5 Steps to a 5 AP Chemistry (2015)
STEP 5. Build Your Test-Taking Confidence
AP Chemistry Practice Exam 1
AP Chemistry Practice Exam 1—Multiple Choice
ANSWER SHEET

The AP exam is a timed exam; keep this in mind as you prepare. When taking the various tests presented in this book, you should follow the AP exam rules as closely as possible. Anyone can improve his or her score by using notes, books, or an unlimited time. You will have none of these on the AP exam, so resist the temptation to use them on practice exams. Carefully time yourself, do not use other materials, and use a calculator only when expressly allowed to do so. After you have finished an exam, you may use other sources to go over questions you missed or skipped. We have seen many students get into trouble because the first time they attempted a test under “test conditions” was on the test itself.
AP Chemistry Practice Exam 1, Section I (Multiple Choice)
Time—1 hour and 30 minutes
NO CALCULATOR MAY BE USED WITH SECTION I
Answer the following questions in the time allowed. You may use the periodic table in the back of the book.
1. Which of the following CANNOT behave as both a Brønsted base and a Brønsted acid?
![]()
![]()
![]()
![]()
2. Which of following are proper laboratory procedures for a titration?
(A) Make sure the color change of the indicator persists for at least 30 s, and allow all materials to cool to room temperature before they are weighed.
(B) Make sure the color change of the indicator persists for at least 30 s, and rinse the buret with deionized water before it is filled with titrant for the first titration.
(C) Allow all materials to cool to room temperature before they are weighed.
(D) Allow all materials to cool to room temperature before they are weighed, and rinse the buret with deionized water before it is filled with titrant for the first titration.
3. In most of its compounds, this element exists as a monatomic cation.
(A) F
(B) S
(C) N
(D) Ca
4. In which of the following groups are the species listed correctly in order of increasing radius?
(A) Sr, Ca, Mg
![]()
![]()
![]()
5. ![]()
After the above chemical equation is balanced, the lowest whole-number coefficient for water is:
(A) 1
(B) 6
(C) 9
(D) 12
6. Which of the following best represents the net ionic equation for the reaction of barium hydroxide with an aqueous potassium sulfate solution to form a precipitate?
![]()
![]()
![]()
![]()
7. A sample of magnesium metal is heated in the presence of nitrogen gas. After the sample was heated, some water was added to it. Which of the following statements is FALSE?
(A) The magnesium reacted with the nitrogen to produce magnesium nitride.
(B) No reaction occurred because nitrogen gas is so unreactive.
(C) The solid did not dissolve in the water.
(D) After the addition of the water, the distinctive odor of ammonia gas was present.
8. A student mixes 50.0 mL of 0.10 M Ni(NO3)2 solution with 50.0 mL of 0.10 M NaOH. A green precipitate forms, and the concentration of the hydroxide ion becomes very small. Which of the following correctly places the concentrations of the remaining ions in order of decreasing concentration?
![]()
![]()
![]()
![]()
9. The addition of concentrated Ba(OH)2(aq) to a 1.0 M (NH4)2SO4 solution will result in which of the following observations?
(A) The odor of ammonia is detected, and a white precipitate forms.
(B) The formation of a white precipitate takes place.
(C) The solution becomes acidic.
(D) The odor of ammonia is detected.
10. Manganese, Mn, forms a number of oxides. A particular oxide is 69.6% Mn. What is the simplest formula for this oxide?
(A) MnO
(B) Mn2O3
(C) Mn3O4
(D) MnO2
11. Sodium sulfate forms a number of hydrates. A sample of a hydrate is heated until all the water is removed. What is the formula of the original hydrate if it loses 56% of its mass when heated?
(A) Na2SO4.H2O
(B) Na2SO4.2H2O
(C) Na2SO4.6H2O
(D) Na2SO4.10H2O
12. ![]()
Copper metal reacts with nitric acid according to the above equation. A 0.30 mol sample of copper metal and 100.0 mL of 3.0 M nitric acid are mixed in a flask. How many moles of NO gas will form?
(A) 0.20 mol
(B) 0.038 mol
(C) 0.10 mol
(D) 0.075 mol
13. Gold(III) oxide, Au2O3, can be decomposed to gold metal, Au, plus oxygen gas, O2. How many moles of oxygen gas will form when 2.21 g of solid gold(III) oxide is decomposed? The formula mass of gold(III) oxide is 442.
(A) 0.00750 mol
(B) 0.0150 mol
(C) 0.00500 mol
(D) 0.00250 mol
14. ![]()
How many moles of MnSO4 are produced when 2.0 mol of KMnO4, 5.0 mol of H2C2O4, and 1.5 mol of H2SO4 are mixed?
(A) 2.0 mol
(B) 1.5 mol
(C) 1.0 mol
(D) 3.0 mol
15. ![]()
Strontium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.100 mol of strontium?
(A) 3.36 L
(B) 5.60 L
(C) 2.24 L
(D) 4.48 L
16. A sample of nitrogen gas is placed in a container with constant volume. The temperature is changed until the pressure doubles. Which of the following also changes?
(A) density
(B) moles
(C) average velocity
(D) number of molecules
17. An experiment to determine the molecular mass of a gas begins by heating a solid to produce a gaseous product. The gas passes through a tube and displaces water in an inverted, water-filled bottle. The mass of the solid is measured, as is the volume and the temperature of the displaced water. Once the barometric pressure has been recorded, what other information is needed to finish the experiment?
(A) the heat of formation of the gas
(B) the density of the water
(C) the mass of the displaced water
(D) the vapor pressure of the water
18. A sample of hydrogen gas initially occupied a volume of 6.00 L at 127°C and 875 mm Hg. The sample was heated, at constant pressure, until it occupied a volume of 15.00 L. Determine the final temperature of the hydrogen gas.
(A) 318°C
(B) 727°C
(C) 45°C
(D) 160°C
19. Which of the following describes the maximum energy available for useful work from a spontaneous reaction?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
20. What is the energy needed to separate the ions in an ionic solid?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
21. What refers to the energy difference between the transition state and the reactants?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) activation energy
22.
![]()
![]()
![]()
Using the information given above, calculate the enthalpy change for the following reaction:
![]()
(A) –135.1 kJ
(B) +135.1 kJ
(C) 270.2 kJ
(D) –270.2 kJ
23. When lithium sulfate, Li2SO4, is dissolved in water, the temperature increases. Which of the following conclusions may be related to this?
(A) Lithium sulfate is less soluble in hot water.
(B) The hydration energies of lithium ions and sulfate ions are very low.
(C) The heat of solution for lithium sulfate is endothermic.
(D) The solution is not an ideal solution.
24. ![]()
Determine ΔH for the above reaction if C2H5OH(l) was formed in the above reaction instead of C2H5OH(g). The ΔH of vaporization for C2H5OH is 43 kJ/mol.
(A) +3 kJ
(B) +89 kJ
(C) –3 kJ
(D) +43 kJ
25. The ground-state configuration of Ni2+ is which of the following?
(A) 1s22s22p63s23p63d84s2
(B) 1s22s22p63s23p63d104s2
(C) 1s22s22p63s23p63d10
(D) 1s22s22p63s23p63d8
26. The maximum number of electrons in an atomic orbital is two as a consequence of which of the following?
(A) Pauli exclusion principle
(B) Hund’s rule
(C) the wave properties of matter
(D) Heisenberg uncertainty principle
27. What idea explains why an oxygen atom is paramagnetic in the ground state?
(A) Pauli exclusion principle
(B) Hund’s rule
(C) the wave properties of matter
(D) Heisenberg uncertainty principle
28. Magnesium reacts with element X to form an ionic compound. If the ground-state electron configuration of X is 1s22s22p5, what is the simplest formula for this compound?
(A) Mg2X3
(B) Mg X2
(C) Mg X4
(D) Mg2X5
29. VSEPR predicts that a BF3 molecule will be which of the following shapes?
(A) tetrahedral
(B) trigonal bipyramidal
(C) square pyramid
(D) trigonal planar
30. Which of the following is polar?
(A) BF3
(B) IF5
(C) CF4
(D) XeF4
31. The only substance listed below that contains ionic, σ, and π bonds is:
(A) C2H4
(B) NaH
(C) NH4Cl
(D) NaC2H3O2
32. Which molecule or ion in the following list has the greatest number of unshared electron pairs around the central atom?
(A) IF7
![]()
(C) BF3
(D) NH3
33. Which of the following processes does not involve breaking an ionic or a covalent bond?
![]()
![]()
![]()
![]()
34. Graphite is:
(A) composed of atoms held together by delocalized electrons
(B) composed of molecules held together by intermolecular dipole-dipole interactions
(C) composed of positive and negative ions held together by electrostatic attractions
(D) composed of macromolecules held together by strong bonds
35. Solid calcium is:
(A) composed of atoms held together by delocalized electrons
(B) composed of molecules held together by intermolecular dipole-dipole interactions
(C) composed of positive and negative ions held together by electrostatic attractions
(D) composed of macromolecules held together by strong bonds
36. CaCO3(s) is:
(A) composed of atoms held together by delocalized electrons
(B) composed of molecules held together by intermolecular dipole-dipole interactions
(C) composed of positive and negative ions held together by electrostatic attractions
(D) composed of macromolecules held together by strong bonds
37. What is the total concentration of cations in a solution made by combining 700.0 mL of 3.0 M (NH4)3PO4 with 300.0 mL of 2.0 M Na2SO4?
(A) 2.7 M
(B) 13 M
(C) 7.5 M
(D) 5.0 M
38. A stock solution that is 0.30 M in Na2SO4 is available. How many moles of solid Na3PO4 must be added to 800.0 mL of this solution to increase the sodium ion concentration to 0.90 M?
(A) 0.060
(B) 0.12
(C) 0.080
(D) 0.16
39. How many milliliters of concentrated ammonia (7.0 M NH3) are needed to prepare 0.250 L of 3.0 M NH3?
(A) 110 mL
(B) 0.11 mL
(C) 200 mL
(D) 150 mL
40. The plot of ln[A] versus time gives a straight line. This implies the rate law is:
(A) rate = (k[A]2
(B) rate = (k[A]
(C) rate = (k[A]0
(D) rate = (k[A]–1
41. The specific rate constant, k, for radioactive lawrencium-256 is 86 h–1. What mass of a 0.0500 ng sample of lawrencium-256 remains after 58 s?
(A) 0.0500 ng
(B) 0.0250 ng
(C) 0.0125 ng
(D) 0.00625 ng
42. 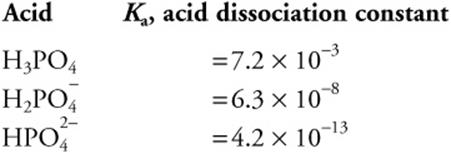
Using the above information, choose the best answer for preparing a pH = 7.9 buffer.
(A) K2HPO4
(B) K3PO4
![]()
![]()
43. What is the ionization constant, Ka, for a weak monoprotic acid if a 0.1 M solution has a pH of 2.0?
![]()
![]()
![]()
![]()
44. Mixing equal volumes of which of the following pairs will produce the most basic solution (highest pH)?
(A) 1 M CH3NH2 (methylamine) and 1 M LiOH (lithium hydroxide)
(B) 1 M C2H5NH2 (ethylamine) and 1 M C2H5NH3NO3 (ethylammonium nitrate)
(C) 1 M CH3NH2 (methylamine) and 1 M HC3H5O2 (propionic acid)
(D) 1 M H2C2O4 (oxalic acid) and 1 M KHC2O4(potassium hydrogen oxalate)
45. An analytical chemist needs a buffer with a pH > 7. He can mix equal volumes of two solutions. Which of the following pairs should he use?
