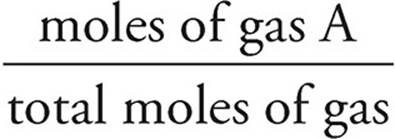Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 4
Big Idea #2: Bonding and Phases
DALTON’S LAW
Dalton’s law states that the total pressure of a mixture of gases is just the sum of all the partial pressures of the individual gases in the mixture.
Dalton’s Law
Ptotal = Pa + Pb + Pc + …
You should also note that the partial pressure of a gas is directly proportional to the number of moles of that gas present in the mixture. So if 25 percent of the gas in a mixture is helium, then the partial pressure due to helium will be 25 percent of the total pressure.
Partial Pressure
Pa = (Ptotal)(Xa)
Xa =