5 Steps to a 5 AP Chemistry (2015)
STEP 4. Review the Knowledge You Need to Score High
CHAPTER 7. Stoichiometry
IN THIS CHAPTER
Summary: The previous chapter on chemical reactions discussed reactants and products in terms of individual atoms and molecules. But an industrial chemist is not interested in the number of molecules being produced; she or he is interested in kilograms or pounds or tons of products being formed per hour or day. How many kilograms of reactants will it take? How many kilograms of products will be formed? These are the questions of interest. A production chemist is interested primarily in the macroscopic world, not the microscopic one of atoms and molecules. Even a chemistry student working in the laboratory will not be weighing out individual atoms and molecules, but large numbers of them in grams. There must be a way to bridge the gap between the microscopic world of individual atoms and molecules, and the macroscopic world of grams and kilograms. There is—it is called the mole concept, and it is one of the central concepts in the world of chemistry.

Keywords and Equations
Avogadro’s number = 6.022 × 1023 mol–1
Molarity, M = moles solute per liter solution
n = moles
![]()
M = molar mass
Moles and Molar Mass
The mole (mol) is the amount of a substance that contains the same number of particles as atoms in exactly 12 grams of carbon-12. This number of particles (atoms or molecules or ions) per mole is called Avogadro’s number and is numerically equal to 6.022 × 1023 particles. The mole is simply a term that represents a certain number of particles, like a dozen or a pair. That relates moles to the microscopic world, but what about the macroscopic world? The mole also represents a certain mass of a chemical substance. That mass is the substance’s atomic or molecular mass expressed in grams. In Chapter 5, the Basics chapter, we described the atomic mass of an element in terms of atomic mass units (amu). This was the mass associated with an individual atom. Then we described how one could calculate the mass of a compound by simply adding together the masses, in amu, of the individual elements in the compound. This is still the case, but at the macroscopic level the unit of grams is used to represent the quantity of a mole. Thus, the following relationships apply:


The mass in grams of one mole of a substance is the molar mass.
The relationship above gives a way of converting from grams to moles to particles, and vice versa. If you have any one of the three quantities, you can calculate the other two. This becomes extremely useful in working with chemical equations, as we will see later, because the coefficients in the balanced chemical equation are not only the number of individual atoms or molecules at the microscopic level, but also the number of moles at the macroscopic level.
How many moles are present in 1.20 × 1025 silver atoms?
Answer:
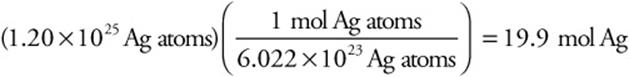
Percent Composition and Empirical Formulas
If the formula of a compound is known, it is a fairly straightforward task to determine the percent composition of each element in the compound. For example, suppose you want to calculate the percentage hydrogen and oxygen in water, H2O. First calculate the molecular mass of water:
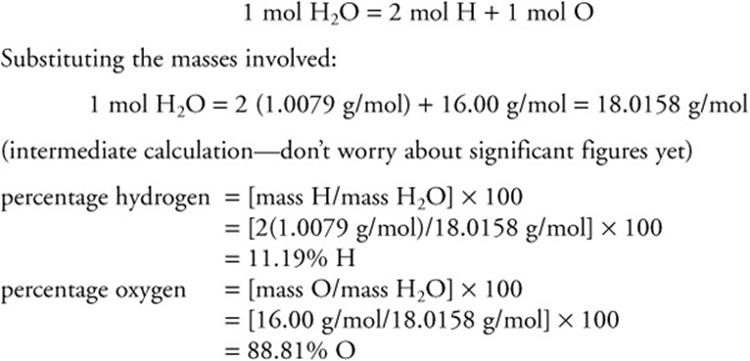
As a good check, add the percentages together. They should equal to 100% or be very close.
Determine the mass percent of each of the elements in C6H12O6
Formula mass (FM) = 180.158 amu
Answer:
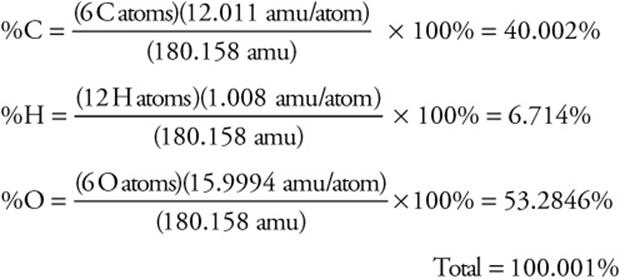
The total is a check. It should be very close to 100%.
In the problems above, the percentage data was calculated from the chemical formula, but the empirical formula can be determined if the percent compositions of the various elements are known. The empirical formula tells us what elements are present in the compound and the simplest whole-number ratio of elements. The data may be in terms of percentage, or mass, or even moles. But the procedure is still the same: convert each to moles, divide each by the smallest number, then use an appropriate multiplier if needed. The empirical formula mass can then be calculated. If the actual molecular mass is known, dividing the molecular mass by the empirical formula mass gives an integer (rounded if needed) that is used to multiply each of the subscripts in the empirical formula. This gives the molecular (actual) formula, which tells which elements are in the compound and the actual number of each.
For example, a sample of a gas was analyzed and found to contain 2.34 g of nitrogen and 5.34 g of oxygen. The molar mass of the gas was determined to be about 90 g/mol. What are the empirical and molecular formulas of this gas?
Answer:
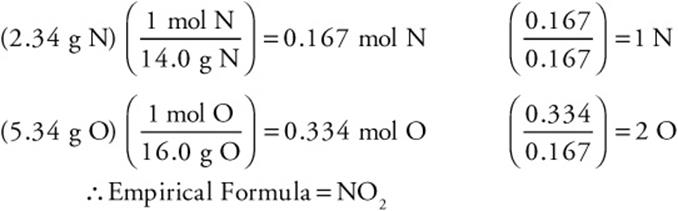
The molecular formula may be determined by dividing the actual molar mass of the compound by the empirical molar mass. In this case the empirical molar mass is 46 g/mol.
Thus 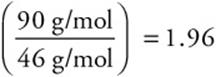 which, to one significant figure, is 2. Therefore, the molecular formula is twice the empirical formula—N2O4.
which, to one significant figure, is 2. Therefore, the molecular formula is twice the empirical formula—N2O4.
![]()
Be sure to use as many significant digits as possible in the molar masses. Failure to do so may give you erroneous ratio and empirical formulas.
Reaction Stoichiometry
As we have discussed previously, the balanced chemical equation not only indicates which chemical species are the reactants and the products, but also indicates the relative ratio of reactants and products. Consider the balanced equation of the Haber process for the production of ammonia:
![]()
This balanced equation can be read as: 1 nitrogen molecule reacts with 3 hydrogen molecules to produce 2 ammonia molecules. But as indicated previously, the coefficients can stand not only for the number of atoms or molecules (microscopic level), they can also stand for the number of moles of reactants or products. The equation can also be read as: 1 mol of nitrogen molecules reacts with 3 mol of hydrogen molecules to produce 2 mol of ammonia molecules. And if the number of moles is known, the number of grams or molecules can be calculated. This is stoichiometry, the calculation of the amount (mass, moles, particles) of one substance in a chemical reaction through the use of another. The coefficients in a balanced chemical equation define the mathematical relationship between the reactants and products, and allow the conversion from moles of one chemical species in the reaction to another.
Consider the Haber process above. How many moles of ammonia could be produced from the reaction of 20.0 mol of nitrogen with excess hydrogen?

Before any stoichiometry calculation can be done, you must have a balanced chemical equation!
You are starting with moles of nitrogen and want moles of ammonia, so we’ll convert from moles of nitrogen to moles of ammonia by using the ratio of moles of ammonia to moles of nitrogen as defined by the balanced chemical equation:
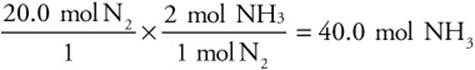
The ratio of 2 mol NH3 to 1 mol N2 is called the stoichiometric ratio and comes from the balanced chemical equation.
Suppose you also wanted to know how many moles of hydrogen it would take to fully react with the 20.0 mol of nitrogen. Just change the stoichiometric ratio:
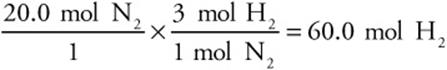
Notice that this new stoichiometric ratio also came from the balanced chemical equation.
Suppose instead of moles you had grams and wanted an answer in grams. How many grams of ammonia could be produced from the reaction of 85.0 g of hydrogen gas with excess nitrogen?
![]()
In working problems that involve something other than moles, you will still need moles. And you will need the balanced chemical equation.
In this problem we will convert from grams of hydrogen to moles of hydrogen to moles of ammonia using the correct stoichiometric ratio, and finally to grams of ammonia. And we will need the molar mass of H2 (2.0158 g/mol) and ammonia (17.0307 g/mol):
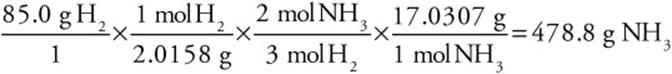
Actually, you could have calculated the actual number of ammonia molecules produced if you had gone from moles of ammonia to molecules (using Avogadro’s number):
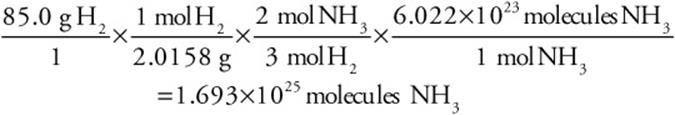
In another reaction, 40.0 g of Cl2 and excess H2 are combined. HCl will be produced. How many grams of HCl will form?
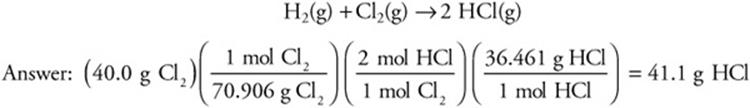
Limiting Reactants
In the examples above, one reactant was present in excess. One reactant was completely consumed, and some of the other reactant would be left over. The reactant that is used up first is called the limiting reactant (L.R.). This reactant really determines the amount of product being formed. How is the limiting reactant determined? You can’t assume it is the reactant in the smallest amount, since the reaction stoichiometry must be considered. There are generally two ways to determine which reactant is the limiting reactant:
1. Each reactant, in turn, is assumed to be the limiting reactant, and the amount of product that would be formed is calculated. The reactant that yields the smallest amount of product is the limiting reactant. The advantage of this method is that you get to practice your calculation skills; the disadvantage is that you have to do more calculations.
2. The moles of reactant per coefficient of that reactant in the balanced chemical equation is calculated. The reactant that has the smallest mole-to-coefficient ratio is the limiting reactant. This is the method that many use.
Let us consider the Haber reaction once more. Suppose that 50.0 g of nitrogen and 40.0 g of hydrogen were allowed to react. Calculate the number of grams of ammonia that could be formed.
First, write the balanced chemical equation:
![]()
Next, convert the grams of each reactant to moles:
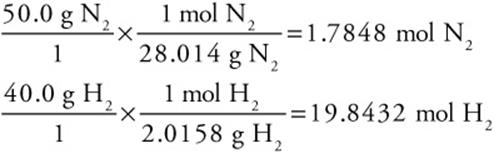
Divide each by the coefficient in the balanced chemical equation. The smaller is the limiting reactant:
For N2: 1.7848 mol N2/1 = 1.7848 mol/coefficient limiting reactant
For H2: 19.8432 mol H2/3 = 6.6144 mol/coefficient
Finally, base the stoichiometry of the reaction on the limiting reactant:
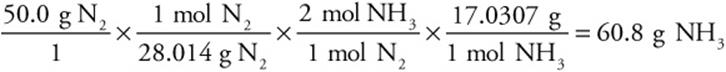
![]()
Anytime the quantities of more than one reactant are given it is probably an L.R. problem.
Let’s consider another case. To carry out the following reaction: ![]() and 50.0 g of H2O were supplied. How many grams of H3PO4 may be produced?
and 50.0 g of H2O were supplied. How many grams of H3PO4 may be produced?
Answer:
1. Convert to moles:
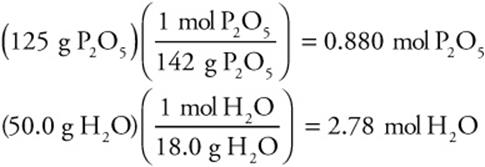
2. Find the limiting reactant:
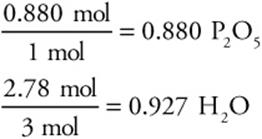
The 1 mol and the 3 mol come from the balanced chemical equation. The 0.880 is smaller, so this is the L.R.
3. Finish using the number of moles of the L.R.:
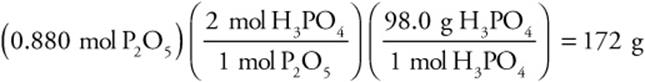
Percent Yield
In the preceding problems, the amount of product calculated based on the limiting-reactant concept is the maximum amount of product that could be formed from the given amount of reactants. This maximum amount of product formed is called the theoretical yield. However, rarely is the amount that is actually formed (the actual yield) the same as the theoretical yield. Normally it is less. There are many reasons for this, but the principal reason is that most reactions do not go to completion; they establish an equilibrium system (see Chapter 15 Equilibrium for a discussion of chemical equilibrium). For whatever reason, not as much as expected is formed. The efficiency of the reaction can be judged by calculating the percent yield. The percent yield (% yield) is the actual yield divided by the theoretical yield, and the result is multiplied by 100% to generate percentage:

Consider the problem in which it was calculated that 60.8 g NH3 could be formed. Suppose that reaction was carried out, and only 52.3 g NH3 was formed. What is the percent yield?
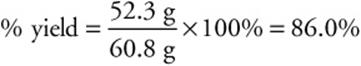
Let’s consider another percent yield problem in which a 25.0-g sample of calcium oxide is heated with excess hydrogen chloride to produce water and 37.5 g of calcium chloride. What is the percent yield of calcium chloride?
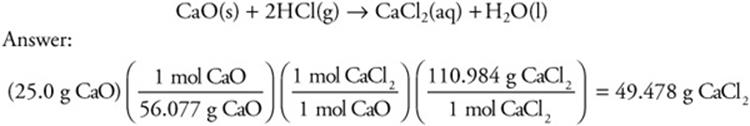
The theoretical yield is 49.5 g.
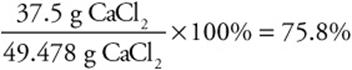
Note: All the units except % must cancel. This includes canceling g CaCl2 with g CaCl2, not simply g.
Molarity and Solution Calculations
We discuss solutions further in the chapter on solutions and colligative properties, but solution stoichiometry is so common on the AP exam that we will discuss it here briefly also. Solutions are homogeneous mixtures composed of a solute (substance present in smaller amount) and a solvent (substance present in larger amount). If sodium chloride is dissolved in water, the NaCl is the solute and the water the solvent.
One important aspect of solutions is their concentration, the amount of solute dissolved in the solvent. In the chapter on solutions and colligative properties we will cover several concentration units, but for the purpose of stoichiometry, the only concentration unit we will use at this time is molarity. Molarity (M) is defined as the moles of solute per liter of solution:
M = mol solute/L solution
Let’s start with a simple example of calculating molarity. A solution of NaCl contains 39.12 g of this compound in 100.0 mL of solution. Calculate the molarity of NaCl.
Answer:
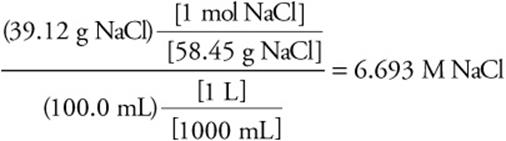
Knowing the volume of the solution and the molarity allows you to calculate the moles or grams of solute present.
Next, let’s see how we can use molarity to calculate moles. How many moles of ammonium ions are in 0.100 L of a 0.20 M ammonium sulfate solution?
Answer:
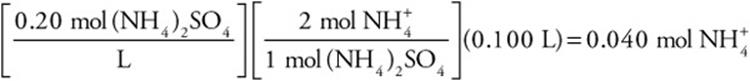
Stoichiometry problems (including limiting-reactant problems) involving solutions can be worked in the same fashion as before, except that the volume and molarity of the solution must first be converted to moles.
If 35.00 mL of a 0.1500 M KOH solution is required to titrate 40.00 mL of a phosphoric acid solution, what is the concentration of the acid? The reaction is:
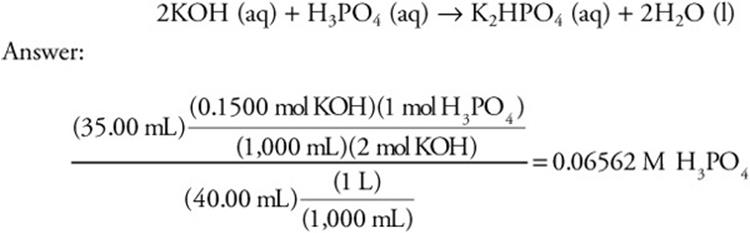
Experimental

Stoichiometry experiments must involve moles. They nearly always use a balanced chemical equation. Measurements include initial and final masses, and initial and final volumes. Calculations may include the difference between the initial and final values. Using the formula mass and the mass in grams, moles may be calculated. Moles may also be calculated from the volume of a solution and its molarity.
Once the moles have been calculated (they are never measured), the experiment will be based on further calculations using these moles.
Common Mistakes to Avoid
![]()
1. Avogadro’s number is 6.022 × 1023 (not 10–23).
2. Be sure to know the difference between molecules and moles.
3. In empirical formula problems, be sure to get the lowest ratio of whole numbers.
4. In stoichiometry problems, be sure to use the balanced chemical equation.
5. The stoichiometric ratio comes from the balanced chemical equation.
6. When in doubt, convert to moles.
7. In limiting-reactant problems, don’t consider just the number of grams or even moles to determine the limiting reactant—use the mol/coefficient ratio.
8. The limiting reactant is a reactant, a chemical species to the left of the reactant arrow.
9. Use the balanced chemical equation.
10. Percent yield is actual yield of a substance divided by the theoretical yield of the same substance multiplied by 100%.
11. Molarity is moles of solute per liter of solution, not solvent.
12. Be careful when using Avogadro’s number—use it when you need or have the number of atoms, ions, or molecules.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry exam. First are 16 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry exam. Following those is a multipart free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 20 minutes. You may not use a calculator. You may use the periodic table and the equation sheet at the back of this book.
1. How many milliliters of 0.100 M H2SO4 are required to neutralize 50.0 mL of 0.200 M KOH?
(A) 25.0 mL
(B) 30.0 mL
(C) 20.0 mL
(D) 50.0 mL
2. A sample of oxalic acid, H2C2O4, is titrated with standard sodium hydroxide, NaOH, solution. A total of 45.20 mL of 0.1200 M NaOH is required to neutralize completely 20.00 mL of the acid. What is the concentration of the acid?
(A) 0.2712 M
(B) 0.1200 M
(C) 0.1356 M
(D) 0.2400 M
3. A solution is prepared by mixing 50.0 mL of 0.20 M arsenic acid, H3AsO4, and 50.0 mL of 0.20 M sodium hydroxide, NaOH. Which anion is present in the highest concentration?
![]()
(B) OH–
![]()
(D) Na+
4. ![]()
This reaction is used in the titration of an iron solution. What is the concentration of the iron solution if it takes 45.20 mL of 0.1000 M ![]() solution to titrate 50.00 mL of an acidified iron solution?
solution to titrate 50.00 mL of an acidified iron solution?
(A) 0.5424 M
(B) 0.1000 M
(C) 1.085 M
(D) 0.4520 M
5. Manganese, Mn, forms a number of oxides. A particular oxide is 63.2% Mn. What is the simplest formula for this oxide?
(A) MnO
(B) Mn2O3
(C) Mn3O4
(D) MnO2
6. Vanadium forms a number of oxides. In which of the following oxides is the vanadium-to-oxygen mass ratio 2.39:1.00?
(A) VO
(B) V2O3
(C) V3O4
(D) VO2
7. How many grams of nitrogen are in 25.0 g of (NH4)2SO4?
(A) 5.30 g
(B) 1.30 g
(C) 0.190 g
(D) 2.65 g
8. Sodium sulfate forms a number of hydrates. A sample of a hydrate is heated until all the water is removed. What is the formula of the original hydrate if it loses 43% of its mass when heated?
(A) Na2SO4·H2O
(B) Na2SO4·2H2O
(C) Na2SO4·6H2O
(D) Na2SO4·8H2O
9. ![]()
Copper metal reacts with nitric acid according to the above equation. A 0.30 mol sample of copper metal and 10.0 mL of 12 M nitric acid are mixed in a flask. How many moles of NO gas will form?
(A) 0.060 mol
(B) 0.030 mol
(C) 0.010 mol
(D) 0.20 mol
10. Gold(III) oxide, Au2O3, can be decomposed to gold metal, Au, plus oxygen gas, O2. How many moles of oxygen gas will form when 221 g of solid gold(III) oxide is decomposed? The formula mass of gold(III) oxide is 442.
(A) 0.250 mol
(B) 0.500 mol
(C) 0.750 mol
(D) 1.00 mol
11. ![]()
When the above equation is balanced, the lowest whole number coefficient for O2 is:
(A) 4
(B) 16
(C) 22
(D) 27
12. 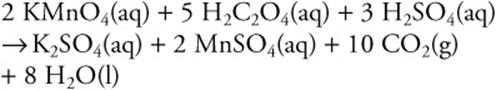
How many moles of MnSO4 are produced when 1.0 mol of KMnO4, 5.0 mol of H2C2O4, and 3.0 mol of H2SO4 are mixed?
(A) 4.0 mol
(B) 5.0 mol
(C) 2.0 mol
(D) 1.0 mol
13. When the following equation is balanced, it is found that 1.00 mol of C8H18 reacts with how many moles of O2?
![]()
(A) 12.5 mol
(B) 10.0 mol
(C) 25.0 mol
(D) 37.5 mol
14. ![]()
Calcium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.200 mol of calcium?
(A) 5.60 L
(B) 4.48 L
(C) 3.36 L
(D) 1.12 L
15. 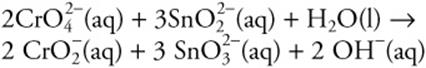
How many moles of OH– form when 50.0 mL of 0.100 M ![]() is added to a flask containing 50.0 mL of 0.100 M
is added to a flask containing 50.0 mL of 0.100 M ![]()
(A) 0.100 mol
(B) 6.66 × 10–3 mol
(C) 3.33 × 10–3 mol
(D) 5.00 × 10–3 mol
16. A solution containing 0.20 mol of KBr and 0.20 mol of MgBr2 in 2.0 liters of water is provided. How many moles of Pb(NO3)2 must be added to precipitate all the bromide as insoluble PbBr2?
(A) 0.10 mol
(B) 0.50 mol
(C) 0.60 mol
(D) 0.30 mol
Answers and Explanations for the Multiple-Choice Questions
1. D—The reaction is ![]()
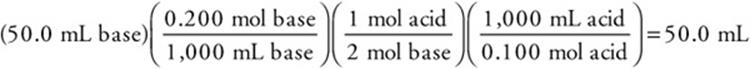
2. C—The reaction is ![]()
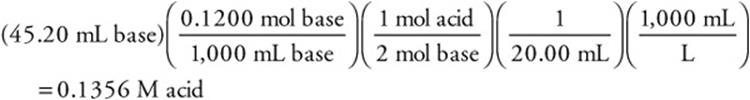
As always, round the values to get an estimate and pick the closest answer.
3. C—Moles acid = (50.0 mL) (0.20 mol acid/1,000 mL) = 0.0100 mol Moles base = (50.0 mL) (0.20 mol base/1,000 mL) = 0.0100 mol
There is sufficient base to react completely with only one of the ionizable hydrogen ions from the acid. This leaves ![]() . Answer D cannot be correct because it is a cation.
. Answer D cannot be correct because it is a cation.
4. A—
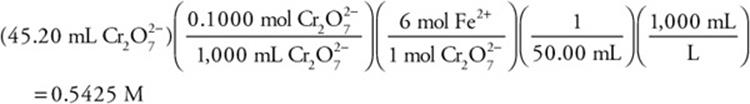
This is a perfect example of where simplification is important. Change the above calculation to
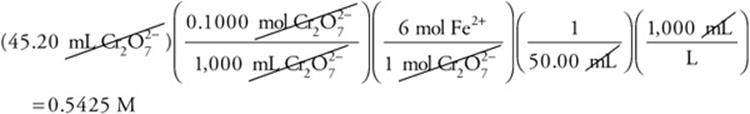
This becomes
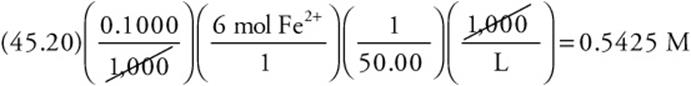
Next round and simplify to
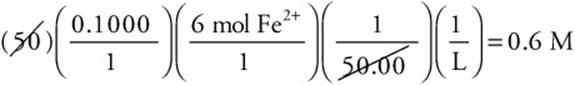
Since the 45.20 was rounded up, the answer is slightly high; therefore, pick the closest answer that is lower.
5. D—63.2% Mn leaves 36.8% O (assuming 100 grams of sample) 63.2/54.94 = 1.15 mole Mn and 36.8/16.0 = 2.30 mole O
Thus, there is 1 Mn/2 O.
6. C—V: 2.39/50.94 = 0.0469 and for O:1.00/16.0 = 0.0625
0.0469/0.0469 = 1 0.0625/0.0469 = 1.33
Multiplying both by 3 gives: 3 V and 4 O.
7. A—(25.0 g (NH4)2SO4)(1 mol(NH4)2SO4/132 g) =
(2 mol N/1 mol(NH4)2SO4)(14.0 g N/1 mol N) = 5.30 g
8. C—[(6 mol H2O)(18 g/mol H2O)]/(250 g Na2SO4 · 6 H2O) × 100% = 43%
9. B—Calculate the moles of acid to compare to the moles of Cu:
![]()
The acid is the limiting reactant, because 0.30 mol of copper requires 0.80 mol of acid. Use the limiting reactant to calculate the moles of NO formed.
![]()
10. C—The balanced chemical equation is:
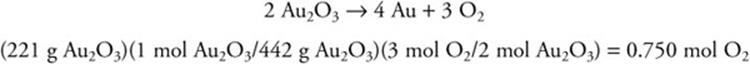
Note the 2:1 relationship between the formula mass and the mass of reactant.
11. D—The balanced equation is:
![]()
12. D—The KMnO4 is the limiting reagent. Each mole of KMnO4 will produce a mole of MnSO4.
13. A—The balanced equation is:
![]()
14. B—(0.200 mol Ca)(1 mol H2/1 mol Ca)(22.4 L at STP/1 mol H2) = 4.48 L
Be careful to only use 22.4 when at STP.
15. C—There are 5.00 × 10–3 mol of ![]() and an equal number of moles of
and an equal number of moles of ![]() . Thus,
. Thus, ![]() is the limiting reactant (larger coefficient in the balanced reaction).
is the limiting reactant (larger coefficient in the balanced reaction).
![]()
16. D—The volume of water is irrelevant. 0.20 mol of KBr will require 0.10 mol of Pb(NO3)2 and 0.20 mol of MgBr2 will require 0.20 mol of Pb(NO3)2. Total the two yields.
Free-Response Question
You have 15 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question 1
The analysis of a sample of a monoprotic acid found that the sample contained 40.0% C and 6.71% H. The remainder of the sample was oxygen.
(a) Determine the empirical formula of the acid.
(b) A 0.2720 g sample of the acid, HA, was titrated with standard sodium hydroxide, NaOH, solution. Determine the molecular weight of the acid if the sample required 45.00 mL of 0.1000 M NaOH for the titration.
(c) A second sample was placed in a flask. The flask was placed in a hot water bath until the sample vaporized. It was found that 1.18 g of vapor occupied 300.0 mL at 100°C and 1.00 atmospheres. Determine the molecular weight of the acid.
(d) Using your answer from part a, determine the molecular formula for part b and for part c.
(e) Account for any differences in the molecular formulas determined in part d.
Answer and Explanation for the Free-Response Question
(a) The percent oxygen (53.3%) is determined by subtracting the carbon and the hydrogen from 100%. Assuming there are 100 grams of sample gives the grams of each element as being numerically equivalent to the percent. Dividing the grams by the molar mass of each element gives the moles of each.

This gives the empirical formula: CH2O.
You get 1 point for correctly determining any of the elements and 1 point for getting the complete empirical formula correct.
(b) Using HA to represent the monoprotic acid, the balanced equation for the titration reaction is:
![]()
The moles of acid may then be calculated:
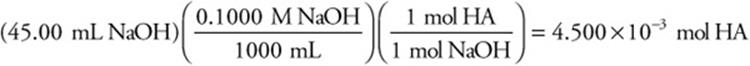
The molecular mass is:
![]()
You get 1 point for the correct number of moles of HA (or NaOH) and 1 point for the correct final answer.
(c) There are several methods to solve this problem. One way is to use the ideal gas equation as done here. The equation and the value of R are in the exam booklet. First find the moles: n = PV/RT. Do not forget, you MUST change temperature to kelvin.
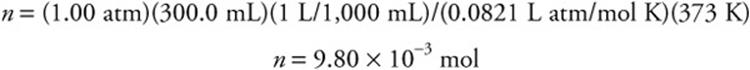
The molecular mass is 1.18 g/9.80 × 10–3 mol = 120 g/mol
You get 1 point for getting any part of the calculation correct and 1 point for getting the correct final answer.
(d) The approximate formula mass from the empirical (CH2O) formula is:
![]()
For part b: (60.44 g/mol)/(30 g/mol) = 2
![]()
For part c: (120 g/mol)/(30 g/mol) = 4
![]()
You get 1 point for each correct molecular formula. If you got the wrong answer in part a, you can still get credit for one or both of the molecular formulas if you used the part a value correctly.
(e) The one formula is double the formula of the other. Thus, the smaller molecule dimerizes to produce the larger molecule.
You get 1 point if you “combined” two of the smaller molecules.
Total your points. There are 9 points possible.
![]() Rapid Review
Rapid Review
• The mole is the amount of substance that contains the same number of particles as exactly 12 g of carbon-12.
• Avogadro’s number is the number of particles per mole, 6.022 × 1023 particles.
• A mole is also the formula (atomic, molecular) mass expressed in grams.
• If you have any one of the three—moles, grams, or particles—you can calculate the others.
• The empirical formula indicates which elements are present and the lowest whole-number ratio.
• The molecular formula tells which elements are present and the actual number of each.
• Be able to calculate the empirical formula from percent composition data or quantities from chemical analysis.
• Stoichiometry is the calculation of the amount of one substance in a chemical equation by using another one.
• Always use the balanced chemical equation in reaction stoichiometry problems.
• Be able to convert from moles of one substance to moles of another, using the stoichiometric ratio derived from the balanced chemical equation.
• In working problems that involve a quantity other than moles, sooner or later it will be necessary to convert to moles.
• The limiting reactant is the reactant that is used up first.
• Be able to calculate the limiting reactant by the use of the mol/coefficient ratio.
• Percent yield is the actual yield (how much was actually formed in the reaction) divided by the theoretical yield (the maximum possible amount of product formed) times 100%.
• A solution is a homogeneous mixture composed of a solute (species present in smaller amount) and a solvent (species present in larger amount).
• Molarity is the number of moles of solute per liter of solution. Don’t confuse molarity, M or [ ], with moles, n or mol.
• Be able to work reaction stoichiometry problems using molarity.
• Always use the balanced chemical equation in reaction stoichiometry problems.