(A) 1 M CH3NH2 (methylamine) and 1 M LiOH (lithium hydroxide)
(B) 1 M C2H5NH2 (ethylamine) and 1 M C2H5NH3NO3 (ethylammonium nitrate)
(C) 1 M CH3NH2 (methylamine) and 1 M HC3H5O2 (propionic acid)
(D) 1 M H2C2O4 (oxalic acid) and 1 M KHC2O4(potassium hydrogen oxalate)
46. It is possible to prepare a buffer by mixing two solutions. Mixing equal volumes of each of the solutions might give such a buffer. Which combination would yield a buffer with a pH < 7?
(A) 1 M CH3NH2 (methylamine) and 1 M LiOH (lithium hydroxide)
(B) 1 M C2H5NH2 (ethylamine) and 1 M C2H5NH3NO3 (ethylammonium nitrate)
(C) 1 M CH3NH2 (methylamine) and 1 M HC3H5O2 (propionic acid)
(D) 1 M H2C2O4 (oxalic acid) and 1 M KHC2O4(potassium hydrogen oxalate)
47. At constant temperature, a change in volume will yield the greatest change in the relative number of moles of the substances present for which of the following?
![]()
![]()
![]()
![]()
48. ![]()
Which species in the above equilibrium behave as bases?
![]()
(B) H2O only
![]()
![]()
49. ![]()
A 1.00 L flask is filled with 0.70 mol of H2 and 0.60 mol of CO, and allowed to come to equilibrium. At equilibrium, there are 0.40 mol of CO in the flask. What is the value of Kc, the equilibrium constant, for the reaction?
(A) 0.74
(B) 3.2
(C) 0.0050
(D) 5.6
50. 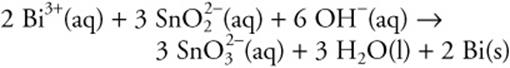
For the above reaction, pick the true statement from the following:
(A) The oxidation number of tin changes from +2 to +4.
(B) The oxidation number of tin changes from +4 to +2.
(C) The Bi3+ is oxidized by the tin.
(D) The OH– reduces the Bi3+.
51. An electrolysis cell was constructed with two platinum electrodes in a 1.00 M aqueous solution of KCl. An odorless gas evolved from one electrode and a gas with a distinctive odor evolved from the other electrode. Choose the correct statement from the following list.
(A) The odorless gas was oxygen.
(B) The odorless gas was evolved at the anode.
(C) The gas with the distinctive odor was evolved at the anode.
(D) The odorless gas was evolved at the cathode.
52. When ![]() decays, it emits two α particles, then a β particle, followed by an α particle. The resulting nucleus is:
decays, it emits two α particles, then a β particle, followed by an α particle. The resulting nucleus is:
![]()
![]()
![]()
![]()
53. Which of the following lists the types of radiation in the correct order of increasing penetrating power?
(A) α, γ, β
(B) β, α, γ
(C) α, β, γ
(D) β, γ, α
54. If 75% of a sample of pure ![]() decays in 24.6 yr, what is the half-life of
decays in 24.6 yr, what is the half-life of ![]()
(A) 24.6 yr
(B) 18.4 yr
(C) 12.3 yr
(D) 6.15 yr
Use the following information to answer questions 55–60.
The reaction of iron metal with hydrochloric acid generates aqueous iron(II) chloride and hydrogen gas. The balanced chemical equation for the reaction is:
![]()
Iron(III) oxide, a component of rust, reacts with hydrochloric acid to generate aqueous iron(III) chloride and water. The balanced chemical equation for the reaction is:
![]()
A student weighed a small flask both with and without a sample of rusty iron and recorded the masses. Next, she connected the flask to the system shown below.
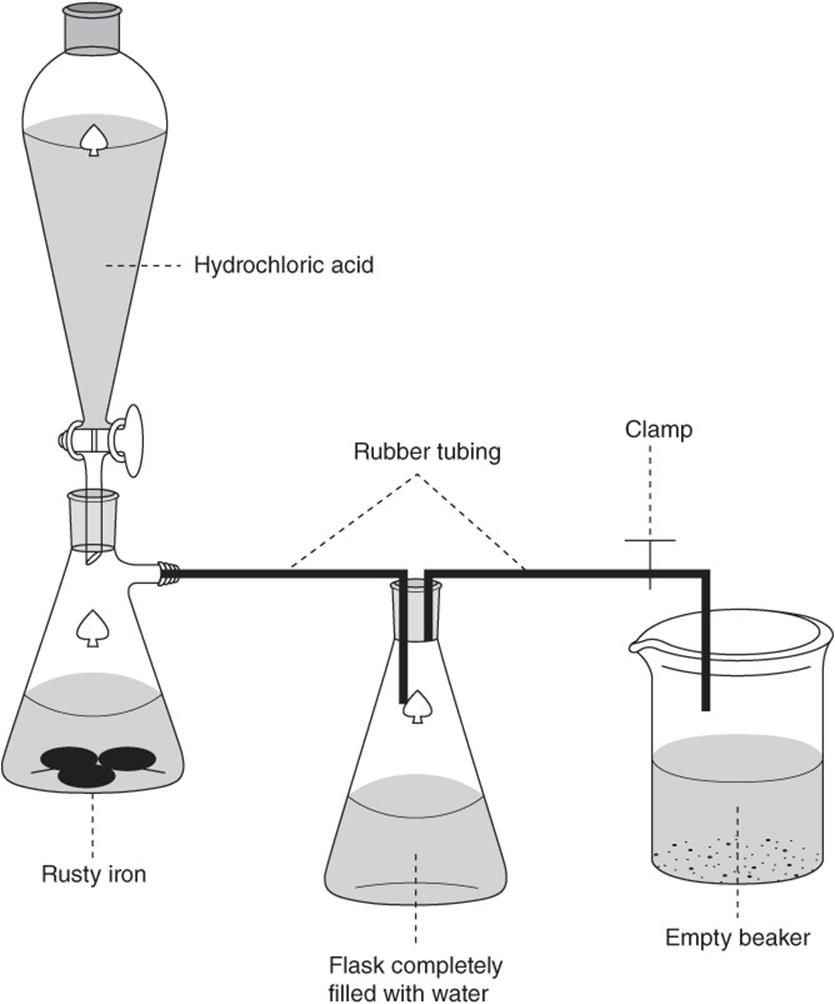
The flask and the rubber tubing leading to the beaker were completely filled with water and then the clamp was removed. Some hydrochloric acid was added to the flask containing the rusty iron. The water level in the second flask dropped as the generated hydrogen gas displaced the water into the beaker. More hydrochloric acid was added until the iron completely dissolved and generation of hydrogen gas ceased. After the system returned to room temperature, the beaker was raised until the water in the beaker was at the same level as in the second flask. When the liquid levels were the same, the clamp was replaced to prevent further transfer. The student completed the following data table in her lab book.

55. What type of reaction generated the hydrogen gas?
(A) single displacement
(B) combination
(C) decomposition
(D) combustion
56. What is the partial pressure of the hydrogen gas in the flask?
(A) 770.9 torr
(B) 752.3 torr
(C) 760.0 torr
(D) 733.7 torr
57. Why was it important to adjust the liquid level in the beaker and the flask to be the same?
(A) to remove excess water from the rubber tubing and into the beaker
(B) to equilibrate the pressure in the flask with the external pressure
(C) to make sure all of the hydrogen gas was out of the rubber tubing
(D) to make sure there was no contamination by the hydrochloric acid
58. Approximately how many moles of hydrogen gas formed?
(A) 0.1 mol
(B) 0.02 mol
(C) 0.005 mol
(D) 0.01 mol
59. If the sample were pure iron, approximately how many moles of hydrogen gas would form?
(A) 0.04 mol
(B) 0.002 mol
(C) 0.02 mol
(D) 0.2 mol
60. In another experiment, a 2.520 g sample of rusty iron generated 0.0205 mol of hydrogen gas. What was the approximate percentage of pure iron in the sample?
(A) 45%
(B) 100%
(C) 25%
(D) 75%
STOP. End of AP Chemistry Practice Exam 1, Section I (Multiple Choice).
![]() Answers and Explanations for Exam 1, Section I (Multiple Choice)
Answers and Explanations for Exam 1, Section I (Multiple Choice)
1. B—All can behave as Brønsted bases (accept a hydrogen ion). Only B cannot behave as an acid (donate a hydrogen ion).
2. A—The buret should be rinsed with titrant, not water.
3. D—The others are normally monatomic anions.
4. C—Increasing sizes indicate decreasing charge, lower position in a column on the periodic table, or position to the left in a period on the periodic table. Note that this type of explanation is unacceptable on the free-response portion of the AP exam.
5. ![]()
6. C—The starting materials are Ba(OH)2 and K2SO4, which must be soluble since they are in solution. The soluble compounds (potassium compounds) and strong bases [KOH and Ba(OH)2] should be separated and the spectator ions eliminated.
7. B—Magnesium nitride does form.
8. D—Based upon solubility, the precipitate cannot contain either sodium or nitrate ions. This only leaves the nickel and hydroxide ions to form the precipitate. Equal moles are mixed, and NaOH is the limiting reagent. Some of the nickel remains, the sodium does not change, and two nitrates are formed per nickel(II) nitrate.
9. 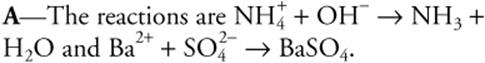
In general, ammonium salts are soluble, so the precipitate could only contain the other cation with an anion other than the hydroxide ion. The problem starts with a Ba(OH)2 solution; therefore, this compound is soluble.
10. B—Percentages: (A) 77.8; (B) 69.6; (C) 72.0; (D) 63.2
Approximate the atomic weights to simplify the calculations.
11. D—Percentage of water: (A) 11; (B) 20; (C) 43; (D) 56
Approximate the calculations using 140 for the molar mass of Na2SO4 and 20 for the molar mass of water.
12. D—Nitric acid is the limiting reagent, and there is 0.30 mol of this compound. The mole ratio from the balanced equation determines the moles of NO.
13. A—(2.21 g)(1 mol/442 g)(3 mol O2/2 mol) = 7.50 × 10–3 mol. Simplify to 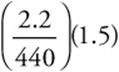 .
.
14. C—Sulfuric acid is the limiting reagent. The moles of limiting reagent times the mole ratio from the balanced equation gives the number of moles formed.
15. C—(0.100 mol Sr)(1 mol H2/1 mol Sr) × (22.4 L/mol) = 2.24 L H2 (Be careful with the 22.4 as it only applies at STP. This is an easy place to make an error from this assumption.)
16. C—The average velocity is related to temperature. Unless the quantity of nitrogen or the volume changes, none of the other values can possibly change.
17. D—The moles of the unknown gas will depend upon its partial pressure, not the total pressure. Water, whenever present, will contribute its vapor pressure. It is necessary to use Dalton’s law to determine the partial pressure of the unknown gas.
18. ![]()
Do the calculation with approximate numbers.
19. A—This describes the free energy.
20. B—This describes the lattice energy.
21. D—This describes the activation energy.
22. 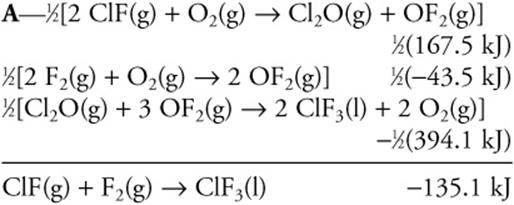
23. A—Exothermic processes, such as this one, shift toward the starting materials when heated.
24. A—Subtract the heat of vaporization from the original value.
25. D—Ni2+ has 26 electrons. The first electrons to leave in the formation of a transition metal cation are the 4s electrons.
26. A—The Pauli exclusion principle limits the number of electrons by requiring that no two electrons in an atom may have the same set of four quantum numbers. Three of the quantum numbers define an orbital and only one (ms) remains, which may only have two different values.
27. B—Hund’s rule says that electrons fill the orbitals individually before pairing. Unpaired ↑ electrons = paramagnetic.
28. B—X is F and forms a –1 ion by gaining one electron. Magnesium ions, Mg2+, form.
29. D—BF3 has three electron pairs around the B.
30. B—Using VSEPR, all the others are nonpolar.
31. D—Ionic bonding requires a metal and a non-metal (usually) or polyatomic ions, which eliminates answer A. There must be internal bonding for σ or π bonds to be present, which eliminates B. Hydrogen cannot form π bonds. Only the acetate ion has resonating bonds (σ and π).
32. D—Draw the Lewis structures. All, except D, have no unshared electron pairs.
33. C—Sublimation usually does not involve bond breaking. In any case, Zn is a metal, and it has no ionic or covalent bonds to break.
34. D—Both diamond and graphite are covalent network solids.
35. A—This is a description of metallic bonding, which is present in all metals such as calcium.
36. C—This is a description of ionic bonding, which is present in ionic compounds such as calcium carbonate.
37. ![]()
38. 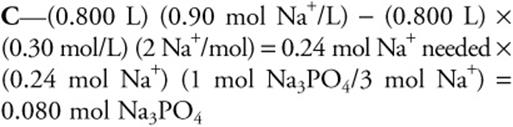
39. ![]()
40. B—This plot gives a straight line only for a first-order reaction.
41. C—t1/2 = (0.693/86 h–1)(3600 s/h) = 29 s. To save time on the exam, you can approximate this equation as t1/2 = (0.7/90)(3600). Dividing 3,600 by 90 gives 40, and 40 times 0.7 = 28. The time is equivalent to two half-lives, so one-fourth of the sample should remain.
42. C—The pKa for ![]() is nearest to the pH value needed. Thus, the simplest buffer would involve this ion.
is nearest to the pH value needed. Thus, the simplest buffer would involve this ion.
43. 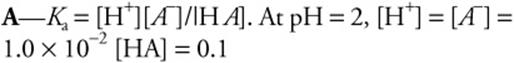
44. A—LiOH is a strong base. The others are acidic or buffer solutions.
45. B—Only B and D are buffers. B is basic, and D is acidic.
46. D—Only B and D are buffers. B is basic, and D is acidic.
47. B—For aqueous solutions or solids, the effect of a pressure change is minimal. If there are equal numbers of moles of gas on each side of the equilibrium arrow, then volume or pressure changes will not affect the equilibrium.
48. ![]() and H3O+ behave as acids, leaving the other species to be bases.
and H3O+ behave as acids, leaving the other species to be bases.
49. D—The loss of 0.20 mol of CO means that 0.40 mol of H2 reacted (leaving 0.30 mol) and 0.20 mol of CH3OH formed. Dividing all the moles by the volume gives the molarity, and Kc = (0.20)/(0.40)(0.30)2 = 5.6
50. A—Assigning oxidation numbers and definitions is required. Bismuth undergoes reduction, and the only other element to change oxidation state is tin.
51. C—Hydrogen (odorless) evolves at the cathode (negative), and chlorine (distinctive odor) evolves at the anode (positive).
52. D—The mass should be 226 – (4 + 4 + 0 + 4) = 214. The atomic number should be 88 – (2 + 2 – 1 + 2) = 83.
53. C—Alpha particles are the least penetrating, and gamma rays are the most penetrating.
54. C—After one half-life, 50% would remain. After another half-life, this would be reduced by one-half to 25%. The total amount decayed is 75%. Thus, 24.6 years must be two half-lives of 12.3 years each.
55. A—The reaction is a single displacement reaction as the iron metal displaces hydrogen gas from the hydrochloric acid.
56. D—The pressure inside the flask is the sum of the partial pressures. Therefore, the pressure of hydrogen gas is the total pressure (752.3 torr) minus the vapor pressure of water (18.6 torr). (The leveling of the water in the beaker and flask adjusted the pressure in the flask to the external [barometric] pressure.)
57. B—If the two liquid levels are the same, the pressures must be the same.
58. D—The ideal gas equation (PV = nRT) gives the moles of hydrogen gas formed.
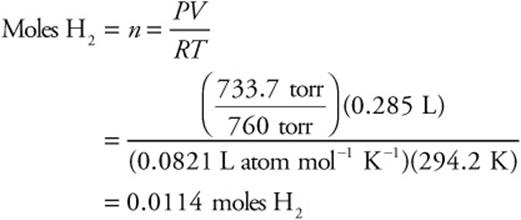
It is easier to calculate the answer by simple rounding as:
59. C—The moles of hydrogen gas formed equal the moles of iron reacting (see the balanced chemical equation). If the sample were pure iron, the mass of iron would be (176.604 – 175.245) g = 1.359 g sample (= Fe). The moles of iron are the mass of iron divided by its molar mass (55.84 g mol–1).
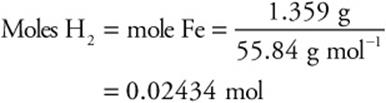
60. A—According to the equation for the formation of hydrogen gas from iron, the moles of iron equals the moles of hydrogen. Therefore, 0.0205 mol of iron reacted. The mass of reacting iron is (0.0205 mol Fe) (55.84 g mol–1) = 1.14 g Fe. The percentage of pure iron in the sample is the mass of the iron divided by the mass of the sample then multiplied by 100%.
