Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 8
Big Idea #6: Equilibrium, Acids and Bases, Titrations, and Solubility
POLYPROTIC ACIDS AND AMPHOTERIC SUBSTANCES
Some acids, such as H2SO4 and H3PO4, can give up more than one hydrogen ion in solution. These are called polyprotic acids.
Polyprotic acids are always more willing to give up their first protons than later protons. For example, H3PO4 gives up an H+ ion (proton) more easily than does 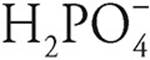 , so H3PO4 is a stronger acid. In the same way,
, so H3PO4 is a stronger acid. In the same way, 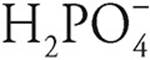 is a stronger acid than
is a stronger acid than 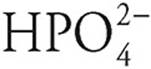 .
.
Substances that can act as either acids or bases are called amphoteric substances.
For instance
· 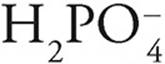 can act as an acid, giving up a proton to become
can act as an acid, giving up a proton to become 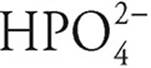 , or it can act as a base, accepting a proton to become H3PO4
, or it can act as a base, accepting a proton to become H3PO4
· 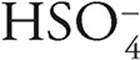 can act as an acid, giving up a proton to become
can act as an acid, giving up a proton to become 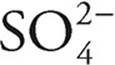 , or it can act as a base, accepting a proton to become H2SO4
, or it can act as a base, accepting a proton to become H2SO4
· H2O can act as an acid, giving up a proton to become OH−, or it can act as a base, accepting a proton to become H3O+