chapters in the book. Remember that it is not necessary to get the correct answer (though it would be nice). You will have time later to work on getting the correct answer. The problems you have trouble with will help direct your studying as you prepare for the AP Exam. If you have trouble (even if you got the correct answer), it indicates that you should spend additional time reviewing the topic. While answering these questions, you may use a calculator and periodic table. For each question, simply circle the letter of your choice, and for each question you have difficulty with, circle the question number.
Some people will wish to repeat some or all questions on this exam before taking the AP Exam.
AP Chemistry Final Practice Exam, Section I (Multiple Choice)
Time—1 hour and 30 minutes
Answer the following questions in the time allowed. You may use the periodic table in the back of the book.
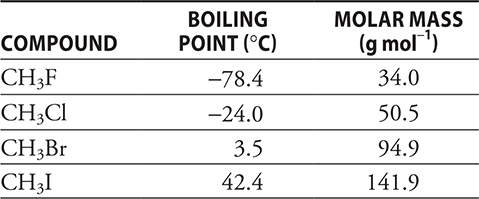
1. Which of the following best explains why the boiling point of CH3F is lower than the other three compounds in the table above?
(A) It is the lightest of the four.
(B) Larger molecules get tangled and cannot escape each other.
(C) It has weaker hydrogen bonds.
(D) It is a more symmetrical molecule.
2. The Dumas method is a simple procedure for the determination of the molar mass of a gas. In this procedure the mass of a gas is divided by the moles of gas determined from the ideal gas equation (n = PV/RT ). The molar masses of some compounds, such as formic acid, HCOOH, illustrated above, show a significant deviation from the “correct” values. Why does the presence of dimers as illustrated make it unlikely to obtain an accurate molar mass of acids, such as formic acid?
(A) Formic acid, like all acids, will lose a hydrogen ion, so the molar mass is that of the formate ion, HCOO-, which is less than that of formic acid.
(B) Formic acid is a liquid at room temperature, and its boiling point is too high to get accurate results.
(C) Acids are too reactive to give accurate results.
(D) The presence of strong intermolecular forces (hydrogen bonding) makes the gas nonideal; therefore, the ideal gas law is not applicable.
3. In which of the following groups are the species listed correctly in order of increasing electron affinity?
(A) Sr, Ca, Ba
(B) Se, Tc, Ba
(C) Mn, Fe, Ni
(D) Cl, Br, I
Use the following information to answer questions 4—10.
For many years, the nitrogen content of food and other substances has been determined by the Kjeldahl method. There are many variations in the method, but the general procedure is as follows: Initially, the sample with a catalyst is heated in boiling sulfuric acid, to convert all the nitrogen present into ammonium sulfate (or ammonium hydrogen sulfate). The sulfuric acid is cooled, diluted, and excess strong base added (for example, NaOH). The strong base neutralizes the remaining sulfuric acid and converts the ammonium ion to ammonia gas. The reactions are:
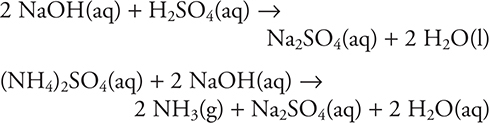
The strong base must be added quickly and the system immediately sealed to prevent the loss of any ammonia gas. The container is heated to drive all remaining ammonia from the solution. The expelled ammonia gas is trapped in excess aqueous hydrochloric acid.
![]()
Finally, the quantity of ammonia generated is determined by back-titrating the excess hydrochloric acid with standard sodium hydroxide.
![]()
A food chemist wishes to use the Kjeldahl method to determine the percent of nitrogen in a sample of dog food. The nitrogen in the dog food is primarily in protein. The chemist weighs three separate samples of dog food into three separate flasks. She then adds a small quantity of selenium to serve as a catalyst and 25 mL of concentrated sulfuric acid. In a fume hood, she heats each of the flasks to boiling (338°C) and lets the samples digest until the samples have dissolved completely.
One by one, she adds the samples to the flask on the left in the figure below. She then pipettes 50.00 mL of standard 0.1000M HCl into the flask at the right of the figure. With everything ready, she temporarily opens the flask on the left and quickly adds excess NaOH solution and closes the flask. Then she heats the left flask until it begins to boil. She boils the flask for 20 minutes and dismantles the apparatus. She stoppers the right-hand flask and sets it aside while she repeats the procedure on the remaining two samples.
Once all three samples have been treated, she titrates each with standard 0.1000 M NaOH using phenolphthalein as the indicator.
The chemist completes the following data table in her lab book.
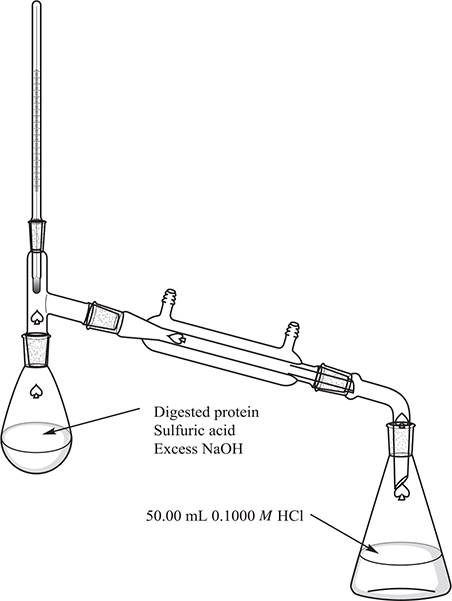
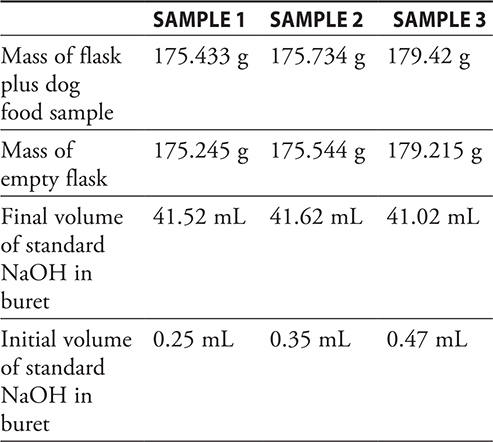
4. What type of reaction generated the ammonia gas?
(A) neutralization
(B) combination
(C) decomposition
(D) combustion
5. Why must the liquid in the right flask be dilute acid?
(A) Dilute acid is necessary to neutralize the excess sodium hydroxide formed.
(B) Dilute acid captures the ammonia gas.
(C) Dilute acid causes the reaction to go to completion.
(D) Dilute acid is easier to handle than many other liquids.
6. Would it be possible to use this procedure to determine the percent of sulfur (as sulfur dioxide) in the dog food?
(A) Yes, because sulfur dioxide is volatile.
(B) No, because sulfur dioxide is not volatile.
(C) Yes, because sulfur dioxide is a base like ammonia.
(D) No, because sulfur dioxide is not a base like ammonia.
7. The flask on the right initially contained 5.000 × 10-3 mole of HCl. How does this value relate to the moles of ammonia plus the moles of NaOH used in the titration?
(A) The moles HCl = moles NaOH.
(B) The moles HCl = moles NH3 + moles NaOH.
(C) The moles HCl = moles NH3.
(D) The relationship is unknown.
8. Approximately how many moles of ammonia gas formed from sample 1?
(A) 0.002 mole
(B) 0.004 mole
(C) 0.005 mole
(D) 0.001 mole
9. If the sample were pure nitrogen, approximately how many moles of ammonia gas would form from sample 3?
(A) 0.040 mole
(B) 0.025 mole
(C) 0.015 mole
(D) 0.150 mole
10. Would it be possible to substitute acetic acid, HC2H3O2, for the hydrochloric acid?
(A) No, because acetic acid is a weak acid and weak acid—base reactions are not efficient.
(B) Yes, because the identity of the acid is irrelevant.
(C) No, because ammonia will not dissolve in an acetic acid solution.
(D) No, because acetic acid is volatile.
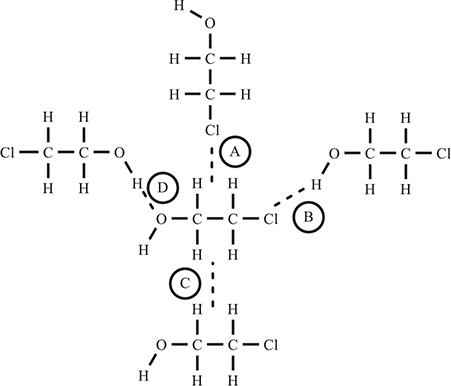
11. A small region in a sample of liquid C2H4ClOH is illustrated above. The lettered (A, B, C, and D) dashed lines represent intermolecular forces influencing the central molecule. Which of the following sets places the intermolecular forces in order of decreasing strength?
(A) D > B > A > C
(B) B > D > A > C
(C) D > B > C > A
(D) D > A > B > C
12. A bottle of wine is claimed to be 50 years old. If the age is correct, the bottle is expected to sell for a minimum of $100,000 at auction. A syringe was inserted through the cork of the bottle and a small sample of wine was removed. Analysis of the wine sample indicated that a small amount of natural radioactive tritium, 3H, was present. If the bottle was really 50 years old, about how much of the original tritium should remain? The half-life of tritium is 12.33 years.
(A) 25%
(B) 12%
(C) 6%
(D) 50%
Use the following information for questions 13-15.
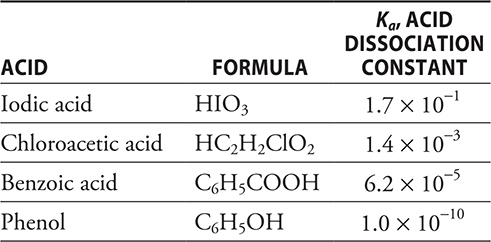
13. A buffered solution with a pH near 5 is needed for an experiment. Using the above information, which of the combinations would be the best choice to prepare the buffer?
(A) HIO3 + KIO3
(B) HC2H2ClO2 + KC2H2ClO2
(C) C6H5OH + C6H5OK
(D) C6H5COOH + C6H5COOK
14. A student wishes to measure the pH of a 0.10 M solution of the sodium salt of each of the acids in the table. The salts chosen were NaIO3, NaC2H2ClO2, C6H5COONa, and C6H5ONa, respectively. Which of the four salt solutions will have the highest pH?
(A) NaIO3
(B) NaC2H2ClO2
(C) C6H5COONa
(D) C6H5ONa
15. Which of the acids in the table would be the easiest to titrate with a weak base like ammonia (Kb = 1.8 × 10-5)?
(A) HIO3
(B) HC2H2ClO2
(C) C6H5COOH
(D) C6H5OH
Use the following information to answer questions 16-20.
pH versus volume of titrant added
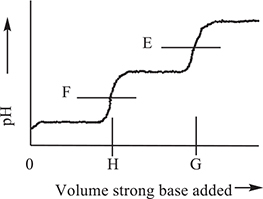
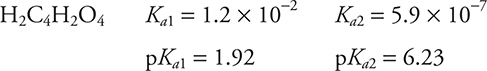
Maleic acid, H2C4H2O4, is a weak diprotic acid. The titration curve shown above is an idealized graph for a diprotic acid. On this graph, E and F represent the pH at the endpoints with the possibility that E may shift slightly and either E or F may not be present. H is the volume of base required to titrate the first hydrogen ion and G is the quantity of base necessary to titrate both hydrogen ions. G is double H.
