Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 4
Big Idea #2: Bonding and Phases
SOLUTIONS
Molarity
Molarity (M) expresses the concentration of a solution in terms of volume. It is the most widely used unit of concentration, turning up in calculations involving equilibrium, acids and bases, and electrochemistry, among others.
When you see a chemical symbol in brackets on the test, that means they are talking about molarity. For instance, “[Na+]” is the same as “the molar concentration (molarity) of sodium ions.”
Molarity (M) = 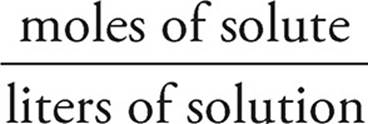
Mole Fraction
Mole fraction (Xs) gives the fraction of moles of a given substance (S) out of the total moles present in a sample.
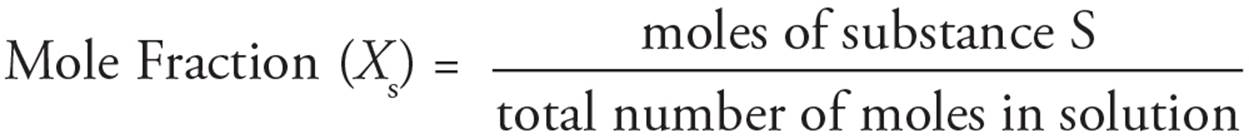
Solutes and Solvents
There is a basic rule for remembering which solutes will dissolve in which solvents.
Like dissolves like
That means that polar or ionic solutes (such as salt) will dissolve in polar solvents (such as water). That also means that nonpolar solutes (such as oils) are best dissolved in nonpolar solvents. When an ionic substance dissolves, it breaks up into ions. That’s dissociation. Free ions in a solution are called electrolytes because they can conduct electricity.
The more ions that are present in an ionic compound, the greater the conductivity of that compound will be when those ions are dissociated. For instance, a solution of magnesium chloride will dissociate into three ions (one Mg2+ and two Cl−). A solution of sodium chloride will dissociate into just two ions (Na+ and Cl−). Thus, a solution of magnesium chloride will conduct electricity better than a solution of sodium chloride if both solutions have identical concentrations.