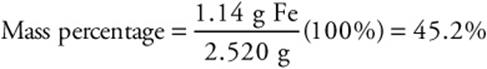
AP Chemistry Practice Exam 1, Section II (Free Response)
Time—1 hour and 30 minutes
Answer the following questions in the time allowed. You may use a calculator and the resources at the back of the book. Write the answers on separate sheets of paper.
Question 1
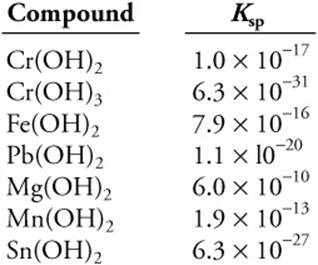
Use the Ksp data given above to answer the following questions.
(a) Excess manganese(II) hydroxide, Mn(OH)2, is added to 100.0 mL of deionized water. What is the pH of the solution?
(b) A solution that is 0.10 M in Mg2+ and 0.10 M in Fe2+ is slowly made basic. What is the concentration of Fe2+ when Mg2+ begins to precipitate?
(c) Two beakers are filled with water. Excess chromium(III) hydroxide is added to one and excess tin(II) hydroxide is added to the other. Which beaker has the higher concentration of metal ions? Calculate the concentration of metal ion in each beaker to support your prediction.
(d) Chromium(III) hydroxide, Cr(OH)3, is less soluble than chromium(II) hydroxide, Cr(OH)2. Explain.
(e) Calculate the grams of lead(II) hydroxide, Pb(OH)2, that will dissolve in 1.00 L of water.
Question 2
![]()
Thermodynamic values related to the above reaction are given in the table below.
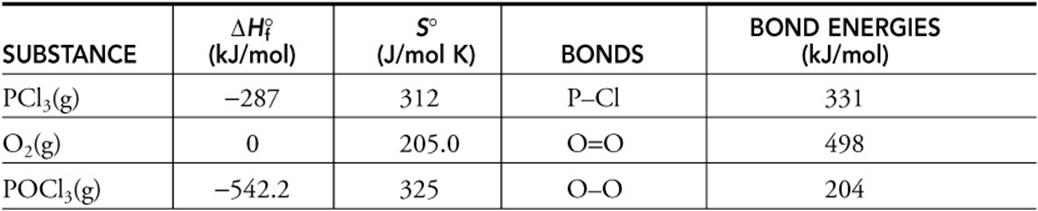
(a) Determine the enthalpy change for the above reaction.
(b) Estimate the PO bond energy.
(c) Is the PO bond a single or a double bond? Justify your answer.
(d) Calculate the entropy change for the reaction.
(e) Is this reaction spontaneous or nonspontaneous at 25°C? Justify your prediction.
Question 3
A sample of a solid, weak monoprotic acid, HA, is available along with a standard sodium hydroxide solution. The sodium hydroxide solution was standardized with potassium hydrogen phthalate (KHP).
(a) List the apparatus required to titrate an HA solution.
(b) Sketch a pH versus volume-of-base-added curve for the titration.
(c) Sketch the titration curve if the unknown acid was really a diprotic acid.
(d) Describe the steps required to determine the molar mass of HA.
(e) How would the molar mass of HA change if the KHP contained an inert impurity?
Question 4
The following equipment is available for the determination of the molar mass of a volatile solid.

(a) Which of the above equipment is necessary for this experiment?
(b) In addition to information that you can determine by using the above equipment, what information is necessary to determine the molar mass of the unknown solid?
(c) Describe how the above equipment may be used to calculate the molar mass of the unknown solid.
Question 5
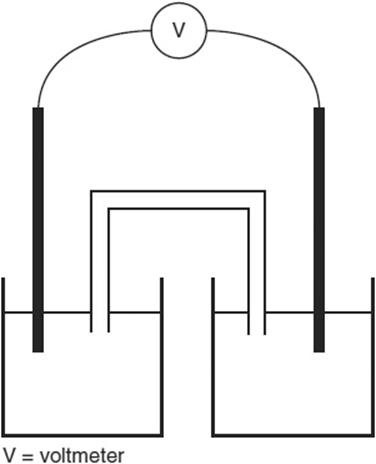
The above galvanic cell is constructed with a cadmium electrode in a 1.0 M Cd(NO3)2 solution in the left compartment and a silver electrode in a 1.0 M AgNO3 solution in the right compartment. The salt bridge contains a KNO3solution. The cell voltage is positive.

(a) What is the balanced net ionic equation for the reaction, and what is the cell potential?
(b) Write the expression for the reaction quotient, Q, of the cell. Explain why any substances from the net ionic equation do not appear in Q.
Question 6
Relate each of the following to atomic properties and the principles of bonding.
(a) Draw the Lewis electron-dot structures for CO2 and CO. Explain the polarity of these compounds.
(b) The compound C2H3F is polar, but compounds with the general formula C2H2F2 are sometimes polar and sometimes nonpolar. Show the structures and explain.
(c) There are two isomers with the formula C2H6O. One of the isomers is more soluble in water than the other isomer. Use the structures of these two compounds to explain the difference in solubility.
Question 7
A flask, filled with water, is inverted into a water bath. Hydrogen gas was introduced to the flask through a tube until it displaced all of the water in the flask. The volume of the sample was 490.0 mL at 26°C, and the pressure in the room was 754 mm Hg. The vapor pressure of water at 26°C is 25.2 mm Hg.
(a) Calculate the number of grams of hydrogen in the flask.
(b) Calculate how many grams of water vapor are present in the flask.
(c) Determine the density (in g L–1) of the gas mixture in the flask.
STOP. End of AP Chemistry Practice Exam 1.
![]() Answers and Explanations for Exam 1, Section II (Free Response)
Answers and Explanations for Exam 1, Section II (Free Response)
Question 1
(a) The volume of the solution is irrelevant. The equilibrium
![]()
The mass-action expression for this equilibrium is:
![]()
Setting [Mn2+] = x and [OH–] = 2x, and inserting into the mass-action expression gives:
![]()
Solving for x gives x = 3.6 × 10–5, and [OH–] = 2x = 7.2 × 10–5.