16. What is the approximate pH at ½ H?
(A) 7
(B) 2
(C) 4
(D) Impossible to predict
17. If G is less than twice H, what would this indicate?
(A) There is an acidic contaminant.
(B) There is a basic contaminant.
(C) The indicator was added too late.
(D) The maleic acid is synthetic.
18. Which of the following bases would be the best choice for the titration?
(A) Mg(OH)2
(B) Na2CO3
(C) NH3
(D) Ba(OH)2
19. In the titration of a sample of maleic acid with standard NaOH, what is the approximate value of F?
(A) < 7
(B) > 7
(C) = 7
(D) unknown
20. The titration of many diprotic acids will not work as well as indicated by the idealized titration curve illustrated earlier. Maleic acid is a good candidate for this type of titration (determining both endpoints). Why is maleic acid a good candidate?
(A) Ka2 and Ka1 are sufficiently close together.
(B) Maleic acid reacts more rapidly than do other acids.
(C) Maleic acid reacts more slowly than do other reactions.
(D) Ka2 and Ka1 are sufficiently far apart.
21. Which of the following CANNOT behave as both a Brønsted base and a Brønsted acid?
(A) HC2O4-
(B) HSO4-
(C) HPO42-
(D) SO32-
22. A student mixes 100.0 mL of 0.100 M sodium oxalate, Na2C2O4, solution with 100.0 mL of 0.100 M silver nitrate solution, AgNO3. A white precipitate immediately forms, and the silver ion concentration drastically decreases. Correctly place the concentrations of the remaining ions in order of decreasing concentration.
(A) [Na+] > [C2O42-] > [NO3-]
(B) [C2O42-] > [NO3-] > [Na+]
(C) [Na+] > [NO3-] > [C2O42-]
(D) [NO3-] > [Na+] > [C2O42-]
23. A chemistry student wishes to determine the molar mass of a volatile liquid. She adds a small quantity of the volatile liquid into a weighed flask and covers the opening with aluminum foil. With a pin, she punches a small hole in the aluminum foil. She then clamps the flask to a ring stand and lowers the flask into a bath of boiling water (temperature measured with a thermometer). After all the liquid has evaporated, she quickly removes the flask from the water bath and cools the flask so that the vapor remaining in the flask condenses. Once the flask has cooled, she dries and reweighs the flask. The mass of the starting material remaining in the flask is determined by subtracting the mass of the empty flask from the mass of the flask with the condensed vapor. She then empties the flask and refills it with water. Once filled she uses a graduated cylinder to determine the volume of water, and hence of the flask, present. What other information is needed to finish the experiment?
(A) the heat of vaporization of the volatile liquid
(B) the original mass of the volatile liquid
(C) the mass of the displaced water
(D) the barometric pressure
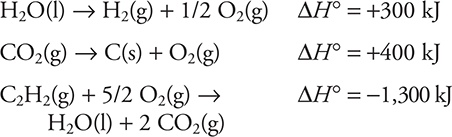
24. Using the information given above, calculate the enthalpy change for the following reaction:
![]()
(A) 500 kJ
(B) -200 kJ
(C) -500 kJ
(D) 200 kJ
25. The solubility of calcium hydroxide, Ca(OH)2, in water is 0.173 g/100 mL H2O at 20°C, and 0.121 g/100 g H2O at 60°C. Which of the following conclusions may be related to this?
(A) The heat of solution of calcium hydroxide is exothermic.
(B) The hydration energies of calcium ions and hydroxide ions are very low.
(C) The heat of solution for calcium hydroxide is endothermic.
(D) The solution is not an ideal solution.
Use the information on the containers in the following diagram to answer questions 26−28.
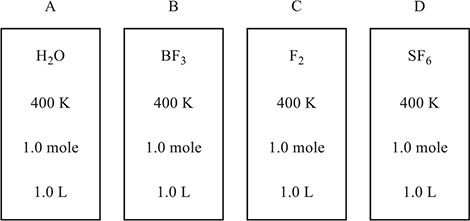
Approximate molar masses: H2O = 18 g mol-1, BF3 = 68 g mol-1, F2 = 38 g mol-1, SF6 = 146 g mol-1
26. Under the conditions indicated, in which of the gas samples is the average velocity of the molecules about half that of water, H2O?
(A) SF6
(B) F2
(C) BF3
(D) They are all at the same temperature; therefore, they have the same average velocity.
27. Which of the four gases will probably show the least deviation from ideal behavior?
(A) F2
(B) H2O
(C) BF3
(D) SF6
28. Container C develops a small leak. Which of the following variables would change?
(A) moles, temperature, and pressure
(B) moles and pressure
(C) temperature and pressure
(D) moles and temperature
29. At high temperatures, ammonia decomposes to the elements. At a certain temperature, the specific rate constant, k, for this first-order reaction is 3.5 × 10-3 s-1. What mass of a 0.100-g sample of starting material will remain after 400 s?
(A) 0.0500 g
(B) 0.0250 g
(C) 0.0125 g
(D) 0.00625 g
Use the information on the containers in the following diagram to answer questions 30−34 concerning the following equilibrium:
2 C2H4(g) + O2(g) ⇆ 2 CH3CHO(g)
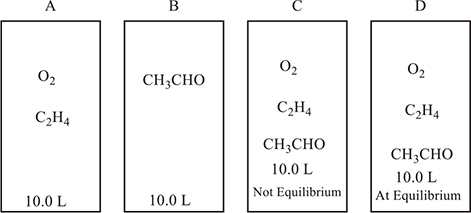
30. Container A initially contained 0.25 mole of O2 and 0.60 mole of C2H4 and then comes to equilibrium. At equilibrium, there is 0.30 mole of C2H4 in the flask. What is the value of Kc, the equilibrium constant, for the reaction?
(A) 0.10
(B) 10
(C) 0.010
(D) 100
31. A 1.00-mole sample of CH3CHO is placed in container B and the system is allowed to go to equilibrium. What can be said about the relative rates of reaction with respect to the various components?
(A) The rate of C2H4(g) formation is numerically equal to the rate of CH3CHO loss.
(B) The rate of O2(g) is numerically equal to the rate of CH3CHO loss.
(C) The rate of C2H4(g) formation is half the rate of O2(g) formation.
(D) The rate of O2(g) formation is equal to the rate of C2H4(g) formation.
32. The mixture in container D is in equilibrium. Which of the following is true?
(A) The rates of the forward and reverse reactions are equal to zero.
(B) The rate of the forward reaction is equal to the rate of the reverse reaction.
(C) The pressure in the system is increasing.
(D) The pressure in the system is decreasing.
33. The mixture in container A goes to equilibrium. If the initial moles of C2H4(g) is twice the initial moles of O2(g), which of the following is true?
(A) Both reactants are limiting; therefore, the reaction will continue until there are zero moles of C2H4 and O2 remaining.
(B) The total pressure of the system decreases until the system reaches equilibrium.
(C) The total pressure of the system increases until the system equals equilibrium.
(D) There is insufficient information.
34. As the mixture in container B approaches equilibrium, the partial pressure of CH3CHO gas decreases by 1.0 atm. Assuming there is no change in temperature, what is the net change in the total pressure of the system?
(A) +1.0 atm
(B) +0.50 atm
(C) −1.0 atm
(D) −0.50 atm
Use the information on standard reduction potentials in the following table to answer questions 35−39.
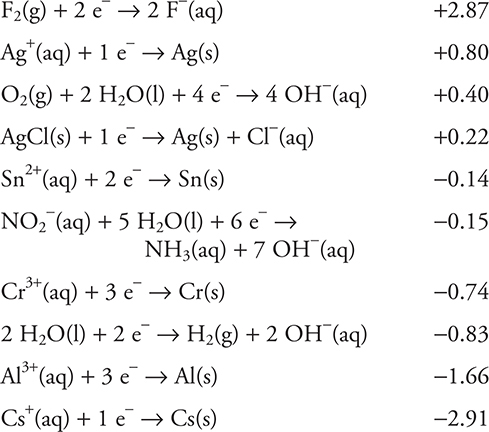
35. A student constructs an electrolysis cell with two inert electrodes in an aqueous solution that is 1.0 M in cesium nitrite, CsNO2, and 1.0 M in cesium hydroxide, CsOH. As the cell operates, an odorless gas evolves from one electrode and a gas with a distinctive odor evolves from the other electrode. Choose the correct statement from the following list.
(A) The odorless gas is oxygen.
(B) The odorless gas is the result of reduction.
(C) The gas with the distinctive odor is the result of oxidation.
(D) The odorless gas evolves at the negative electrode.
36. Another student constructs a galvanic cell involving a tin, Sn, electrode in a 1.0 M tin(II) chloride, SnCl2, solution and a chromium, Cr, electrode in a 1.0 M chromium(III) sulfate, Cr2(SO4)3, solution. What is the cell potential?
(A) +0.60 V
(B) −0.60 V
(C) +0.88 V
(D) 0.00 V
37. Another student attempted to prepare an electrolysis cell to produce aluminum metal, Al, from an aqueous solution of aluminum chloride, AlCl3, using a 6.0 V battery. The cathode compartment of the electrolysis contained 1.0 M aluminum chloride, and the anode compartment contained 1.0 M calcium chloride, CaCl2. The student was unsuccessful. Why was the student unable to produce aluminum metal?
(A) The voltage from the battery was insufficient to force the reaction to occur.
(B) Reduction of chloride ions occurred in preference to reduction of aluminum ions.
(C) Aluminum chloride solutions do not conduct electricity.
(D) Reduction of water occurred in preference to reduction of aluminum ions.
38. Which of the substances in the table would be capable of reducing the aluminum ions in solid aluminum chloride, AlCl3, to aluminum metal? Assume the cell potentials in the table also apply to the solid state.
(A) Cr(s)
(B) Cs(s)
(C) Ag(s)
(D) none
39. A student constructs a galvanic cell that has a chromium, Cr, electrode in a compartment containing a 1.0 M chromium(III) nitrate, Cr(NO3)3, solution and a silver, Ag, electrode in a compartment containing 1.0 M silver nitrate, AgNO3, solution. A salt bridge containing a 1.0 M potassium chloride, KCl, solution connects the two compartments. The student used the following two half-reactions from the table:
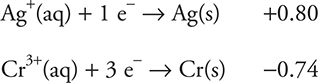
When the student measures the cell potential, the value is far from the ideal predicted value. What is the cause of this discrepancy?
(A) The initial concentrations should have been lower than 1.0 M.
(B) The initial concentrations should have been higher than 1.0 M.
(C) The potassium chloride in the salt bridge interfered with the reaction.
(D) The student did not allow the cell to come to equilibrium.
Use the information on the acids in the following diagram to answer questions 40−41.
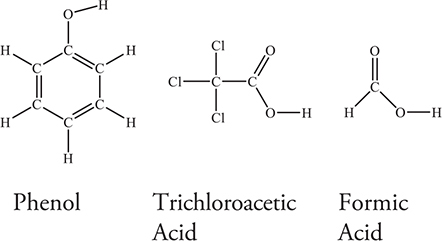
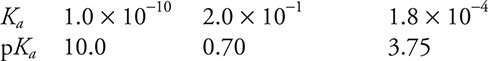
40. Sample solutions of each of the three acids shown above were titrated with 0.10 M sodium hydroxide, NaOH. Each of the acid solutions had a concentration of 0.10 M. Which of the acid titrations had the highest pH at the endpoint?
