5 Steps to a 5 AP Chemistry (2015)
STEP 5. Build Your Test-Taking Confidence
AP Chemistry Practice Exam 2
AP Chemistry Practice Exam 2—Multiple Choice
ANSWER SHEET
The AP exam is a timed exam; keep this in mind as you prepare. When taking the various tests presented in this book, you should follow the AP exam rules as closely as possible. Anyone can improve his or her score by using notes, books, or an unlimited time. You will have none of these on the AP exam, so resist the temptation to use them on practice exams. Carefully time yourself, do not use other materials, and use a calculator only when expressly allowed to do so. After you have finished an exam, you may use other sources to go over questions you missed or skipped. We have seen many students get into trouble because the first time they attempted a test under “test conditions” was on the test itself.
AP Chemistry Practice Exam 2, Section I (Multiple Choice)
Time—1 hour and 30 minutes
NO CALCULATOR MAY BE USED WITH SECTION I
Answer the following questions in the time allowed. You may use the periodic table in the back of the book.
1. Chlorine forms a number of oxyacids. Which of the following is the correct order of increasing acid strength?
(A) HClO3 < HClO4 < HClO < HClO2
(B) HClO4 < HClO3 < HClO2 < HClO
(C) HClO < HClO2 < HClO3 < HClO4
(D) HClO4 < HClO3 = HClO2 < HClO
2. Oxidation of which of the following substances will yield a stronger acid?
(A) H2CO3
(B) HNO3
(C) HIO
(D) HIO4
3. A student prepared five vinegar samples by pipetting 10.00 mL samples of vinegar into five separate beakers. Each of the samples was diluted with deionized water, and phenolphthalein was added as an indicator. The samples were then titrated with standard sodium hydroxide until the appearance of a permanent pink color indicated the end point of the titration. The following volumes were obtained.
Volumes of standard NaOH
Sample 1: 43.28 mL
Sample 2: 43.27 mL
Sample 3: 50.00 mL no color change
Sample 4: 43.26 mL
Sample 5: 43.24 mL
Which of the following is the most likely cause for the variation in the results?
(A) Too much deionized water was added to the third sample.
(B) The student forgot to add phenolphthalein to the third sample.
(C) More acetic acid was present in the third vinegar sample.
(D) The student did not properly rinse the buret with sodium hydroxide solution.
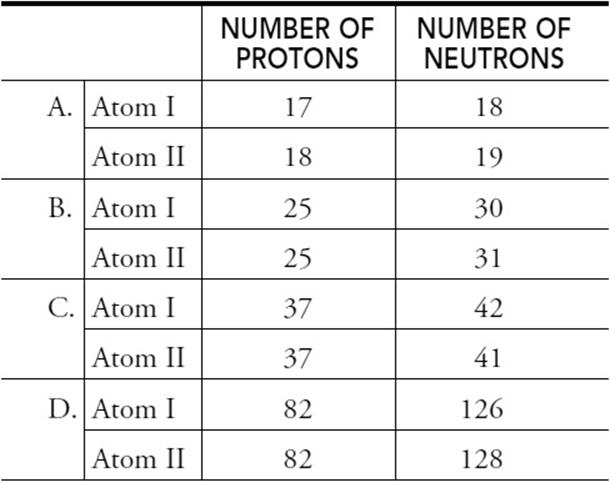
4. Choose the group that does not contain isotopes of the same element.
5. Choose the ion with the largest ionic radius.
(A) S2–
(B) H+
(C) Li+
(D) O2–
6. You are given an aqueous solution of BaCl2. The simplest method for the separation of BaCl2 from the solution is:
(A) filtration of the solution
(B) adding water to the solution
(C) evaporation of the solution to dryness
(D) electrolysis of the solution
7. Which of the following is the correct net ionic equation for the addition of aqueous potassium sulfate to a solution of barium chloride?
![]()
![]()
![]()
![]()
8. The addition of concentrated Ba(NO3)2(aq) to a 1.0 M (NH4)2SO4 solution will result in which of the following observations?
(A) Nothing happens because the two solutions are immiscible.
(B) The formation of a white precipitate takes place.
(C) An odorless gas forms and bubbles out of the mixture.
(D) The solution becomes basic.
9. How many milliliters of 0.300 M H2SO4 are required to neutralize 50.0 mL of 0.600 M KOH?
(A) 20.0 mL
(B) 50.0 mL
(C) 30.0 mL
(D) 60.0 mL
10. Heating will decompose silver oxide, Ag2O, to silver metal, Ag, plus oxygen gas, O2. How many moles of oxygen gas will form when 4.64 g of solid silver oxide is decomposed? The formula mass of silver oxide is 232 g/mol.
(A) 0.100 mol
(B) 0.0100 mol
(C) 0.0200 mol
(D) 0.0150 mol
11. 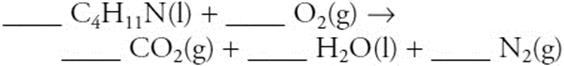
When the above equation is balanced, the lowest whole-number coefficient for N2 is:
(A) 2
(B) 22
(C) 16
(D) 27
12. 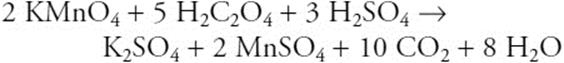
How many moles of MnSO4 are produced when 2.0 mol of KMnO4, 10 mol of H2C2O4, and 6.0 mol of H2SO4 are mixed?
(A) 1.0 mol
(B) 2.0 mol
(C) 3.5 mol
(D) 2.5 mol
13. ![]()
Radium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.0100 mol of radium?
(A) 0.560 L
(B) 0.224 L
(C) 0.448 L
(D) 0.112 L
14. Three containers are used for three different gases. The volumes of the containers are not fixed. The containers are at the same temperature and pressure. One container has 2.0 g of hydrogen, another has 32.0 g of oxygen, and the third has 44.0 g of carbon dioxide. Pick the FALSE statement from the following list:
(A) The densities increase in the order hydrogen < oxygen < carbon dioxide.
(B) The number of molecules in all the containers is the same.
(C) The average speed of all the molecules is the same.
(D) The volume of all three containers is the same.
15. If a sample of SO2 effuses at a rate of 0.0035 mol per hour at 20°C, which of the gases below will effuse at approximately double the rate under the same conditions?
(A) H2
(B) O2
(C) CO
(D) CH4
16. The average _____________ is the same for any ideal gas at a given temperature.
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) ionization energy
17. What describes the maximum energy available for useful work from a spontaneous reaction?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) ionization energy
18. What is the energy necessary to separate cations from anions in an ionic solid?
(A) free energy
(B) lattice energy
(C) kinetic energy
(D) ionization energy
19. When cerium(III) acetate, Ce(C2H3O2)3, dissolves in water, the temperature increases. Which of the following conclusions may be related to this?
(A) The hydration energies of cerium(III) ions and acetate ions are very low.
(B) Cerium(III) acetate is less soluble in hot water.
(C) The solution is not an ideal solution.
(D) The heat of solution for cerium(III) acetate is endothermic.
20. Choose the reaction expected to have the greatest increase in entropy.
![]()
![]()
![]()
![]()
21. A certain reaction is nonspontaneous under standard conditions, but becomes spontaneous at lower temperatures. What conclusions may be drawn under standard conditions?
(A) ΔH < 0, ΔS < 0, and ΔG = 0
(B) ΔH > 0, ΔS < 0, and ΔG > 0
(C) ΔH < 0, ΔS < 0, and ΔG > 0
(D) ΔH > 0, ΔS > 0, and ΔG > 0
22. ![]()
Determine ΔH for the above reaction if N2O5(s) were formed in the above reaction instead of N2O5(g). The ΔH of sublimation for N2O5 is 54 kJ/mol.
(A) +54 kJ
(B) +219 kJ
(C) +165 kJ
(D) –219 kJ
23. Which of the following groups contains only atoms that are diamagnetic in their ground state?
(A) He, Co, and Sr
(B) Zn, Mg, and Xe
(C) O, Be, and Ne
(D) Ca, Mn, and Ar
24. A halogen has this electron configuration.
(A) 1s21p62s22p3
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
25. A transition metal atom might have this configuration.
(A) 1s22s22p63s23p64s2
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p64s23d104p2
(D) 1s22s22p5
26. A transition metal ion could have this configuration.
(A) 1s21p62s22p3
(B) 1s22s22p63s23p64s23d104p65s24d1
(C) 1s22s22p63s23p63d3
(D) 1s22s22p5
27. What explains why the exact position of an electron is not known?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
28. What explains the observation that an atomic orbital can hold no more than two electrons?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
29. The 4s orbital fills before the 3d orbitals. Why?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
30. Which of the following does NOT have one or more π bonds?
(A) HNO2
(B) N2
(C) N2H4
(D) HNO3
31. The unusually high melting point of hydrogen fluoride is due to which of the following?
(A) ionic bonds
(B) hybrid orbitals
(C) resonance structures
(D) hydrogen bonding
32. Which of the following explains why the bonds in BF3 are all the same?
(A) ionic bonds
(B) hybrid orbitals
(C) resonance structures
(D) hydrogen bonding
33. Which of the following has more than one unshared pair of valence electrons on the central atom?
![]()
(B) NO2
(C) NH3
![]()
34. Which types of hybridization of carbon are in the compound propane, CH3CH2CH3?
(A) sp3 only
(B) sp2 and sp
(C) sp3, sp2, and sp
(D) sp2 only
35. The approximate boiling points for hydrogen compounds of some of the elements in the nitrogen family are: (SbH3 15°C), (AsH3 –62°C), (PH3 –87°C), and (NH3, –33°C). The best explanation for the fact that NH3 does not follow the trend of the other hydrogen compounds is:
(A) NH3 is the only one that is nearly ideal in the gas phase.
(B) NH3 is the only one that is a base.
(C) NH3 is the only one that is water-soluble.
(D) NH3 is the only one to exhibit hydrogen bonding.
36. Which of the following explains why copper is ductile?
(A) London dispersion forces
(B) covalent bonding
(C) hydrogen bonding
(D) metallic bonding
37. Which of the following best explains why 1-butanol, CH3CH2CH2CH2OH, has a higher boiling point (117°C) than its isomer, methyl propyl ether, CH3OCH2CH2CH3 (39°C)?
(A) the lack of hydrogen bonding in 1-butanol
(B) the presence of hydrogen bonding in 1-butanol
(C) the higher molecular mass of 1-butanol
(D) the lower specific heat of 1-butanol
38. All the following substances will dissolve in water. Pick the nonelectrolyte.
(A) Ca(NO3)2
(B) KOH
(C) C2H5OH
(D) HCl
39. To prepare 4.0 L of a 0.50 molar KClO3 solution (molecular mass 122.6 g/mol), a student should follow which of the following procedures?
(A) The student should weigh 245.2 g of solute and add 4.0 L of water.
(B) The student should weigh 61.3 g of solute and add sufficient water to obtain a final volume of 4.0 L.
(C) The student should weigh 61.3 g of solute and add 4.0 Kg of water.
(D) The student should weigh 245.2 g of solute and add sufficient water to obtain a final volume of 4.0 L.
40. For the following reaction, H2(g) + I2(g) → 2 HI(g), the rate law is: Rate = k[H2][I2]. If a small amount of iodine vapor (I2) is added to a reaction mixture that was 0.10 M in H2 and 0.20 M in I2, which of the following statements is true at constant temperature?
(A) Both k and the reaction rate decrease.
(B) Both k and the reaction rate increase.
(C) Only the rate increases; k remains the same.
(D) Only k increases; the reaction rate remains the same.
41. ![]()
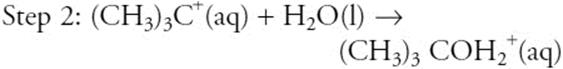
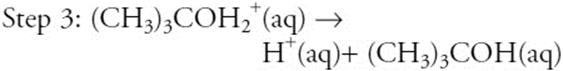
The above represents a proposed mechanism for the hydrolysis of (CH3)3CBr. What are the overall products of the reaction?
![]()
![]()
![]()
![]()
42. The following table gives the initial concentrations and rate for three experiments.
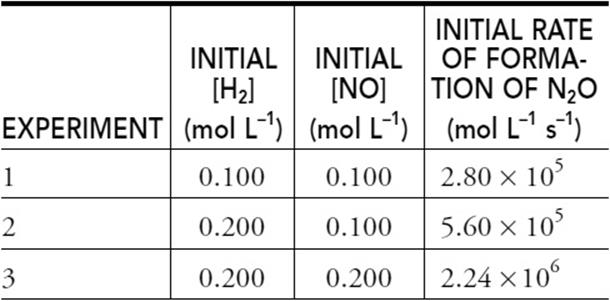
The reaction is H2(g) + 2 NO(g) → N2O(g) + H2O(g). What is the rate law for this reaction?
(A) Rate = k[NO]
(B) Rate = k[NO]2[H2]2
(C) Rate = (k [H2]
(D) Rate = (k [NO]2[H2]
43. A solution of a weak base is titrated with a standard solution of a strong acid. The progress of the titration is followed with a pH meter. Which of the following observations would occur?
(A) At the equivalence point, the pH is below 7.
(B) The pH of the solution gradually decreases throughout the experiment.
(C) At the equivalence point, the pH is 7.
(D) The pOH at the equivalence point equals the pKb of the base.
44. When potassium carbonate dissolves in water:
(A) the solution is neutral
(B) the solution is basic because of hydrolysis of the ![]() ion
ion
(C) the solution is basic because of hydrolysis of the K+ ion
(D) the solution is acidic because of hydrolysis of the K+ ion
45. Determine the OH–(aq) concentration in 0.10 M pyridine (C5H5N) solution. (The Kb for pyridine is 9 × 10–9.)
![]()
![]()
![]()
![]()
46. ![]() exothermic
exothermic
An equilibrium mixture of the reactants is placed in a sealed container at 150°C. The amount of the product may be increased by which of the following changes?
(A) decreasing the volume of the container
(B) decreasing the volume of the container and raising the temperature of the container
(C) raising the temperature of the container
(D) adding 1 mol of Ar(g) to the container and decreasing the volume of the container
The follow information applies to questions 47–50.
The following reaction takes place in a voltaic cell:
![]()
The cell has its voltage measured and found to be +1.10 volts.
47. What happens to the voltage when deionized water is added to the zinc compartment?
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases, but stays positive.
48. What happens to the cell voltage when the copper electrode is made larger?
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases, but stays positive.
49. What happens to the cell voltage when the salt bridge is replaced with a zinc wire?
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases, but stays positive.
50. What happens to the cell voltage after the cell has operated for 15 min?
(A) There is no change in the voltage.
(B) The voltage becomes zero.
(C) The voltage increases.
(D) The voltage decreases, but stays positive.
51. How many moles of Cr may be deposited on the cathode when 0.60 F of electricity is passed through a 1.0 M solution of Cr3+?
(A) 0.60 mol
(B) 0.20 mol
(C) 1.0 mol
(D) 0.30 mol
52. 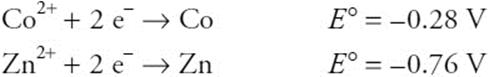
Given the above standard reduction potential, estimate the approximate value of the equilibrium constant for the following reaction:
![]()
(A) 104
(B) 1016
(C) 10–16
(D) 10–8
53. ![]()
For the above reaction, choose the correct statement from the following list.
(A) This reaction occurs at the anode.
(B) The iodine is reduced from +5 to –1.
(C) The iodine is oxidized from –1 to 0.
(D) This reaction occurs at the cathode.
54. The formation of ![]() from
from ![]() occurs by:
occurs by:
(A) electron capture
(B) α decay
(C) β decay
(D) positron decay
55. The transition state is higher in energy than the reactants by an amount called:
(A) the heat of reaction
(B) the reaction energy
(C) the free energy
(D) the activation energy
Use the following information to answer questions 56–60
When heated, most metals will react with oxygen in the air to form oxides. A few metals, when heated in air, will form an oxide contaminated with the metal nitride. It is often possible to remove the contaminating nitride by the addition of water or dilute acid and reheating to give the pure oxide. A student conducts an experiment to determine the formula of a metal oxide by collecting the following data:

The metal is finely powdered to ensure complete reaction. After an initial heating to form an oxide-nitride mixture, the cooled sample was treated with water to react with the nitride and for a gas that turned red litmus paper blue. A final careful reheating removed the unreacted water to leave the pure metal oxide.
56. What type of reaction occurs when heating the metal with air to form an oxide-nitride mixture?
(A) decomposition
(B) neutralization
(C) precipitation
(D) combination
57. What is the approximate percent oxygen in the final metal oxide?
(A) 26%
(B) 74%
(C) 50%
(D) 15%
58. The treatment of the metal nitride released a gas. What was the gas?
(A) N2
(B) NO
(C) NH3
(D) H2O
59. In another experiment on the same metal, a different oxide formed. This oxide was 21% oxygen. What was the formula of the second oxide?
(A) M2O3
(B) MO
(C) MO2
(D) M2O
60. What would be the approximate percent oxygen if the oxide were Fe3O4? (Molar masses: O = 16.00 g mol–1, Fe = 55.85 g mol–1, and Fe3O4 = 231.55 g mol–1)
(A) 28%
(B) 7%
(C) 72%
(D) 57%
STOP. End of AP Chemistry Practice Exam 2, Section I (Multiple Choice).
![]() Answers and Explanations for Exam 2, Section I (Multiple Choice)
Answers and Explanations for Exam 2, Section I (Multiple Choice)
1. C—In general, the more oxygen atoms present, the stronger the oxyacid.
2. C—Only C can be oxidized. In the other cases, the central element is already in its highest oxidation state.
3. B—The phenolphthalein is the source of the color change.
4. A—If the numbers of protons are equal, the atoms are isotopes.
5. A—Anions are usually larger than cations. If the charges are equal, then lower on the periodic table is larger. S is lower on the periodic table than O. This explanation will work for multiple-choice questions; however, free-response questions require more detail.
6. C—Solutions do not readily separate by any means; therefore, it is necessary to remove either the solvent or solute from each other.
7. A—Soluble compounds, in this case, the starting materials and potassium compounds exist as separate ions. Separate the soluble compounds, and remove the spectator ions to get the net ionic equation.
8. B—Aqueous solutions will mix, which eliminates A. The only gas that might form would be ammonia, which is far from odorless (eliminating C). The ammonium sulfate solution is slightly acidic, and mixing will not change that (eliminating D). This only leaves B. Solid BaSO4 forms.
9. B—(50.0 mL)(0.600 mol KOH/1,000 mL) (1 mol H2SO4/2 mol KOH)(1,000 mL/0.300 mol H2SO4)
10. B—(4.64 g oxide)(1 mol oxide/232 g oxide) (1 mol O2/2 mol oxide) = 0.0100 mol
11. 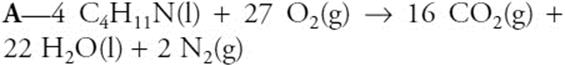
12. B—Based upon the number of moles given and the balanced chemical equation, KMnO4 is the limiting reactant. The limiting reagent and the mole ratio will determine the amount produced.
13. B—(0.0100 mol Ra)(1 mol H2/1 mol Ra) (22.4 L/mol H2) = 0.224 L (The 22.4 L/mol assumption only works at STP.)
14. C—The average kinetic energy, not the average speed, is the same. There is one mole of gas in each container.
15. D—Use Graham’s law. To double the rate, the molar mass must be 1/(2)2, the mass of SO2.
16. C—This is one of the consequences of kinetic molecular theory.
17. A—The description gives a property of free energy.
18. B—The description refers to ionic materials, which involves the lattice energy.
19. B—This is an exothermic process (temperature of the solution increases), and exothermic processes do not proceed as well at higher temperatures.
20. B—The reaction that has the greatest increase in the number of moles of gas has the greatest entropy change.
21. C—To become spontaneous at a lower temperature means entropy impeded the original reaction (entropy was negative). The enthalpy must be negative; otherwise, it would be impossible for a negative entropy reaction to become spontaneous. Nonspontaneous under standard conditions means: ΔG>0. If ΔG = 0, then there is an equilibrium.
22. D—Subtract 54 × 2 mol N2O5 from the observed value (use Hess’s law).
23. B—These have completely filled shells or subshells—all the electrons are paired, which makes the species diamagnetic.
24. D—All halogens are ns2np5.
25. B—A partially filled d orbital is required with an s1 or s2.
26. C—This is V2+. Most transition metal ions have an s0 in the outer shell.
27. D—This is a restatement of the uncertainty principle.
28. A—This is an application of the definition.
29. B—This is a consequence of the better shielding of d electrons over s electrons.
30. C—There are only single bonds (σ) present in the Lewis structure of this substance.
31. D—Hydrogen bonding may occur when hydrogen is attached directly to N, O, or F.
32. B—B has three equivalent sp2 orbitals.
33. A—Draw the Lewis structures. C and D have one unshared pair, and B has a lone electron.
34. A—All three of the carbon atoms have four single bonds.
35. D—Hydrogen bonding is possible when hydrogen is attached to N, O, and F. Hydrogen bonding leads to higher boiling points than predicted from the trend.
36. D—This is a consequence of metallic bonding. The atoms can move past each other without breaking bonds.
37. B—The presence of the —OH group makes hydrogen bonding possible in the 1-butanol.
38. C—A is a soluble ionic compound (contains the nitrate ion); all the others are acids or bases. Acids, bases, and soluble salts are electrolytes. C is a neutral molecular compound.
39. D—(0.50 mol/L) (4.0 L) (122.6 g/mol) = 245.2 g and dilute to volume.
40. C—Placing a larger number into the rate law will give a larger rate. The rate constant, k, is constant (unless the temperature changes or a catalyst is added).
41. D—Add the equations together and cancel any species that appear on both sides.
42. D—The reaction is first-order in H2 and second order in NO. Comparing the rates of experiments 1 and 2 shows that doubling the H2 doubles the rate, which is first-order behavior. Comparing the rates of experiments 2 and 3 shows that doubling the NO quadruples, 22, the rate, which is second-order behavior.
43. A—Strong acids and strong bases have pH = 7 at the equivalence point. The presence of a weak base lowers this value.
44. B—The carbonate ion is the conjugate base of a weak acid and will produce a basic solution through hydrolysis. The potassium ion comes from a strong base and will not undergo hydrolysis or change the pH.
45. ![]()
46. A—Decreasing the volume will force the equilibrium to shift to the side with fewer moles of gas. Ar is not in the equilibrium; therefore, it has no effect. Heating an exothermic equilibrium will shift the reaction to the left (less product forms).
47. C—This will lower the concentration of Zn2+, causing a shift to the right.
48. A—The size of the electrode is irrelevant.
49. B—A source of cations and anions is needed.
50. D—As the cell begins to run, the voltage decreases.
51. B—(0.60 F) (1 mol Cr/3 F)
52. ![]()
53. D—Reduction reactions like this one always occur at the cathode.
54. B—Mass difference = 234 – 230 = 4, and atomic number difference = 92 – 90 = 2. These correspond to an α particle.
55. D—This is the definition of the activation energy.
56. D—This is a combination reaction where the metal combines with either oxygen or nitrogen to form either the metal oxide or metal nitride.
57. A—The percent oxygen is 100% times the grams of oxygen divided by the mass of metal oxide. The mass of oxygen is (mass of crucible plus metal oxide) – (mass of crucible plus metal) = (53.855 – 53.660) g = 0.195 g oxygen. The mass of the metal oxide is (mass of crucible plus metal oxide) – (mass of crucible) = (53.855 – 53.120) g = 0.735 g metal oxide. Finally,
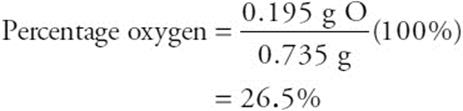
58. C—This is the only one of the gases that is basic (turns red litmus paper blue).
59. B—It is necessary to determine the empirical formula of the compound. If the sample is 21% oxygen, then it is 79% metal. Assuming 100 g of metal, the masses of O and M are 21 g and 79 g, respectively. Converting each of these to moles gives:
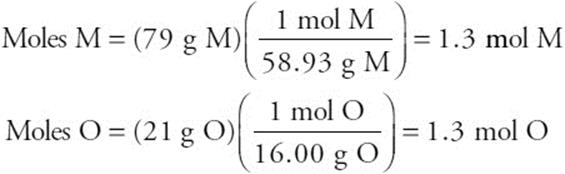
Since the moles are the same, the formula must be MO.
60. A—The general equation to determine the percent oxygen in the sample is:
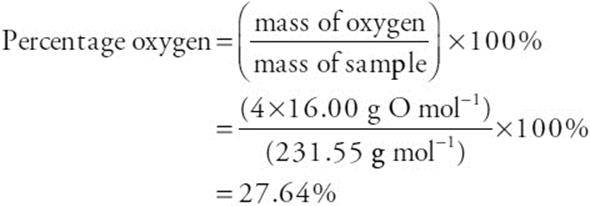
AP Chemistry Practice Exam 2, Section II (Free Response)
Time—1 hour and 30 minutes
Answer the following questions in the time allowed. You may use a calculator and the resources at the back of the book. Write the answers on separate sheets of paper.
Question 1
![]()
The value of Kp for the above equilibrium is 0.262 at 1,270 K.
(a) Write the equilibrium constant expression for the above equation.
(b) What is the value of Kp for the following equilibrium at 1,270 K? Justify your answer.
![]()
(c) Methane gas is introduced to a rigid container. The container is heated to 1,270 K, and the initial partial pressure of the methane is 0.200 atm at this temperature. What is the partial pressure of H2 once equilibrium has been established?
(d) In a separate experiment, a container with the initial equilibrium established is heated to 1,350 K. At the higher temperature, the amount of CH4 present has decreased, and the amount of H2 present has increased. Is the initial equilibrium endothermic or exothermic? Justify your answer.
(e) A small amount of carbon is introduced to a rigid container that is already at equilibrium. What changes, if any, would there be in the partial pressure of H2? Justify your answer.
Question 2
The bromate ion will oxidize bromide ions to elemental bromine. It is necessary to add an acid to catalyze the reaction. Using the data in the following table, determine the rate law.
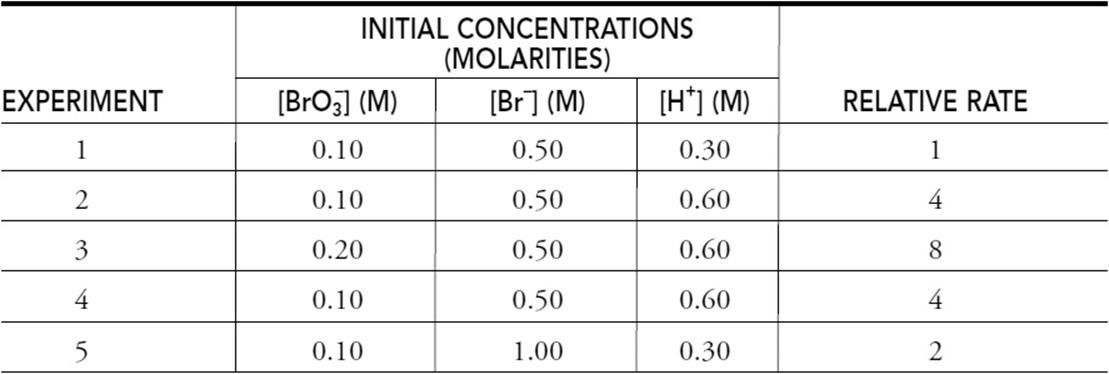
(a) Determine the order of the reaction for ![]() , Br–, and H+. Justify your answers.
, Br–, and H+. Justify your answers.
(b) Write the rate law for the reaction.
(c) What are the units on the rate constant?
(d) Assuming the reaction is exothermic. Draw and label the potential energy-level diagram for the reaction.
Question 3
![]()
The above reaction is to be used in the analysis of a sample containing oxalate ion, ![]()
(a) Outline the general procedure for the standardization of a potassium permanganate, KMnO4, solution beginning with primary standard sodium oxalate, NaC2O4.
(b) Outline the calculation of the concentration of the potassium permanganate solution.
(c) Show how to calculate the percent sodium oxalate in an unknown.
(d) List the problems arising if the primary sodium oxalate solution was not dried before being weighed.
(e) Normally, sulfuric acid, H2SO4, is used to supply the hydrogen ions for the reaction. What would be the problem of substituting hydrochloric acid, HCl, for sulfuric acid?
Question 4
![]()
Thermodynamic values related to the above reaction are given in the following table.
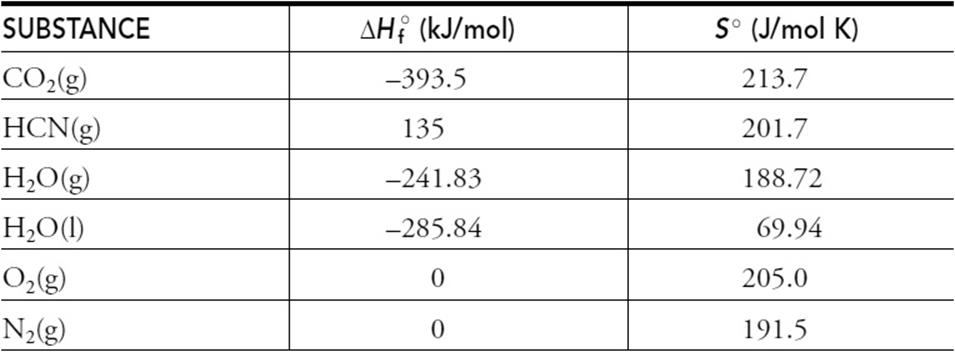
(a) Calculate the enthalpy change for this reaction.
(b) Calculate the entropy change for this reaction.
(c) Calculate the standard free energy for this reaction at 25°C.
(d) Calculate the standard free-energy change, at 25°C, if liquid water formed instead of water vapor.
Question 5
Answer each of the following with respect to chemical bonding and structure.
(a) The nitrite ion, ![]() , and the nitrate ion,
, and the nitrate ion, ![]() , both play a role in nitrogen chemistry.
, both play a role in nitrogen chemistry.
i. Draw the Lewis (electron-dot) structure for the nitrite ion and the nitrate ion.
ii. Predict which ion will have the shorter bond length, and justify your prediction.
(b) Using Lewis (electron-dot) structures, explain why the ClF3 molecule is polar and the BF3 molecule is not polar.
(c) Consider the following substances and their melting points:
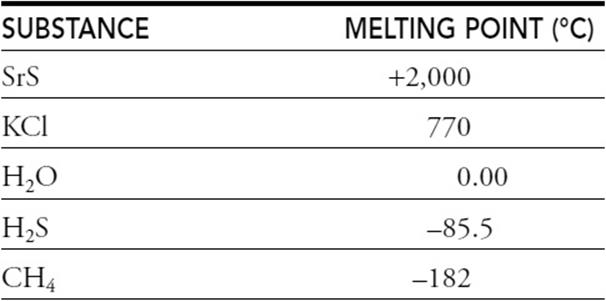
Explain the relative values of the melting points of these substances.
Question 6
Five beakers (A–E) are on a countertop. Each contains 200 mL of a 0.10 M solution. Beaker A contains Ba(OH)2; beaker B contains (NH4)2SO4; beaker C contains (CH3)2CHOH; beaker D contains K3PO4; and beaker E contains FeSO4.
(a) Which beaker has the lowest pH? Explain.
(b) Which two beakers may be mixed to produce a gas with a characteristic odor? Write a chemical equation for the reaction.
(c) Which two solutions are basic?
(d) Which beaker will NOT give a precipitate containing the hydroxide ion when added to beaker A?
Question 7
A water-soluble sample of a solid containing some barium nitrate, Ba(NO3)2, is to be analyzed for barium. The barium is to be precipitated as barium sulfate, BaSO4. Answer the following questions about this experiment.
(a) List the apparatus needed for this experiment.
(b) Outline the steps in this experiment.
(c) Set up the calculations needed to determine the percent barium in the sample.
(d) What changes would be required in the procedure if the original sample also contained lead, which also forms a precipitate with sulfate ion?
STOP. End of AP Chemistry Practice Exam 2.
![]() Answers and Explanations for Exam 2, Section II (Free Response)
Answers and Explanations for Exam 2, Section II (Free Response)
Question 1
![]()
You get 1 point for the correct equation. The “= 0.262” is optional.
![]()
When reversing an equilibrium, you take the reciprocal of the equilibrium constant.
You get 1 point for the answer and 1 point for the explanation.
![]()
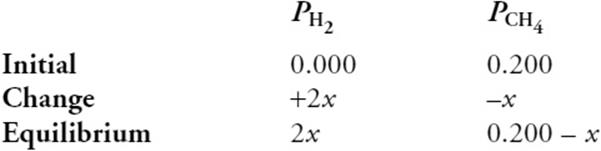
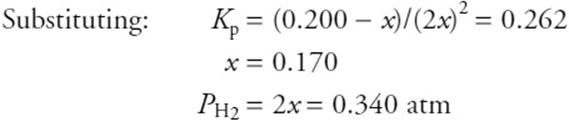
You get 1 point for the correct answer, you get 1 point for the table, and you get 1 point for the setup. You could correctly use the equation and answer from part c.
(d) The reaction is exothermic. The change in the amounts of CH4 and H2 indicate the reaction has shifted to the left. Exothermic reactions shift to the left as the temperature increases.
You get 1 point for answering exothermic and 1 point for the explanation. You may get the explanation point even if you answered endothermic.
(e) There would be no change. Solids do not shift equilibria.
You get 1 point for “no change” and 1 point for the explanation.
Total your points for each part. There are 10 possible points. Subtract 1 point if you do not have the correct number of significant figures in all cases.
Question 2
(a) The orders for Br– and ![]() are 1. The order for H+ is 2.
are 1. The order for H+ is 2.
If you get all three of these correct, you get 1 point.
Comparing experiments 1 and 5: The concentration of Br– is doubled, and the rate is doubled. This leads to Br– having an order of 1.
You get 1 point for this reasoning.
Comparing experiments 2 and 3: The concentration of ![]() is doubled, and the rate is doubled. This leads to
is doubled, and the rate is doubled. This leads to ![]() having an order of 1.
having an order of 1.
You get 1 point for this reasoning.
Comparing experiments 1 and 2: The concentration of H+ is doubled, and the rate is quadrupled (22). This leads to H+ having an order of 2.
You get 1 point for this reasoning.
![]()
This answer is worth 1 point. You will still get 1 point if you use incorrect orders from part a.
(c) Rearrange the rate law to: 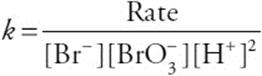
The rate has units of M s–1, and everything else has units of M. Plugging the units in gives:
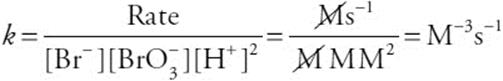
You get 1 point for the units.
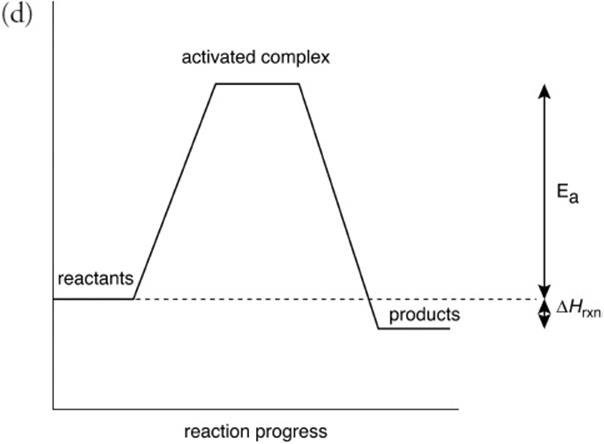
You get 1 point for showing the products being lower than the reactants. You get 1 point for labeling the activation energy. You get 1 point for correctly labeling ΔH.
Total your points for the different parts. There are 9 possible points. Subtract 1 point if you did not report the correct number of significant figures in part c.
Question 3
(a) 1. Samples of primary standard sodium oxalate are weighed into beakers, and deionized water is added to dissolve each of the samples.
2. A buret is rinsed with the potassium permanganate solution and then filled.
3. The potassium permanganate solution is titrated into the oxalate sample until a permanent pink color from the permanganate solution appears.
All three of these will get you 2 points. One or two of these will get you 1 point. Other items, such as heating the oxalate solution before the titration, are not necessary.
(b) (Grams sample) ![]()
This entire setup will get 2 points (the 134 is optional). You get 1 point if you miss a step.
(c) Percent sodium oxalate = (grams sodium oxalate/grams sample) × 100%
You get 1 point for this answer.
(d) If the primary standard were not dried, the sample would contain less sodium oxalate than indicated by the mass. The calculated concentration of the potassium permanganate solution would be too low.
This item is worth 1 point.
A low concentration for the potassium permanganate solution would yield a low percentage of sodium oxalate in the sample.
This item is worth 1 point.
(e) Hydrochloric acid might react with permanganate ion; therefore, there should be a test before using it.
This answer is worth 1 point.
Total your points for the various parts. There are 8 possible points.
Question 4
All necessary equations for this problem are in the exam booklet (and the tables at the end of this book).
![]()
The setup (products – reactants) is worth 1 point, and the answer is worth 1 point. You do not need to get the exact answer, but you should be able to round to this one.
![]()
The setup (products – reactants) is worth 1 point, and the answer is worth 1 point. You do not need to get the exact answer, but yours should round to this one.
![]()
The setup (putting values into the equation) is worth 1 point if you remember to change the temperature to kelvin and the joule to kilojoule conversion. An additional 1 point comes from the answer. If you got the wrong value in either part a or b, but used it correctly, you will still get the point for the answer. The free-energy equation is part of the material supplied in the exam booklet.
(d) This requires a repeat of parts a–c using the values for H2O(l).

You get 1 point for the work and 1 point for the answer.
Total your points for each part. There are 8 possible points. Subtract 1 point if all reported answers did not have the correct number of significant figures.
Question 5
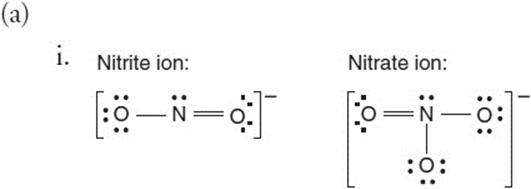
Give yourself 1 point for each structure that is correct. The double bonds could be between the nitrogen and any of the oxygen atoms, not just the ones shown. Only one double bond per structure is acceptable.
ii. If you predicted the nitrite ion has the shorter bond length, you have earned 1 point. The explanation must invoke resonance. You do not need to show all the resonance structures. You need to mention that the double bond “moves” from one oxygen to another. In the nitrite ion, each N—O bond is a double bond half the time and a single bond the other half. This gives an average of 1.5 bonds between the nitrogen and each of the oxygen atoms. Similarly, for the nitrate ion, each N—O bond spends one-third of the time as a double bond, and two-thirds of the time as a single bond. The average N—O bond is 1.33. The larger the average number of bonds, the shorter the bond is.
This explanation will get you 1 point.
(b) Give yourself 1 point for each correct Lewis structure.
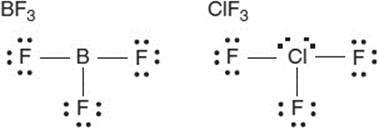
The BF3, with three bonding pairs and no nonbonding pairs on the central atom, is not polar. The ClF3, with five pairs about the central atom, is polar because of the two lone pairs.
Give yourself 1 point for this explanation.
(c) You get 1 point for saying that the two compounds with the highest melting points are ionic and the other compounds are molecular.
You get 1 point if you say that SrS is higher than KCl because the charges on the ions in SrS are higher.
You get 1 point if you say H2O is higher than the lowest two because of hydrogen bonding.
You get 1 point if you say H2S is higher than CH4 because H2S is polar and CH4 is nonpolar.
Total your points for the different parts. There are a maximum of 11 points.
Question 6
(a) Beaker B has the lowest pH. The ![]() ion is the conjugate acid of a weak base, and as an acid (weak), it will lower the pH. There are no other acids present.
ion is the conjugate acid of a weak base, and as an acid (weak), it will lower the pH. There are no other acids present.
You get 1 point for the correct beaker. You get 1 point for the explanation.
(b) Solutions A and B produce a gas with a characteristic odor when mixed. The reaction is:
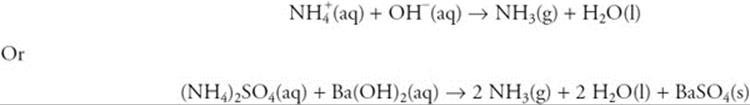
You get 1 point for the correct two solutions, and you get 1 point for either of the balanced equations.
(c) Solutions A and D—solution A is a strong base and solution D contains the conjugate base, ![]() , of a weak acid. The conjugate bases of weak acids undergo hydrolysis to produce basic solutions.
, of a weak acid. The conjugate bases of weak acids undergo hydrolysis to produce basic solutions.
You get 1 point for each of these you get correct.
(d) Solution C will give no precipitate, as it is a nonelectrolyte that will not react. All other beakers, except E, will generate soluble hydroxide or release ammonia gas. Beaker E gives Fe(OH)2, the only insoluble hydroxide that might form. Note that if you did not know about the solubility of barium hydroxide, the fact that the problem gave you a solution means that it must be soluble.
You get 1 point for choosing solution C.
Total your points for the different parts. There are 7 possible points.
Question 7
(a) analytical balance, beaker(s), crucible and cover, desiccator, drying oven, funnel, Meker burner, support stand, triangle, crucible support
You get 1 point for the following set: analytical balance, crucible and cover, and funnel.
You get 1 point for any others you list. There is a maximum of 2 points.
You can only get the second point if you have the entire first set.
(b) 1. *Weigh a sample of the solid (into a beaker).
2. Heat the crucible and lid with the Meker burner repeatedly until constant weight is achieved. (If you do not know what a Meker burner is, just remember, a burner is a burner is a burner.)
3. *Weigh the crucible and lid.
4. Add sufficient deionized water to dissolve the sample.
5. (Warm the solution.)
6. *Slowly add a solution containing the sulfate ion (usually sodium sulfate) to the solution.
7. Allow the precipitate to settle, and check for complete precipitation.
8. *Filter the precipitate and place it in the crucible.
9. Heat the crucible and lid with the sample to constant weight.
10. *Weigh the cooled crucible, lid, and sample.
You get 2 points for everything in order. Information in parentheses is optional.
The first two items may be in any order. You get 1 point if the order is wrong or if any of the starred items is missing.
(c) Mass of precipitate (ppt) = (crucible + lid + sample) – (crucible + lid)

Give yourself 1 point for each correct equation. The values 137.33 and 233.34 are optional.
(d) Since lead also precipitates as the sulfate, it would be necessary to remove the lead before the precipitation of the barium with sulfate.
Give yourself 1 point for this answer.
There is a total of 8 points possible.
Scoring Sheet for Chemistry Practice Exam 2
Multiple-Choice Section
![]()
Free-Response Section
Approximate Conversion Scale:
