Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 5
Big Idea #3: Chemical Reactions, Energy Changes, and Redox Reactions
ELECTROLYTIC CELLS
In an electrolytic cell, an outside source of voltage is used to force an unfavored redox reaction to take place. Most electrolytic cells occur in aqueous solutions which are created when a chemical dissolves in water; either the ions or the water molecules can be reduced or oxidized.
Let’s look at a solution of nickel (II) chloride as an example. To determine which substance is reduced, you must compare the reduction potential of the cation with that of water. The half-reaction with the more positive value is the one that will occur.
|
Ni2+ + 2e− → Ni(s) |
E ° = −0.25 V |
|
2H2O(l) + 2e− → H2(g) + 2OH− |
E ° = −0.80 V |
In this case, the Ni2+ reduction will occur. For the oxidation, the oxidation potential of the anion versus that of water must be examined. As with before, the half-reaction with the most positive value is the one that will occur. Remember that potentials are always given as reduction potentials, so you must flip the sign when you flip the equation to an oxidation.
|
2Cl− → Cl2(g) + 2e− |
E ° = −1.36 V |
|
2H2O(l) → O2(g) + 4H+ + 4e− |
E ° = −1.23 V |
So, in this case, the water itself would be reduced. When the equations are balanced for electron transfer, the net ionic equation looks like this:
2Ni2+ + 2H2O(l) → 2Ni(s) + O2(g) + 4H+
E = −0.25 V + −1.24V = −1.49 V
The anode and cathode in an electrolytic reaction are usually just metal bars that conduct current and do not take part in the reaction. In the above reaction, solid nickel is being created at the cathode, and oxygen gas is being evolved at the anode. The sign of your total cell potential (E) for an electrolytic reaction is always negative.
Occasionally, a current will either be run through a molten compound or pure water. In this case, you do not need to determine which redox reactions are taking place as you will only have one choice for each.
The AN OX/RED CAT rule applies to the electrolytic cell in the same way that it applies to the galvanic cell.
Electroplating
Electrolytic cells are used for electroplating. You may see a question on the test that gives you an electrical current and asks you how much metal “plates out.”
There are roughly four steps for figuring out electrolysis problems.
1. If you know the current and the time, you can calculate the charge in coulombs.
Current
I = ![]()
I = current (amperes, A)
q = charge (coulombs, C)
t = time (sec)
2. Once you know the charge in coulombs, you know how many electrons were involved in the reaction.
Moles of electrons = 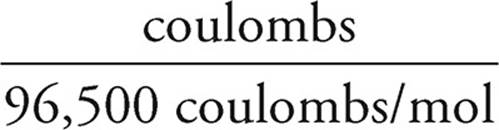
3. When you know the number of moles of electrons and you know the half-reaction for the metal, you can find out how many moles of metal plated out. For example, from this half-reaction for gold
Au3+ + 3e− → Au(s)
you know that for every 3 moles of electrons consumed, you get 1 mole of gold.
4. Once you know the number of moles of metal, you can use what you know from stoichiometry to calculate the number of grams of metal.
For instance, if a current of 2.50 A is run through a solution of iron (III) chloride for 15 minutes, it would cause the following mass of iron to plate out:
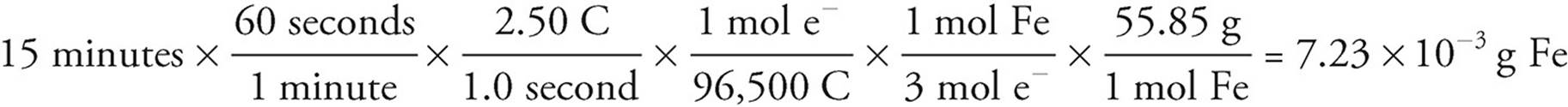
It is particularly important to keep track of your units in an electroplating problem; there are many different conversions before you come up with your final answer. In general, as long as all of your conversions are set up correctly your final answer will have the correct units.