(A) formic acid
(B) phenol
(C) trichloroacetic acid
(D) They all had a pH of 7 at the endpoint.
41. A student prepares three buffer solutions. Each solution is 1.0 M in one of the acids in the table and 1.0 M in its corresponding sodium salt. Which of the solutions has the greatest buffer capacity with respect to added NaOH and why?
(A) The phenol buffer does because it is the strongest acid.
(B) The trichloroacetic acid buffer does because it is the strongest acid.
(C) The formic acid buffer does because it donates both of its hydrogen atoms.
(D) All are the same.
42. Sulfurous acid is an unstable acid in solution, which decomposes to water and gaseous sulfur dioxide. The sulfur dioxide will escape from the solution. The decomposition of the acid lowers its concentration over time. What effect will the decomposition of one-third of the acid have on the agreement between the endpoint of the titration and the equivalence point during a titration with standard sodium hydroxide?
(A) The endpoint would remain near the ideal equivalence point.
(B) The endpoint would be before the ideal equivalence point.
(C) The endpoint would be after the ideal equivalence point.
(D) It is impossible to determine.
43. Three 20.00 mL samples of approximately 0.10 Mp-cresol, CH3C6H4OH, Ka = 6.7 × 10-11 were removed from a container and placed in separate 250 mL flasks. The samples were titrated with standard 0.1025 M sodium hydroxide, NaOH, solution. Thymolphthalein was the acid—base indicator used in the titration. The samples required 21.75, 22.38, and 31.75 mL to reach the endpoint. Which of the following might explain why the third sample required significantly more of the base to reach the endpoint?
(A) The indicator was added too soon.
(B) The wrong indicator was used.
(C) There was an acid contaminating the unclean flask.
(D) There was a base contaminating the unclean flask.
44. During the study of the reaction AB → A + B, a chemist constructs several graphs. The graph [AB] of versus time and the graph of ln [AB] versus time both give a curved line; however, the graph of 1/[AB] versus time gives a straight line. This implies the rate law is
(A) Rate = k[AB]
(B) Rate = k[AB]0
(C) Rate = k[AB]2
(D) Rate = k[AB]-1
45. The photoelectron spectrum of boron has three peaks. One peak is less intense than the other two. Which peak is the less intense peak?
(A) the 2p peak
(B) the 2s peak
(C) the 1s peak
(D) the 1p peak
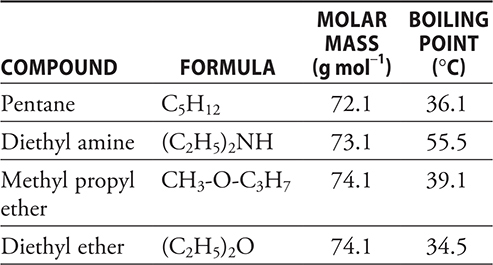
46. According to the data in the table above, which of the compounds has the strongest intermolecular forces?
(A) pentane
(B) diethyl amine
(C) methyl propyl ether
(D) diethyl ether
Use the following information to answer questions 47 and 48.
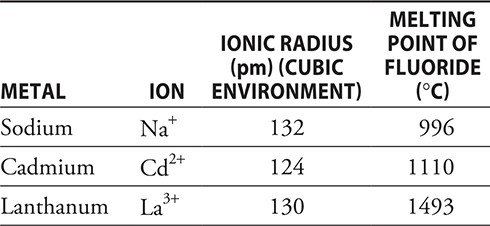
47. Each of the ions in the table form stable fluorides (NaF, CdF2, and LaF3). Lanthanum fluoride, LaF3, has a melting point higher than that of the other fluorides. Which of the following is the best explanation of why this is true?
(A) Lanthanum is a lanthanide element, and the melting points of these elements are always high.
(B) There is more fluorine in the formula LaF3 than in the other formulas.
(C) Lanthanum had the highest charge; therefore, it has the highest lattice energy.
(D) Alkali metals like sodium and transition metals like cadmium tend to have low melting points.
48. The lithium ion, Li+, is smaller than the sodium ion. Predict how the melting point of lithium fluoride, LiF, compares to that of sodium fluoride?
(A) It is higher because smaller ions have a higher lattice energy.
(B) It is the same because the charges are the same.
(C) It is lower because smaller ions have a smaller lattice energy.
(D) It is impossible to predict because there is insufficient information in the problem.
49. During the investigation of a chemical reaction by a chemistry student, he was able to determine that the reaction was nonspontaneous at 1 atm and 298 K. However, he learned that cooling the system with dry ice (−78°C) caused the reaction to become spontaneous. Which of the following combinations must apply to this reaction?
(A) ΔH < 0, ΔS < 0, and ΔG = 0
(B) ΔH > 0, ΔS < 0, and ΔG > 0
(C) ΔH < 0, ΔS < 0, and ΔG > 0
(D) ΔH > 0, ΔS > 0, and ΔG > 0
50. A 0.060 M solution of a weak acid has a pH of 2.0. What is the ionization constant, Ka for this acid?
(A) 2.0 × 10-1
(B) 1.7 × 10-1
(C) 5.0 × 10-3
(D) 2.0 × 10-3
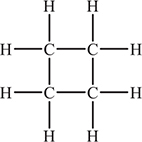
51. Cyclobutane, shown above, is a relatively unstable compound. As seen in the diagram, the four carbon atoms form the corners of a square and each carbon atom has two hydrogen atoms attached to complete an octet of electrons around the carbon atoms. Based upon this structure, why is cyclopropane a relatively unstable compound?
(A) The bonds do not match the angles.
(B) Compounds that have identical atoms bonded to each other are relatively unstable.
(C) There is no resonance to stabilize the compound.
(D) Hydrocarbon compounds are relatively unstable in general.
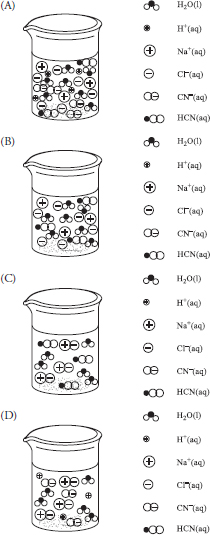
52. A chemist has a 500 mL beaker with 0.0010 mole of sodium cyanide, NaCN, on the bottom. She adds 250 mL of water and 0.0010 mole of hydrochloric acid. She then tests the solution and finds that it conducts electricity. Which of the following best represents the major species in the beaker after the addition of the acid?
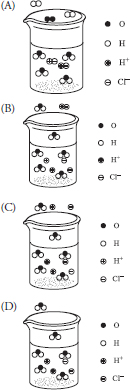
53. A dilute aqueous solution of hydrochloric acid, HCl, is heated to the boiling point. Which of the following best represents this system?
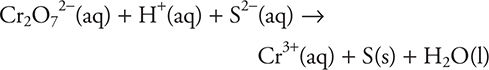
54. What is the coefficient of H+ when the above reaction is balanced?
(A) 2
(B) 14
(C) 18
(D) 6
55. There are three steps in the formation of a solution. It is necessary to overcome the intermolecular forces present within the solute. It is also necessary to overcome the intermolecular forces present within the solvent. Both these steps require energy related to the strength of the intermolecular forces. The final step in the formation of a solution involves the creation of new intermolecular forces between the solute and solvent. This energy release is related to the strength of the intermolecular forces created. Which of the following illustrates a situation most likely to release the greatest amount of energy to form the new intermolecular forces?
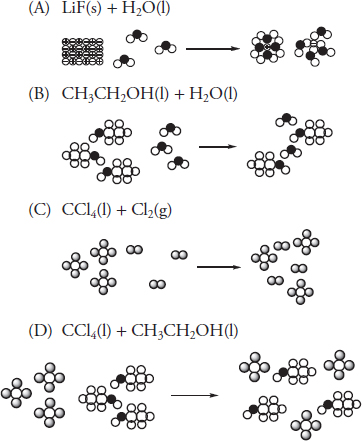
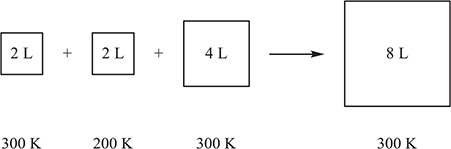
56. Each of the containers to the left of the arrow are filled with an ideal gas. The contents in the left three containers are transferred to the empty container on the right. The volumes of the original containers are exactly the values indicated. The pressure in the first three containers is 0.75 atm. What is the pressure in the container on the right?
(A) 3.0 atm
(B) 4.0 atm
(C) 1.1 atm
(D) 0.50 atm
![]()
57. The diagram above shows the structure of molecules of CS2 and CSe2. The melting point of CS2 is −112.1°C and CSe2 has a melting point of −43.7°C. Which of the following is the best explanation of why the melting point of CSe2 is higher?
(A) The molar mass of CSe2 is greater.
(B) CS2 has weaker covalent bonds than CSe2.
(C) Only CS2 can form intermolecular dipole-dipole forces.
(D) CSe2 has stronger intermolecular forces because it is polar and CS2 is not.
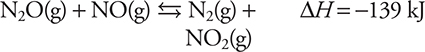
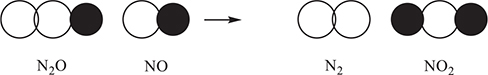
58. The above reaction was the subject of a kinetics experiment. The reaction was shown to obey the rate law Rate = k[N2O][NO]. Which of the following is the most likely to illustrate an effective collision?
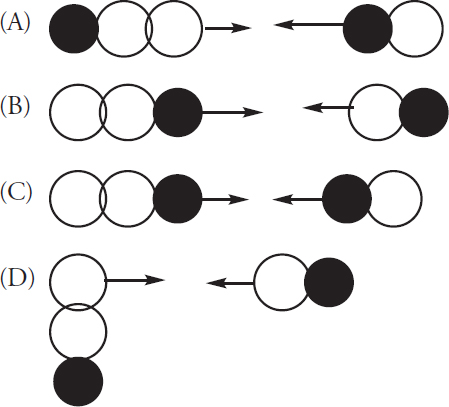
![]()
The proposed mechanism for the above reaction is as follows:
59. What is the rate law expression for this reaction?
(A) Rate = k[CHCl3][Cl]
(B) Rate = k[CHCl3][Cl2]1/2
(C) Rate = k[Cl2]
(D) 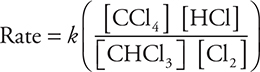
60. A 20.0-g sample of chromium metal was heated to 75.0°C. This sample was clamped in contact with a 25.00-g sample of beryllium metal originally at 25.0°C. The final temperature of the two metals was 34.9°C, and no heat was lost to the surroundings. What is one possible conclusion from this experiment?
(A) The heat lost by X was greater than the heat gained by Y.
(B) The metals have different heat capacities.
(C) The heat lost by X was less than the heat gained by Y.
(D) The final temperature was incorrectly determined, as it should be the average (50.0°C).
STOP. End of AP Chemistry Final Practice Exam, Section I (Multiple Choice).
![]() Answers and Explanations for Final Practice Exam, Section I (Multiple Choice)
Answers and Explanations for Final Practice Exam, Section I (Multiple Choice)
1. A—The boiling points are related to the strength of the intermolecular forces. For all four compounds dipole—dipole forces and London dispersion forces are present. If dipole—dipole forces were the primary factor, the sequence of boiling points should be the reverse of what is shown with CH3F having the highest boiling point. London dispersion forces depend upon the number of electrons present. The number of electrons present in the molecule increases with the boiling point, which indicates that, for these molecules, London dispersion forces are the key. You covered this material when you worked through Chapter 9.
2. D—Strong hydrogen bonds (dotted lines) hold two molecules of formic acid together. Ideal gases have no intermolecular forces. Therefore, the ideal gas law used in the experiment is invalid because of the presence of strong intermolecular forces. You covered this in Chapters 8 and 9.
3. C—Increasing ionization energy applies to an element higher in a column on the periodic table, or in a position farther to the left in a period on the periodic table. Note that this type of explanation is unacceptable on the free-response portion of the AP Exam, where your explanation would require additional information such as a discussion of radii and effective nuclear charges. You covered this in Chapter 12.
4. A—The reaction is neutralization reaction between the ammonium ion (acid) and the sodium hydroxide (base). You saw the different types of reactions in Chapter 12.
5. B—Since ammonia is a base, it will react with the acid. The ammonia is captured by its reaction with the dilute acid; otherwise, part of the ammonia would escape.
6. D—The procedure works because the gas (ammonia) is a base that will react with the acid. Sulfur dioxide is an acidic oxide; therefore, it will neither react with nor be trapped by the dilute acid.
7. B—Both reactions with HCl have 1:1 stoichiometry, so moles acid = moles base. The ammonia first reacts with some of the HCl, then the NaOH reacts with the remainder.
8. D—The moles of HCl (5.000 × 10-3) − moles NaOH titration (4.127 × 10-3) = moles NH3 (8.73 × 10-4).
The moles of HCl were given in the preceding question (or could be calculated from the molarity and volume). The moles of NaOH are calculated as:
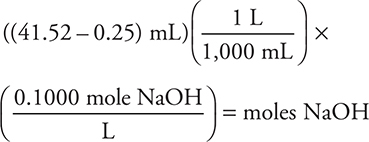
Only an approximate answer is needed.
It is easier to calculate the answer by simple rounding as:
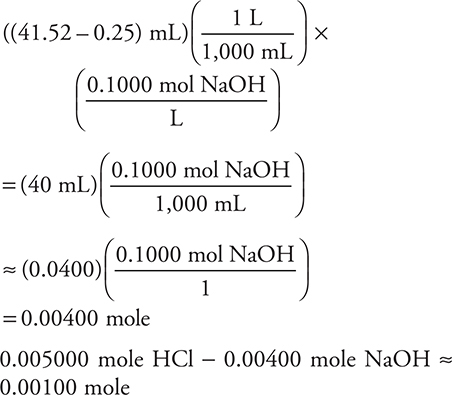
This was covered in Chapter 6. It is not necessary to write down everything when taking the multiple- choice portion of the exam; however, we have seen numerous wrong answers from people not writing down their steps. There is ample room for you to write the steps in the exam booklet.
9. C—The mole—mole ratio between N and NH3 is 1:1. The mass of sample 2 is (179.425 − 179.215) g = 0.210 g N. Convert this to moles and use the mole—mole ratio to determine the moles of ammonia:
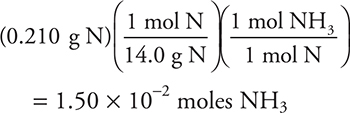
Only an approximate answer is needed.
It is easier to calculate the answer by simply rounding as (and factoring out a 7):
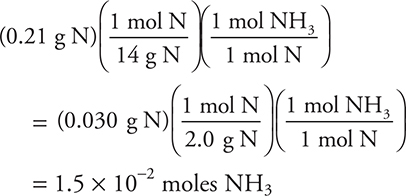
10. A—Sulfur dioxide gas is soluble in water and, while less soluble in dilute acid, some would still dissolve to give an erroneous volume (smaller).
11. A—The intermolecular forces in order of decreasing strength are: D = hydrogen bonding (strongest), B = dipole—dipole force, A = dipole—induced dipole force, and C = London dispersion force (weakest). If you have trouble here, you should go back to where you covered intermolecular forces the first time you covered the AP material (Chapter 9).
12. C—The decay reaction is first-order and 50 years amounts to about four half-lives. After one half-life, 50% would remain. Another half-life would reduce this by one-half to 25%, a third half-life would reduce the remaining material by 12.5%, and the fourth half-life would reduce the amount remaining to 6.26%. The closest answer is (C). Note, this is not a nuclear chemistry problem; it is a kinetics problem. Do not be misled by the presence of a radioactive substance; all radioactive substances follow first-order kinetics (Chapter 13).
13. C—The best choice to prepare the buffer is the one where the pKa is closest to the desired pH. It is possible to estimate the pKa for an acid (without a calculator) by taking the negative of the exponent for the different Ka values. This gives 1 for HIO3, 3 for HC2H2ClO2, 5 for C6H5COOH, and 10 for C6H5OH. The C6H5COOH has the value closest to the desired pH. It would not be a good choice since the pKa is so far from the pH; however, it is the best choice. Note that the formulas of the acids are irrelevant to this set of questions; only the Ka values are needed to answer these questions. This shows that if you do not know the formula of a substance in a question, you can still do the problem.
14. D—These are salts of a weak acid and a strong base. The anion in such salts undergo hydrolysis. The hydrolysis of an anion (conjugate base) yields hydroxide ion (increases the pH). This hydrolysis is a Kb equilibrium. The smaller the Ka of the acid, the larger the Kb of the conjugate base. A larger Kb means a stronger base and the higher the pH.
15. A—In order to get good results when titrating a weak base, it is important to use as strong an acid as possible to get a sharp endpoint. HIO3 is the strongest acid in the table.
16. B—The position ½ H is halfway to the first equivalence point. This is in the first buffer region of the titration. The pH at the halfway point is equal to pKa (= −log Ka). The value of pKa is 1.92. You covered this in Chapter 16.
17. A—To reach H, it is necessary to convert all the ascorbic acid to the hydrogen ascorbate ion, which means the moles of hydrogen ascorbate ion formed must equal the moles of ascorbic acid originally present. The moles of hydrogen ascorbate ion formed would require the same number of moles of base as that required to convert the ascorbic acid to hydrogen ascorbate. Equal moles would mean equal volumes added. If G is not twice H, there must be some contaminant present to react with the base.
18. D—In any acid—base titration, it is always easier to use a strong base (and a strong acid). Barium hydroxide, Ba(OH)2, is the only strong base among the choices. You covered the reasons for this in Chapter 16.
19. B—The titration of a weak acid with a strong base always has an equivalence point above 7. A pH = 7 occurs in the titration of a strong acid with a strong base.
20. D—The Ka values differ by a factor of over 20,000, which is great enough to distinguish the two equivalence points. Acid—base reactions, including maleic acid, are normally very fast reactions.
21. D—All the substances can behave as Brønsted bases (accept a hydrogen ion), because they are the conjugate bases of weak acids. Only D cannot behave as an acid (donate a hydrogen ion), because it has no hydrogen ion to donate.
22. C—Initially, doubling the volume will result in halving the concentrations (0.0500M). Next, consider the reaction. The balanced equation is: Na2C2O4(aq) + 2 AgNO3(aq) → Ag2C2O4(s) + 2 NaNO3(aq). The silver ion is the limiting reagent (check this for yourself using a calculator—if you do not recall how to determine the limiting reagent, take the Diagnostic Exam in the Appendix of this book), so very little Ag+(aq) remains in solution (due to its Ksp, there must be a Ksp because there was a precipitate). The precipitation of silver oxalate reduces the oxalate concentration from 0.0500 M. The nitrate does not change (soluble), remaining 0.0500 M. Since two sodium ions (soluble) are formed per sodium oxalate, after mixing the sodium ion concentration was 0.100 M and does not change (soluble). The concentrations are Na+ = 0.100 M, NO3- = 0.0500 M, and C2O42- < 0.0500 M (there is no need to calculate the actual value; however, you should be able to use the procedure seen the first time you covered this material.) (The fact that neither the oxalate nor the nitrate ion separate is considered prior knowledge information that you learned before starting AP Chemistry.)
23. D—This is the Dumas method. To determine the molar mass of the gas it is necessary to know the mass of the gas (determined) and the moles of the gas. The ideal gas equation is necessary to determine the number of moles of gas present. To use the equation, it is necessary to know the temperature (measured), volume (determined = volume from graduated cylinder), and pressure of the gas. The pressure of the gas is equal to the barometric pressure, which still needs to be determined.
24. D—This is a Hess’s law problem, requiring you to manipulate the given equations to produce the desired equation. It is possible to begin with any of the given equations. Take the given equations in order. Reverse the first equation, double the second equation, and reverse it (doubles the ΔH° and reverses the sign), and reverse the third equation (reverses the sign of ΔH°). The results are:
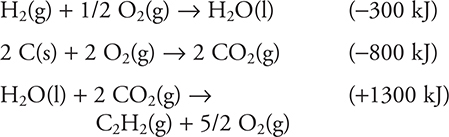
Add the three equations together and cancel any species appearing on opposite sides of the reaction arrow. The (2 + 1/2) O2(g) on the reactant side cancels the 5/2 O2(g) on the product side. The H2O(l) on the product cancels the H2O(l) on the reactant side. Finally, the 2 CO2(g) on each side cancel. The only substances not cancelled are: H2(g), 2 C(s), and C2H2(g). If you get anything not identical to equation sought, you made an error.
25. A—Since the compound is less soluble in hot water, the solution process must be exothermic. Exothermic processes shift toward the starting materials (solid calcium hydroxide) at higher temperatures. This is an application of Le Châtelier’s principle to a solubility equilibrium.
26. C—The gases are all at the same temperature; therefore their average kinetic energies are the same. Since kinetic energy is equal to ½ mv2, ½ m1v12 = ½ m2v22, the subscripts refer to two different gases. Setting m1 = 18 g mol-1 and v2 = ½ v1 gives: ½ 18v12 = ½ m2(½ v1)2; this leads to: 18v12 = m2 (1/4 v12). Rearranging and canceling v1 yields m2 = 4(18) = 72 g mol-1. The molar mass of BF3 is the closest to this value.
27. A—The smaller the molecule and the less polar (more nonpolar) the gas is, the smaller the deviation from ideal gas behavior. While water is the smallest, it is extremely polar. The next largest molecule is F2, which is nonpolar. This is an application of the kinetic molecular theory you covered in Chapter 10.
28. A—Escaping gas would decrease the number of moles. Less gas remaining in the container would mean less pressure. The faster-moving gas molecules would escape faster, lowering the average velocity of those remaining in the container. A lower average velocity means a lower temperature.
29. C—It is necessary to use the half-life relationship for first-order kinetics. This relationship is ![]()
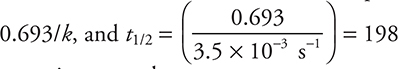 s. To save time, on the exam you can approximate this equation as
s. To save time, on the exam you can approximate this equation as 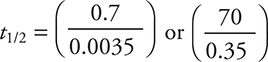 or
or 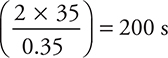 . If the half-life is ≈ 200 s, then the time (400 s) is equivalent to about two half-lives, so one-fourth of the sample should remain. Remaining mass
. If the half-life is ≈ 200 s, then the time (400 s) is equivalent to about two half-lives, so one-fourth of the sample should remain. Remaining mass 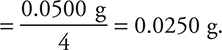
30. D—The loss of 0.30 mol of C2H4 means that 0.15 mol of O2 reacted (leaving 0.10 mol) and 0.30 mol of CH3CHO formed. Dividing all the moles by the volume gives the molarity: [C2H2] = 0.030 M, [O2] = 0.010 M, and [CH3CHO] = 0.030 M. Next enter the values into the mass action expression:
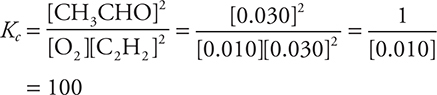
31. A—Based upon the stoichiometry of the reaction, C2H4(g) will form at the same rate as CH3CHO(g) disappears, while the rate of O2(g) formation will equal half the rate of CH3CHO(g) loss. The numerical values are the same; however, the rates have opposite signs.
32. B—At equilibrium, there is no net change because the forward and reverse reactions are going at the same rate. At equilibrium, the forward and reverse reactions will be going at the same rate and the pressure will not be changing.
33. B—As the reaction approaches equilibrium, there is a net decrease in the number of moles of gas present. A decrease in the number of moles of gas will lead to a decrease in pressure. If the system is in equilibrium, there must be some of the reactants and products present (nothing can be 0).
34. B—The loss of 1.0 atm of CH3CHO(g), based on stoichiometry, leads to the formation of 1.0 atm of C2H4(g) and 00.5 atm of O2(g); therefore, the net change is (−1.0 + 1.0 + 0.50) atm = 0.50 atm.
35. A—Oxygen (odorless) evolves at the anode (positive) and ammonia (distinctive odor) evolves at the cathode (negative). From the choices given, the only gas with a distinctive odor that could form is ammonia, NH3. For this reason, the nitrite ion half-reaction must be the cathode reaction. The anode half-reaction must be the reverse of one of the reactions in the table. The only one of the half-reactions given that generates an odorless gas is the one involving oxygen, O2. Do not forget, as you saw in Chapter 17, that an electrolysis cell is nonspontaneous with a negative cell potential. The cesium half-reaction will not occur under the conditions in this cell.
36. A—The tin electrode is the cathode (−0.14 V), and the chromium electrode is the anode (reverse sign, +0.74 V). Cell voltage = (−0.14 + 0.74) V = +0.60 V. The standard voltage of a galvanic cell is always positive, so you would know you made a mistake if you reversed the wrong half-reaction.
37. D—The formation of aluminum metal is a reduction and must occur in the cathode compartment. It is necessary to consider all possible half-reactions that might occur during electrolysis. Of the half-reactions listed, only the reduction of water and the reduction of aluminum ions are applicable to this experiment. For any electrolysis, the half-reaction requiring the least amount of energy will take place. So, while it is possible to reduce both water and the aluminum ion, the reduction potential for water is lower (requiring less energy), so water will reduce in preference to aluminum ion.
38. B—In order for substance A to reduce substance B, substance A must be a stronger reducing agent than substance B. Aluminum is a strong reducing agent as indicated by the large negative potential in the table (−1.66 V). The only substance in the table that has a more negative potential is cesium, Cs (−2.91 V).
39. C—Silver appears twice in the list of half-reactions, once in the simple reduction of silver ions and once in the reduction of silver chloride, AgCl. The AgCl half-reaction clearly shows that this compound is a solid. This solid will form as the silver ion in the silver nitrate solution reacts with chloride ion from the potassium chloride in the salt bridge. The formation of solid silver chloride will alter the concentration of the silver ion, which leads to a change in the cell potential.
40. B—The weakest acid (smallest Ka) will have the highest pH at the endpoint. Do not worry about the structures of the acids; the Ka is the key. While you do not need to know organic chemistry for the AP Exam, such molecules may still appear as examples of other chemistry topics.
41. D—The buffer capacity only depends on the number of moles present. All three solutions have the same number of moles.
42. B—Less acid would require less than the ideal amount of base to reach the endpoint. Therefore, the endpoint would occur too soon.
43. C—An acid contaminant must be present, and the excess acid would require additional base to titrate this acid in addition to the p-cresol. Do not be distracted by the “OH” in phenol—it has a Ka; therefore, it must be an acid. The key is to recognize the principles (an acid has a Ka, and in situations like this, all acids will behave the same). The use of an organic compound is irrelevant as you are being asked about acid—base reactions, not organic chemistry.
44. C—Since only the plot of 1/[AB] versus time yielded a straight line, this implies that the reaction is second-order in AB. If the reaction were first-order, the graph of ln [AB] versus time would have given a straight line. If the reaction were a different order, a different graph would give a straight line. You were first introduced to this material in Chapter 13.
45. C—The electron configuration of boron is 1s22s22p1. The 1s and 2s orbitals both contain two electrons; thus, they should be equally intense. The 2p orbital with only one electron (half that of the other orbitals) should have less (half) the intensity. Note that answer (D) is impossible as there is no such thing as a 1p orbital; therefore, it cannot be the answer.
46. B—The compound with the highest boiling point has the strongest intermolecular forces. This is only valid if the molar masses are similar, which they are in the problem. Do not be distracted by the name of the compound or its formula.
47. C—The melting points of ionic materials depend upon the lattice energy. The higher the lattice energy, the higher the melting point is. Lattice energies depend upon the sizes of the ions and the magnitude of the charge. All three metal ions are approximately the same size; therefore, the size factor is minimal. This leaves the magnitude of the charge, and lanthanum, with the largest charge, which should have the highest lattice energy. Lattice energy was introduced to you in Chapter 9.
48. A—The lattice energy depends upon both the charge and the size of the ions involved. The greater the charge is, the greater the lattice energy will be (higher melting point). There is an inverse relationship between the lattice energy and the size of the ion. Therefore, the smaller the ion is, the greater the lattice energy will be (higher melting point).
49. A—The key relationship is ΔG = ΔH − TΔS. If the reaction is nonspontaneous at 1 atm and 298 K, then ΔG > 0 under these conditions. For this reaction to become spontaneous (ΔG < 0) at a lower temperature the reaction must be impeded by entropy (entropy was negative). If the entropy were negative, the enthalpy must also be negative, or the reaction could never be spontaneous. You covered these relationships in Chapter 14.
50. D—Enter the information into the Ka expression. A pH of 2.0 means that [H+] = 10-2.0 M or 1.0 × 10-2. 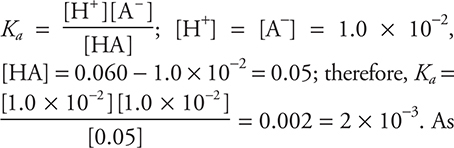 always, on the multiple-choice part of the exam, you must estimate.
always, on the multiple-choice part of the exam, you must estimate.
51. A—A carbon atom with four single bonds should be tetrahedral. Tetrahedral atoms have an ideal bond angle of 109.5°. However, the carbon atoms in cyclobutane are at the corners of a square, where the ideal angle is 90°. The discrepancy between the two ideal bond angles leads to the relative instability of cyclobutane. This is a VSEPR problem, not an organic chemistry problem. Organic compounds serve as many good examples, such as this one, which do not involve organic chemistry.
52. B—The reaction is:
NaCN(s) + HCl(aq) → NaCl(aq) + HCN(aq)
The only species existing in solution in relatively large amounts are H2O, Na+, Cl-, and HCN. The mole ratio is 1:1 and since equal moles of both reactants are added, both reactants are limiting. The NaCl formed is a strong electrolyte and will be completely dissociated in solution (making the solution conducting). The HCN is a weak acid, which means that very little dissociates and in solution, it exists primarily as unionized molecules.
53. B—This answer shows the hydrogen ions and chloride ions as separate ions in solution, which is a property of strong electrolytes. Molecules of hydrogen chloride are present in the gas phase. The water molecules are present in both the liquid and gas phase. Other diagrams incorrectly show water decomposing, HCl molecules in solution, and hydrogen chloride vaporizing as separate ions.
54. B—The balanced equation is:
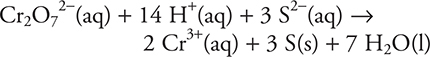
55. A—In diagram A, the forces forming are ion-dipole forces. In diagram B, the forces forming are hydrogen bonding. In diagram C, the forces forming are London dispersion forces. In diagram D, the forces forming are dipole-induced dipole forces. The strongest forces forming are ion—dipole forces. The strongest interaction forming will release the greatest amount of energy.
56. C—There are several ways of solving this problem. One way is to determine the moles present in the original containers, which must be the same as in the final container. In each case, moles = n = PV/RT. Numbering the containers from left to right as 1, 2, 3, and 4 gives (followed by estimating):
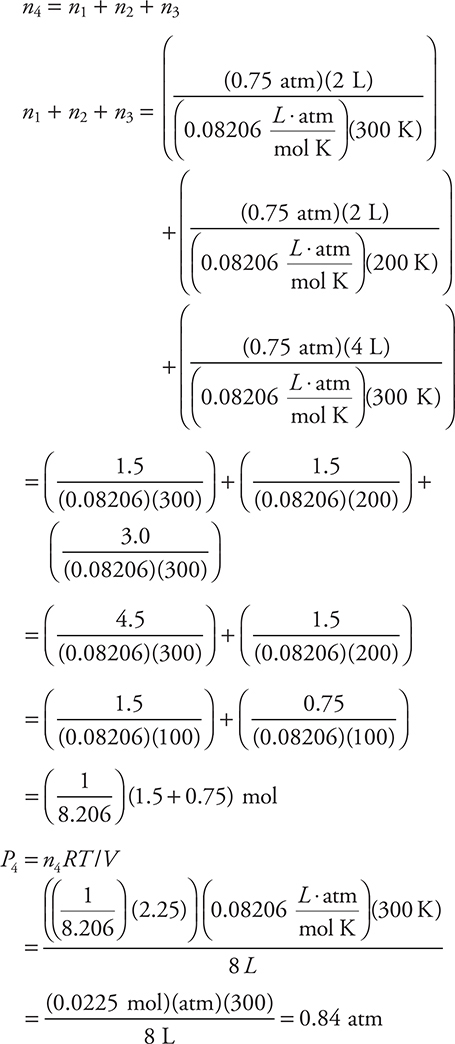
Extra detail is given in this solution. On the exam, it is not necessary to write out all these steps. Take shortcuts.
57. A—Stronger intermolecular forces lead to higher melting points. Both molecules are nonpolar; therefore, the key intermolecular forces are London dispersion forces. The greater molar mass of CSe2 leads to stronger London dispersion forces. This illustrates why comparisons should only be made between molecules of similar molecular masses.
58. B—The rate law shows that the rate law is first order in each of the reactants. The reaction involves the transfer of an oxygen atom from N2O to NO to form NO2, leaving an N2. To form NO2, the N from the NO must bond to the O from N2O. To form the new NO bond, the N from NO must come in contact with the O of N2O.
59. B—Only the slow step in a mechanism leads to the rate law. The CHCl3 goes into the mechanism. However, the other substance (Cl) in the slow step is an intermediate and cannot be in the final answer. The intermediate, Cl, must be replaced with a reactant; since Cl is ½ Cl2, the chlorine goes into the rate law as [Cl2]1/2. Answer (D) contains an equilibrium constant expression, which does not apply to kinetics problems like this one.
60. B—The heat lost must be identical to the heat gained (Law of Conservation of Energy). For the final temperature to be the average, the metals would need to have equal specific heats. The masses given in the problem are the same, and it is extremely unlikely that two different metals would have the same specific heat.
STOP. End of AP Chemistry Final Practice Exam, Section I (Multiple Choice).
![]() AP Chemistry Final Practice Exam, Section II (Free Response)
AP Chemistry Final Practice Exam, Section II (Free Response)
Time—1 hour and 45 minutes
Answer the following questions in the time allowed. You may use a calculator and the resources at the back of the book. Write the answers on separate sheets of paper.
Question 1
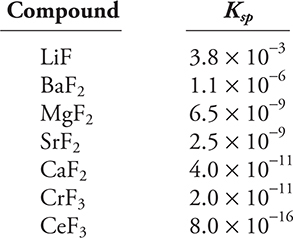
Use the Ksp data given above to answer the following questions.
A chemist is investing the chemistry of metal fluoride compounds. Some of her data are in the Ksp table given above. In addition to this information, she knows that sodium fluoride, NaF, is soluble in water and that hydrofluoric acid, HF, has Ka = 6.8 × 10-4 at 25°C.
(a) (i) Write a balanced chemical equation for the dissolution equilibrium of barium fluoride, BaF2, in water.
(ii) Write the Ksp relationship for the dissolution equilibrium of barium fluoride.
(iii) What is the concentration of fluoride ions in a saturated solution of barium fluoride?
(b) What is the solubility of strontium fluoride, SrF2, in a 0.10 M strontium nitrate, Sr(NO3)2, solution?
(c) (i) How does the solubility of magnesium fluoride, MgF2, in 1.0 M nitric acid compare to its solubility in pure water?
(ii) Explain your answer to part (c) (i).
(d) Which of the following will produce a higher fluoride ion concentration in solution, calcium fluoride, CaF2, or chromium(III) fluoride, CrF3? What is the fluoride ion concentration for each of these two compounds?
(e) Write a balanced net ionic equation for the addition of a sodium fluoride solution to a cerium(III) nitrate, Ce(NO3)3 solution. Recall that sodium fluoride, NaF, is soluble in water.
Question 2
![]()
A series of experiments were conducted to study the above reaction. The initial concentrations and rates are reported in the table below.
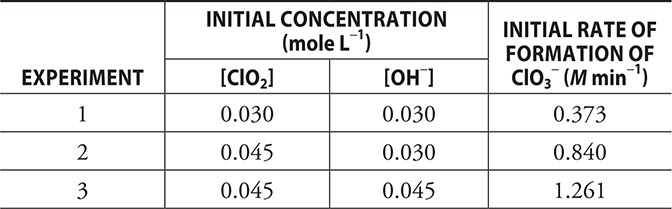
(a) (i) Determine the order of the reaction with respect to each reactant. Make sure you explain your reasoning.
(ii) What is the rate law for the reaction?
(b) Determine the value of the rate constant, making sure the units are included.
(c) Calculate the initial rate of disappearance of ClO2 in Experiment 1.
(d) The following has been proposed as a mechanism for this reaction.
Step 1: ClO2(aq) + ClO2(aq) → Cl2O4(aq)
Step 2: Cl2O4(aq) + OH -(aq) → ClO3-(aq) + HClO2(aq)
Step 3: HClO2(aq) + OH -(aq) → ClO2-(aq) + H2O(l)
Which step is the rate-determining step? Show that this mechanism is consistent with both the rate law for the reaction and with the overall stoichiometry.
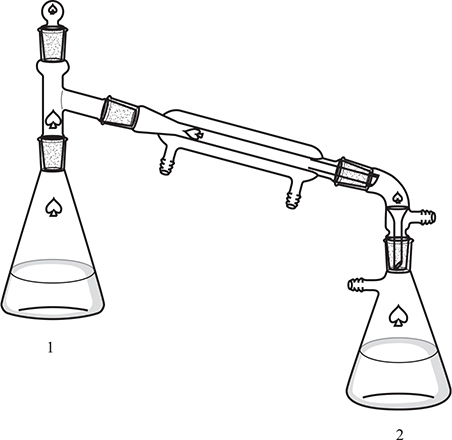
Question 3
A student wishes to analyze a sample of ammonium chloride, NH4Cl, contaminated with sodium chloride, NaCl. She constructs the apparatus shown above to carry out this analysis. She weighs 1.032 g of sample into flask 1 and dissolves the solid in 50 mL of water. Then she adds 50.00 mL of 0.2000 M sulfuric acid, H2SO4, to flask 2. In the next step, she quickly adds an excess of concentrated sodium hydroxide, NaOH, solution to flask 1 and quickly seals the system. She then heats flask 1 to boiling and distills over about 25 mL of water to flask 2; during this process all the ammonia, NH3, generated in flask 1 is transferred to flask 2. After the distillation is complete, she disassembles the apparatus and adds a small amount of Congo red indicator to flask 2 and titrates the solution in flask 2 with standard sodium hydroxide solution. The titration requires 39.35 mL of 0.1500 M sodium hydroxide solution to reach the endpoint.

(a) (i) Calculate the moles of sulfuric acid originally in flask 2.
(ii) Calculate the moles of sulfuric acid reacting with the sodium hydroxide solution.
(iii) Calculate the moles of ammonia that reacted with the sulfuric acid.
(b) (i) Calculate the mass of ammonium chloride in the sample. (Molar mass of ammonium chloride = 53.50 g mol-1.)
(ii) Calculate the percent ammonium chloride in the sample.
(c) (i) Strong acid—strong base titrations commonly use phenolphthalein as an indicator. Give a good reason why the student chose to use Congo red instead. The pKa of the ammonium ion is 9.25.
(ii) A second student used phenolphthalein in place of Congo red and got significantly different results. Was the second student’s percent higher or lower than the student using Congo red? What was the cause of this discrepancy?
(d) In the box below, sketch the Lewis electron-dot structure of ammonium chloride.

Question 4
The following equipment is available for the determination of the enthalpy of solution (molar) for an unknown salt, AX. Assume the salt is soluble in water.

(a) Which of the above equipment is necessary for the determination of the enthalpy of solution?
(b) List the measurements necessary to determine the enthalpy of solution for the unknown salt.
(c) List what additional information is necessary to determine the enthalpy of solution.
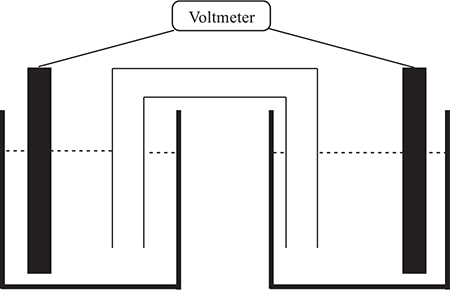
Question 5
The above schematic is one form of a galvanic cell. The electrode on the left is platinum metal in a solution that is 1.0 M in iron(II) sulfate, FeSO4, and 1.0 M in iron(III) chloride, FeCl3. The electrode on the right is either pure silver metal or silver metal, with a coating of solid silver chloride, AgCl, depending upon the experiment. In either case, the silver electrode is in a 1.0 M silver nitrate, AgNO3, solution. The salt bridge contains 2.0 M potassium nitrate. The potentially important half-reactions are:
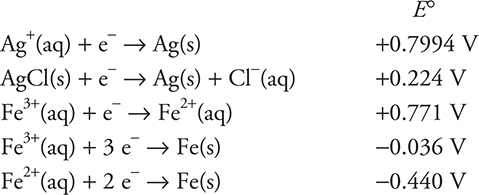
(a) In the first experiment, a pure silver electrode was used. The voltmeter indicated the predicted voltage. Write an equation for the cell reaction.
(b) In the second experiment, a silver electrode coated with silver chloride was used. A 2.0 M solution of potassium chloride, KCl, was substituted for the potassium nitrate in the salt bridge. When connected, the voltmeter showed a voltage markedly different from the predicted value. Write the cell reaction for this experiment? What caused the voltage to deviate from the predicted value?
(c) When a cell is not standard, it is necessary to adjust the cell voltage to reflect the variation from standard conditions. Show the mathematical relationship necessary to calculate the nonstandard cell potential for the cell in part (b). There is no need to enter the numerical values or to calculate an answer.
Question 6
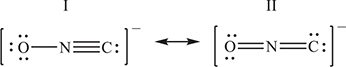
Fulminates, ONC-, salts of the extremely unstable fulminic acid, HONC, are used in detonators for other explosives. Two resonance structures for the fulminate ion are shown above.
(a) Explain which of the two resonance forms is the more stable. The explanation must be justified with formal charges.
(b) The cyanate ion, OCN-, is isomorphous with the fulminate ion, except that the carbon and nitrogen atoms exchange places. Like the fulminate ion, the cyanate ion has more than one resonance form. In each of the boxes below, draw a different resonance form of the cyanate ion.
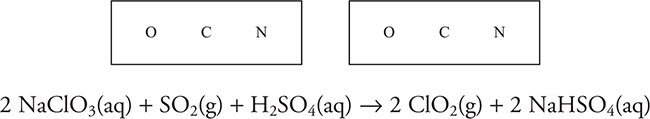
(c) It is possible to prepare chlorine dioxide, ClO2, by the above reaction. The best results occur when sodium chloride, NaCl, is added as a catalyst. This major industrial chemical is used directly or indirectly as a bleach. The observed bond angle is 118°. Explain the observed bond angle.
Question 7
A student in Denver, Colorado, used the following reaction to generate oxygen gas in a gas generator:
![]()
The reaction is catalyzed by the presence of iron(III) ions from a small amount of iron(III) chloride. The gas generator was connected by a tube to an inverted flask, filled with water, in a water bath. The gas displaced most of the water by the time the reaction ended. The flask was sealed while still inverted in the water bath. The flask was turned upright still containing the oxygen gas and the liquid water that had not been expelled. A graduated cylinder was used to measure the volume of water remaining in the flask. The flask was then refilled with water and a graduated cylinder was used to determine the total volume of the flask. The difference between the two volumes was the volume of oxygen in the flask. The volume of the sample was 375 mL at 26°C, and the pressure in the room was 635 mm Hg. The vapor pressure of water at 26°C is 25 mm Hg.
A second student conducted a similar experiment under identical conditions beginning with the following reaction:
![]()
The second student’s results were dramatically different from those of the first student (not as good), indicating that there was something drastically different.
(a) How many grams of oxygen are in the flask? Show all calculations.
(b) How many hydrogen atoms were in the oxygen gas in the flask after the reaction ended?
(c) What is the most likely reason why the second student did not get good results? Explain your reasoning.
STOP. End of AP Chemistry Final Practice Exam, Section II (Free Response).
![]() Answers and Explanations for Final Practice Exam, Section II (Free Response)
Answers and Explanations for Final Practice Exam, Section II (Free Response)
Question 1
(a) (i) ![]()
You get 1 point for this answer. The equilibrium arrow and the ionic charges must be present.
(ii) ![]()
You get 1 point for this answer. The charges must be included, and the fluoride ion concentration must be squared. You can still get 1 point if your expression correctly utilizes a wrong answer from part (a) (i).
(iii) ![]()
Setting ![]() and inserting into the mass-action expression gives:
and inserting into the mass-action expression gives:
![]()
Solving for x gives x = 6.5 × 10-3, and [F-] = 2x = 1.3 × 10-2 M.
You get 2 points for the correct [F-]. If your only mistake was forgetting to double the F-, you get 1 point. You can still get 1 point if you correctly used your wrong answer from part (a) (ii).
(b) Strontium nitrate is soluble in water (obviously because you are given a solution), which separates into strontium and nitrate ions. The strontium ion concentration is 0.10 M and it is a common ion affecting the equilibrium.
![]()
Setting ![]() and
and ![]() and inserting into the mass-action expression gives:
and inserting into the mass-action expression gives:
(0.10 + x)(2x)2 = 2.5 × 10-9. Assuming x is much smaller than 0.10 allows a simplification of the calculation to (0.10)(2x)2 = 0.4 x2 = 2.5 × 10-9.
Solving for x gives x = 7.9 × 10-5 M, which leads to [F-] = 2x = 1.6 × 10-4 M.
You get 1 point for the correct [F-].
(c) (i) Magnesium fluoride is more soluble in nitric acid than in pure water.
You get 1 point for this answer.
(ii) Hydrofluoric acid is a weak acid (Ka = 6.8 × 10-4); therefore, according to Le Châtelier’s principle, the solubility equilibrium will be displaced to the right as fluoride ions combine with hydrogen ions from the nitric acid to form unionized hydrofluoric acid. The reactions are:
![]()
You get 1 point for this answer.
(d) If the two compounds have the same stoichiometry, the larger Ksp would generate the higher fluoride ion concentration. However, this is not the case, so it is necessary to calculate the fluoride ion concentration for each.
![]()
Setting [Ca2+] = x and [F-] = 2x, and inserting into the mass-action expression gives:
![]()
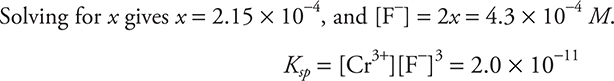
Setting ![]() and inserting into the mass-action expression gives:
and inserting into the mass-action expression gives:
![]()
Solving for ![]() (more soluble).
(more soluble).
You get 1 point for doing the calculations and showing CrF3 is more soluble and 1 additional point for two correct answers.
(e) It is easier to begin with the balanced molecular equation:
![]()
Converting the molecular equation to a complete ionic equation:
![]()
The ionic compounds NaF and Ce(NO3)3 should be separated into their ions since you are supplied with solutions, which means they must be soluble, and the sodium nitrate is also shown in the ionic form since you are told it is soluble in water. There is a Ksp for CeF3 given in the table; therefore, it does not separate into ions.
Removing the spectator ions ![]() from the complete ionic equation leaves the net ionic equation:
from the complete ionic equation leaves the net ionic equation:
![]()
You get 1 point for this answer.
Total your points for the different parts. There is a maximum of 10 points possible. Subtract 1 point if all answers did not have the correct number of significant figures. You covered this material with the Solubility Equilibria in Chapter 15.
Question 2
(a) (i) This part of the problem begins with a generic rate equation: ![]() It is necessary to begin by determining the values of the exponents, the orders (m and n). It does not matter which is determined first. We will begin with ClO2; therefore, then it is necessary to pick two experiments from the table where the ClO2 concentration changes, but the OH- concentration does not change. This would be Experiments 1 and 2. Experiment 2 has 1.5 times the concentration of ClO2 as Experiment 1. This factor (1.5) times the ClO2 concentration has increased the rate by 2.25 times. The relationship between the concentration (× 1.5) and the rate (× 2.25 = × 1.52) indicates that the order for ClO2 is 2 (= m). Moving the other reactant, using Experiments 2 and 3 (only the OH- concentration changes), we see that increasing the concentration by a factor of 1.5 increases the rate by the same factor. Thus, the order for OH- is 1 (= n). Give yourself 1 point for each order you got correct if you explained how you determined the order. The detail does not need to be in as much detail as given here.
It is necessary to begin by determining the values of the exponents, the orders (m and n). It does not matter which is determined first. We will begin with ClO2; therefore, then it is necessary to pick two experiments from the table where the ClO2 concentration changes, but the OH- concentration does not change. This would be Experiments 1 and 2. Experiment 2 has 1.5 times the concentration of ClO2 as Experiment 1. This factor (1.5) times the ClO2 concentration has increased the rate by 2.25 times. The relationship between the concentration (× 1.5) and the rate (× 2.25 = × 1.52) indicates that the order for ClO2 is 2 (= m). Moving the other reactant, using Experiments 2 and 3 (only the OH- concentration changes), we see that increasing the concentration by a factor of 1.5 increases the rate by the same factor. Thus, the order for OH- is 1 (= n). Give yourself 1 point for each order you got correct if you explained how you determined the order. The detail does not need to be in as much detail as given here.
(ii) Entering the orders from part (i) into the generic rate law gives: ![]() which is usually simplified to:
which is usually simplified to: ![]() Give yourself 1 point if you got this correct. If one or both of your orders from part (i) is incorrect, but you entered your answers correctly here, you still get 1 point for part (ii).
Give yourself 1 point if you got this correct. If one or both of your orders from part (i) is incorrect, but you entered your answers correctly here, you still get 1 point for part (ii).
(b) Any one of the three experiments may be used to calculate the rate constant. If the problem asked for an average rate constant, you would need to calculate a value for each of the experiments and then average the values.
The rate law should be rearranged to: 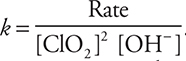 Then enter the appropriate values into this rearranged equation. Using Experiment 1 as an example:
Then enter the appropriate values into this rearranged equation. Using Experiment 1 as an example:
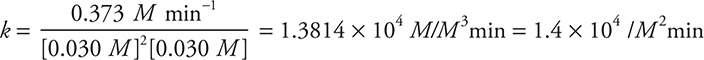
The answer could also be reported as 1.4 × 104 L2/mol2min. You should not forget that M = mol/L.
Give yourself 1 point for the correct numerical value, unless you ended with the wrong number of significant figures. Give yourself 1 point for the correct units. If your rate law from part (a) (ii) was incorrect, but you used it correctly, you can still earn 1 or both points.
(c) The rate is based upon ClO3-; therefore, it is necessary to convert to ClO2. The coefficients from the equation say that for every mole of ClO3- that forms, two moles of ClO2 reacted. Thus the rate of ClO2 is twice the rate of ClO3-. Do not forget that since ClO3- is forming, it has a positive rate, and since ClO2 is reacting it has a negative rate. This gives:
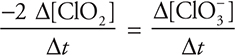
Rearranging and plugging in the rate from Experiment 1 gives:
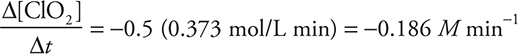
Give yourself 2 points if you got the entire answer correct. You get only 1 point if the sign is incorrect or the units are missing. If you got the significant figures wrong, deduct 1 point.
(d) The rate-determining step must match the rate law. One approach is to determine the rate law for each step in the mechanism. This gives:
Step 1: ![]()
Step 2: ![]()
Step 3: ![]()
The Cl2O4 and [HClO2] in steps 2 and 3 are intermediates. An intermediate cannot appear in the rate law; therefore, the [Cl2O4] and [HClO2] will need to be replaced with reactants. In the far-right side of steps 2 and 3, this conversion from intermediate to reactant has been performed. Step 2 gives a rate-law matching the one derived in part (a). Give yourself 1 point if you picked step 2, or if you picked a step with a rate-law that matches your wrong answer for part (a). Give yourself 1 more point if you explained the substitution of reactants for intermediates.
To see if the stoichiometry is correct, simply add the three steps together and cancel the intermediates (materials that appear on both sides of the reaction arrow).
Step 1: ![]()
Step 2: ![]()
Step 3: ![]()
Total: 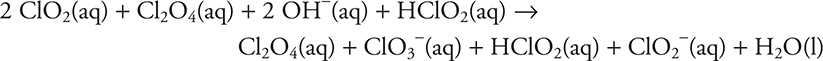
After removing the intermediates (Cl2O4 and HClO2):
![]()
As this matches the original reaction equation, the mechanism fulfills the overall stoichiometry requirement. Give yourself 1 point if you have done this.
The total is 10 points for this question. You saw most of the material when you first went through the material in the Kinetics chapter (Chapter 13).
Question 3
(a) (i) The moles of sulfuric acid originally present in 50.00 mL of 0.2000 M H2SO4: 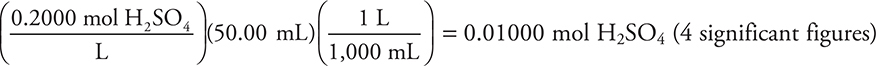 (You can simplify this calculation as
(You can simplify this calculation as 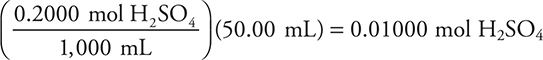 You get 1 point for this answer.
You get 1 point for this answer.
(ii) You need the balanced chemical equation for the reaction:
![]()
The titration required 39.35 mL of 0.1500 M NaOH, and the calculation is:
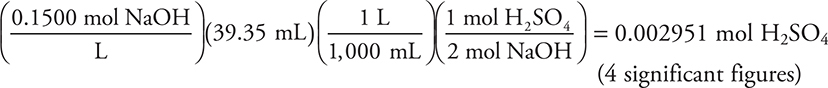
You get 1 point for this answer.
(iii) You need the balanced chemical equation for the reaction:
![]()
The moles of H2SO4 involved in this reaction is the moles originally present (0.01000 mole) minus the moles reacting with the NaOH (0.002951 mole).
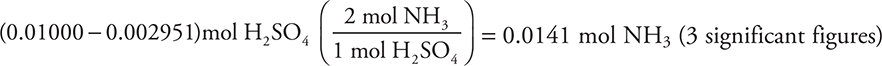
You get 1 point for this answer. If you miscalculated either of the preceding two answers but use your results correctly in this step, you still earn this point. The subtraction step resulted in the loss of one significant figure.
(b) (i) 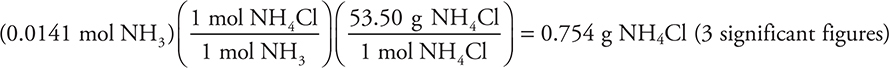
You get 1 point for this answer.
(ii) 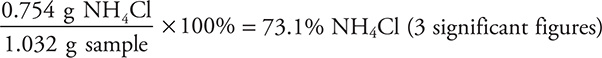
You get 1 point for this answer. If you got the wrong result for part (b) (i), but used it correctly here, you still get the point.
(c) (i) The sodium hydroxide solution will react with not only the hydrochloric acid but also the ammonium ion. The Congo red endpoint is below where the ammonium ion begins to react (as noted from the pKa); therefore, Congo red is a better measure of the amount of HCl reacting than phenolphthalein, which gives an endpoint after at least some of the ammonium ion has reacted.
You get 1 point for this answer.
(ii) The percent will be lower. It will be necessary to use more sodium hydroxide solution to reach the endpoint, making it appear that less ammonia reacted with the H2SO4. If there were less ammonia, then the percentage would be lower.
You get 1 point for predicting the percent will be lower and 1 point for the explanation.
(d) 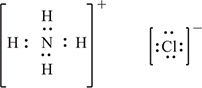
You get 1 point for having an octet on the nitrogen and an octet on the chlorine with no additional electrons shown.
You get 1 point for showing an ionic structure with no hint of a covalent bond between the ammonium ion and the chloride ion. The brackets are not essential but are present here as an aid to stress that these are ions with no covalent (electron-sharing).
Total your points. There is a maximum of 10 possible points. Subtract 1 point if any of your answers does not have the correct number of significant figures.
Question 4
(a) Analytical balance, thermometer, polystyrene cup, support stand and clamp, beakers, or flasks.
You must have these five items to get 1 point. There is no deduction if you have an additional item. You do not get the point if you list any item that is not on the list.
(b) The values needed are the masses of the sample and water, and the temperature change. These require the following measurements:
There are two ways to determine the masses (your choice), and you only need to use one.
Using the balance
1. Determine the mass of an empty beaker or flask; then redetermine the mass after the unknown solid has been added to the beaker or flask.
Determine the mass of an empty polystyrene cup; then redetermine the mass after a quantity of water has been added to the cup.
2. Place an empty beaker or flask on the balance and tare the balance; then add the unknown solid and read the mass off the balance.
Place an empty polystyrene cup on the balance and tare the balance; then add water and read the mass of the balance.
Using the thermometer
Place the thermometer in the water (in the cup) and record the initial temperature.
Leave the thermometer in the water and add the unknown solid. Wait until the temperature stops changing and record the final temperature.
The use of the balance is worth 1 point. The use of the thermometer is worth 1 point. Statements such as “Measure the temperature change” earn no credit.
(c) The specific heat of water is needed, and the molar mass of the unknown solid are needed.
This complete list is worth 1 point.
Total your points. There are 4 possible points. We have seen students taking the AP Exam have trouble with a problem like this because they described the procedure instead of listing only the measurements. This is a “Laboratory” question, which will appear at least once on the free-response portion of the exam. You covered the basic principles necessary for the question in Chapter 20.
Question 5
(a) To get the cell reaction, the correct two half-reactions are needed. Since a pure silver electrode is present, it is necessary to use the silver half-reaction that does not contain chloride. There is no iron metal present; therefore, neither of the two iron half-reactions containing iron metal can be used. The two half-reactions must be:

The iron half-reaction has a lower standard potential, so it should be changed from a reduction to an oxidation (reversed) before it is added to the silver half-reaction. Both half-reactions involve one electron; therefore, the two may be simply added.
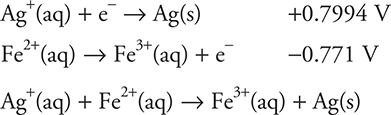
You get 1 point for the correct equation.
(b) To get the cell reaction, the correct two half-reactions are needed. Since a silver electrode with AgCl is present, it is necessary to use the silver half-reaction. There is no iron metal present; therefore, neither of the two iron half-reactions containing iron metal can be used. The two half-reactions must be:
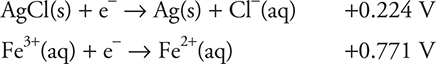
The silver chloride half-reaction has a lower standard potential, so it should be changed from a reduction to an oxidation (reversed) before it is added to the iron half-reaction. Both half-reactions involve one electron; therefore, the two may be simply added.
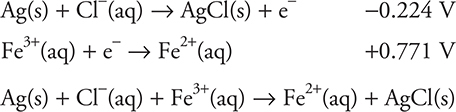
You get 1 point for the correct equation.
As indicated by the silver chloride half-reaction, AgCl is a solid (precipitate). Some chloride ions will diffuse from the salt bridge and react with the silver ions present in the silver nitrate solution. This reaction will precipitate some of the silver ion from the solution. Precipitation of some of the silver ion from the solution will alter the concentration from standard (1 M). Any change from standard will alter the voltage.
You get 1 point for this explanation. Simply saying the cell is not standard does not earn this point.
(c) If the cell is not standard, it is necessary to use the Nernst equation to make the adjustment. The equation is present in the exam booklet (in the back of this book).
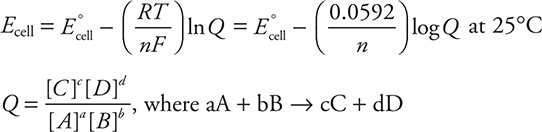
Using the cell reaction from (b), it is only necessary to determine Q.
You get 1 point for the correct setup for Q. Q cannot contain any solids (Ag or AgCl).
Total your points; there are 4 points possible. This is normally one of the last topics covered in an AP Chemistry course, so you may need to go back to the last few class lectures to clarify any difficulties you had with this question. You also covered this material when you first went through Chapter 17 in this book.
Question 6
(a) Here are the two Lewis structures with formal charges included. (There may be other Lewis structures; however, you are specifically told to deal with these two.) While not specifically required, you should calculate the formal charge for each of the six atoms, and it will simplify your justification if you include your answers as indicated below. Recall that for an atom, the formal charge equals the number of valance electrons minus the number of electrons in lone pairs minus one-half the electrons involved in bonding pairs to that atom. (You may wish to confirm your understanding of formal charges by confirming the formal charges shown below.)
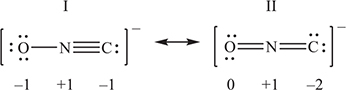
Structure I is the more stable structure. The presence of a −2 formal charge indicates that Structure II is less stable.
You get 1 point for the correct choice including the formal charge argument. Any mention of another structure is irrelevant. Any justification based upon the bonding is irrelevant; besides, both structures contain two σ bonds and two π bonds. In both structures, all the atoms have a complete octet of electrons.
(b) There are three reasonable resonance forms, which are pictured below. Other forms are either incorrect or different views of the forms shown below. You need to reproduce any two of them. In all structures all atoms obey the octet rule (failure to do this is a common reason for a structure being incorrect). The exact placement of the lone pairs is irrelevant if they are on the appropriate atoms. The square brackets are optional. Make sure that you do not accidently draw two views of the same structure. The only bonding schemes are (left to right): [1 and 3], [2 and 2], and [3 and 1].
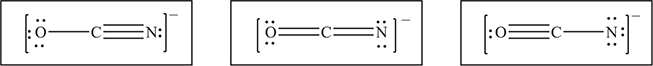
You get 1 point for each correct Lewis structure for a maximum of 2 points. Refrain from including all three structures in your answer, because if one is wrong, you may lose a point.
(c) The simplest/easiest way to explain the bond angle is to begin with a Lewis structure and utilize VSEPR. The Lewis structure is:
![]()
This is an odd electron atom molecule (19 valence electrons); therefore, the less electronegative element (Cl) will probably not have an octet. Chlorine is the central atom and the placement of four electron groups around it would normally lead to a bond angle of 109.5° (sp3 hybridized). However, the lone electron will not repel as efficiently as a pair of electrons. The less effective repulsion allows the bond angle to increase to the observed 118°.
An alternate explanation is that the chlorine atom is sp2 hybridized with the lone electron in an unhybridized p orbital. In this explanation, the angle is slightly less than the ideal 120° because of the presence of the lone pair. In this situation, the lone electron does not influence the angle.
You get 1 point for either explanation.
Total your points for the problem. There is a maximum of 4 possible points. Much of the material in this problem is based upon pre-AP material, with the final answers being based upon the additional understanding you gained by taking an AP Chemistry course.
Question 7
(a) The quickest way to answer this question is to use the ideal gas equation. The values necessary for this calculation are:
Ptotal = 635 mm Hg
Poxygen = 635 − 25 = 610 mm Hg (Dalton’s law)
P = 610 mm Hg/760 mm Hg = 0.803 atm (The 760 mm Hg = 1 atm is in the exam booklet and in the back of this book.)
V = 375 mL = 0.375 L
T = 26°C = 299 K
R = 0.08206 L atm mol-1 K-1 (given in the exam booklet and in the back of this book)
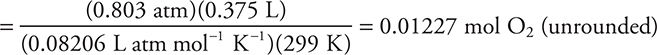
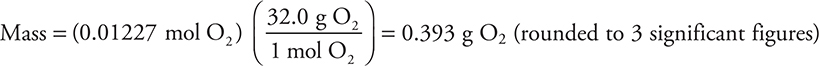
Give yourself 1 point for using Dalton’s law to determine the partial pressure of oxygen. Give yourself 1 point for the correct answer (no deduction for rounding differently). You must include ALL parts of the calculation (including “=”). There is no deduction for combining steps. You cannot get this point if you did not subtract the vapor pressure of water from the total pressure. If you went directly through 22.4 L mole-1, you receive 0 points because the conditions are not STP.
If you used a different approach and got the correct answer, you can still earn 1 point.
(b) The hydrogen atoms in the flask present in the water molecules are from the vapor pressure of water. This calculation also utilizes the ideal gas equation to calculate the moles of water present. These moles are then converted to moles of hydrogen atoms, which are converted to hydrogen atoms through Avogadro’s number.
The variables used in part (a) are the same here except it is necessary to use only the vapor pressure of water.
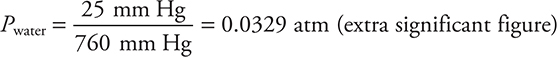
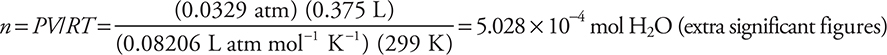
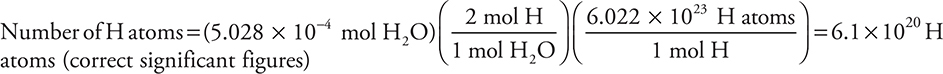
Give yourself 1 point for the correct answer (no deduction for rounding variations).
(c) Unlike oxygen gas, sulfur dioxide gas is soluble in water because it, like many nonmetal oxides, is capable of reacting with water to form an acid (acid anhydride). Oxygen is only slightly soluble in water.
Give yourself 1 point for indicating the problem is the solubility of sulfur dioxide in water.
Total your points for this question. There are 4 points possible. Deduct 1 point if you reported the wrong number of significant figures in any answer.
Scoring and Interpretation
After finishing and scoring this diagnostic exam, you need to analyze your results. During this analysis, the key is not whether you got the question correct or not, but how well you understood the question. First, you should pay attention to any area where you had difficulty (even if you got the correct answer). This should not be limited to unfamiliar material, which you will no doubt see. Determine where this material is covered in the book. Plan on spending additional time on the chapters/sections in question. There may be some material that you do not recognize simply because it was not covered in your class. Save your results for comparison whenever you retake this exam.
If you did not do as well as you would have liked, don’t panic. There is plenty of time for you to prepare for the exam. This diagnostic exam is a guide to allow you to know which path you need to follow as you prepare for the AP Exam. This exam is not intended to be a predictor of your success.
As stated earlier, it may be useful for you to retake this exam before you start your final review. When doing so, focus on the areas where you did not improve as much as you would like.
You are about to begin your 5 Steps to a 5. Remember, this test is to help you organize your study plan and not to predict your results on the exam.
Good luck!