You get 1 point for the correct [OH–].
There are two common ways to finish the problem. You do not need to show both.
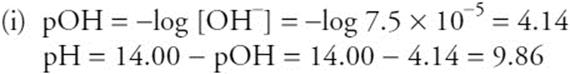
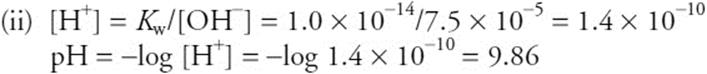
You get 1 point for the correct pH. If you got the wrong [OH–] value, but use it correctly, you still get 1 point.
(b) The important equilibria are: ![]() , where M = Mg or Fe. It is necessary to determine the hydroxide ion concentration when the magnesium begins to precipitate.
, where M = Mg or Fe. It is necessary to determine the hydroxide ion concentration when the magnesium begins to precipitate.
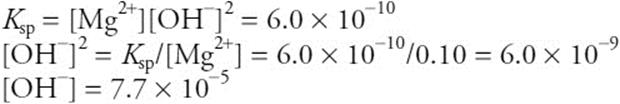
Using this value with the iron equilibrium gives:
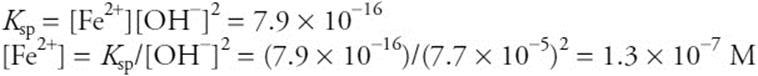
You get 1 point for the correct [OH–] and 1 point for the correct [Fe2+]. Alternately, you get 1 point if you did only part of the procedure correctly. There is a maximum of 2 points for this part.
(c) Using the appropriate mass-action expressions:
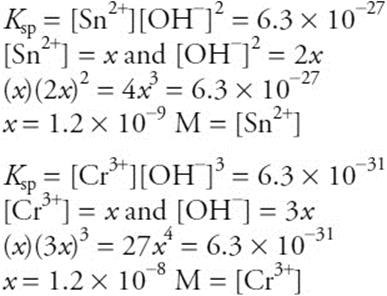
The tin(II) hydroxide beaker has the lower metal ion concentration.
You get 1 point for the correct beaker. You also get 1 point for each metal ion concentration you got correct. Your answers do not need to match exactly, but they should round to the same value.
(d) The higher the charge on the cation, the less soluble a substance is, because there is a greater attraction between the ions (higher lattice energy).
You get 1 point for this answer.
(e) The mass-action expression is:
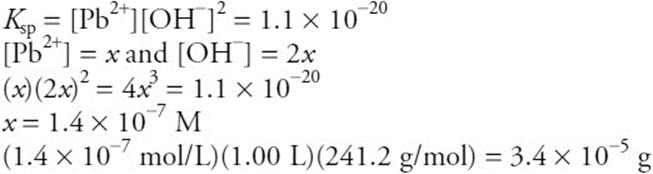
You get 1 point for the correct answer (or an answer that rounds to this answer). You get 1 point for the setup.
Total your points for the different parts. There is a maximum of 10 points possible. Subtract one point if all answers did not have the correct number of significant figures.
Question 2
![]()
The setup (products – reactants) is worth 1 point, and the answer is worth 1 point. You do not need to get the exact answer, but your answer should round to this one.
(b) The answer from part a equals the bonds broken minus the bonds formed. Both phosphorus molecules have three P–Cl bonds, and O2 has an O![]() O bond.
O bond.
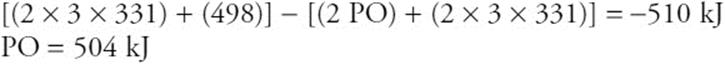
The setup (broken – formed) is worth 1 point, and the answer is worth 1 point. You do not need to get the exact answer, but your answer should round to this one. If your answer from a was wrong, but you use it correctly in this part, you still get your answer point.
(c) It is a double bond. The value from part b is much higher than the single-bond values from the table.
You get 1 point for the correct prediction, and 1 point for the explanation. If you got the wrong answer for part b, you can still get 1 or 2 points if you used the answer correctly on this part.
![]()
The setup (products – reactants) is worth 1 point, and the answer is worth 1 point. You do not need to get the exact answer, but your answer should round to this one.
(e) It is necessary to calculate the free-energy change.
![]()
The negative value means the reaction is spontaneous.
You get 1 point for the prediction that the reaction is spontaneous. The setup (plugging into the equation) is worth 1 point if you remember to change the temperature to kelvin and the joule to kilojoule conversion. An additional 1 point comes from the answer. If you got the wrong value in either part a or b, but used it correctly, you will still get the point for the answer. The free-energy equation is part of the material supplied in the exam booklet.
Total your points for the different parts. There is a maximum of 11 points possible. Subtract 1 point if all your answers do not have the correct number of significant figures.
Question 3

You get 1 point if you have ALL the starred items. You get 1 point for the other items. There is a maximum of 2 points. If you have only some of the starred items, your maximum is 1 point.
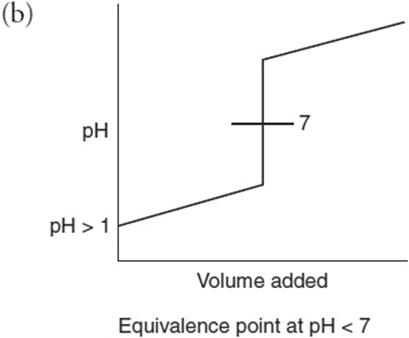
You get 1 point for this graph. You get 1 point for noting that the equivalence point is greater than 7.
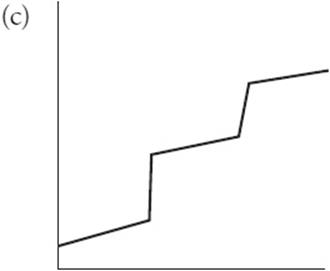
You get 1 point for this graph. You must show two regions.
(d) Weigh a sample of HA.
Titrate HA versus standard NaOH to find the volume of NaOH solution required to neutralize the acid.
Multiply the concentration of the NaOH solution times the volume used to get the moles of NaOH.
The moles of HA is the same as the moles of NaOH. Divide the mass of HA by the moles of HA.
You get 2 points if you list all five steps. If you miss one or more steps, you get only 1 point. You get 0 points if you get none of the steps correct. There are no bonus points for more steps or more details.
(e) If the KHP contained an inert impurity, the concentration of the NaOH solution would be too low. If the concentration of the NaOH solution were too low, then more solution would be needed for the titration of HA. This would yield a lower number of moles of HA, giving a higher molar mass.
You get 1 point for the NaOH concentration being low. You get 1 point for predicting a higher molar mass. If you incorrectly predicted the NaOH concentration to be too high, you can get 1 point if you predicted a lower molar mass.
Total your points. There is a maximum of 9 possible points.
Question 4

You must have analytical balance, thermometer, glass tubing, hot plate, and flask(s) to get 1 point. Having the remaining items will get you a second point.
(b) You need the atmospheric pressure (from a barometer).
You get 1 point for this answer.
(c) 1. Determine the mass of the flask with a stopper and a small piece of glass tubing.
2. Add a small quantity of the unknown solid.
3. Place the stoppered flask (vapor may exit through the glass tube) in a hot water bath consisting of a large beaker partially filled with water and sitting on a hot plate.
4. Continue heating until the solid has completely vaporized. Use the thermometer to measure the temperature (T) of the water, and remove the flask from the hot water bath to cool. Dry the flask.
5. Weigh the flask after it cools to room temperature.
6. Fill the flask with water and use the graduated cylinder to determine the volume (V) of the flask. (As an alternative, it is possible to determine the volume using a pipette.)
7. Record the atmospheric pressure (P).
8. Use the ideal gas equation to determine the moles of solid (n = PV/RT). Use the gas constant and the values of T, V, and P previously determined.
9. Determine the mass (m) of the solid by subtracting the mass of the empty flask from that of the cooled flask.
10. Divide the mass of the sample (m) by the moles (n) determined from the ideal gas equation.
There are 10 steps; you must have each of the steps to get one of the variables (T, V, P, n, and m) to get 1 point. The other steps will get you 1 more point.
Total your points. There are 5 possible points.
Question 5
(a) Use the two half-reactions provided.

It is necessary to reverse the first reaction, the silver half-reaction needs to be doubled, and the reactions and voltages added:

You get 1 point for the correct equation. You also get 1 point for the correct cell voltage.
![]()
This answer is worth 1 point.
The remaining substances (Cd and Ag) are solids. Solids do not appear in Q expressions.
You get 1 point for the explanation.
Total your points; there are 4 points possible. Subtract 1 point if all answers do not have the correct number of significant figures.
Question 6
![]()
CO2 is linear and nonpolar. The different electronegativities of C and O make CO polar.
You get 1 point for each correct Lewis structure and 1 point if you explain both polarities correctly. There is a maximum of 3 points.
(b) There is one compound with the formula C2H3F, and there are three compounds with the formula C2H2F2. The structures are:
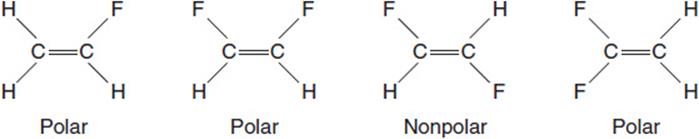
The fluorine atoms are the most electronegative atoms present, and the bonds to them are polar covalent. The only nonpolar compound is the result of the polar C´F bonds pulling equally in opposite directions.
You get 1 point for ALL the structures, and 1 point for a correct explanation. There is a maximum of 2 points.
(c) The structures are CH3—O—H3 and CH3CH2OH.
The first compound (dimethyl ether) is polar, but not as soluble in water as the second compound (ethanol), which is capable of hydrogen bonding to water.
You get 1 point if you show both structures, and you get 1 point for a correct explanation. The names shown in parentheses are not required.
Total your points for the problem. There is a maximum of 7 possible points.
Question 7
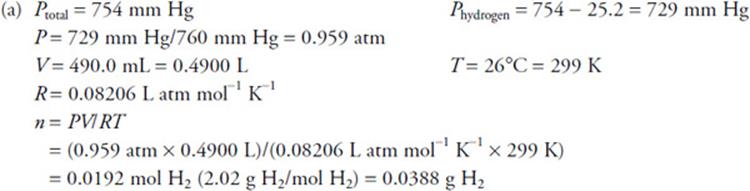
Give yourself 1 point for the correct answer (no deduction for rounding differently). You must include ALL parts of the calculation (including “=”).
Give yourself 1 point for the correct equation, or for any other correct calculation. Do not give yourself more than 2 points total for this part.

Give yourself 1 point for the correct answer (no deduction for rounding differently). You must include ALL parts of the calculation (including “=”). Give yourself 1 point for the correct equation, or for any other correct calculation. Do not give yourself more than 2 points total for this part.
(c) Total mass = 0.0388 g H2 + 0.0119 g H2O = 0.0507 g
Density = 0.0507 g/0.4900 L = 0.103 g/L
Give yourself 1 point for the correct answer (no deduction for rounding differently). You must include ALL parts of the calculation (including “=”). Give yourself 1 point for the correct equation, or for any other correct calculation. Do not give yourself more than 2 points total for this part.
Total score = sum of parts a-c. If any of the final answers has the incorrect number of significant figures, subtract 1 point.
Scoring Sheet for Chemistry Practice Exam 1
Multiple-Choice Section
![]()
Free-Response Section
Approximate Conversion Scale:
