5 STEPS TO A 5: 500 AP Chemistry Questions to Know by Test Day! (2012)
ANSWERS
Chapter 1: Atomic Theory and Structure
1. (B) The mass number of cadmium is 112, not the atomic mass (the weighted average of the naturally occurring isotopes). The mass number will always be a whole number because it is the sum of the number of protons and neutrons (collectively called the nucleons, referring to their location in the nucleus) in an atom. The number of electrons and protons will always be the same in a neutral atom because they are the only negatively and positively charged (respectively) particles in the atom. The atomic number is the number of protons. It determines the identity of the atom, so finding cadmium on the periodic table will tell us its atomic number. If we subtract the atomic number from the mass number, we get the number of neutrons in that particular isotope.
2. (E) Location E on the periodic table is in the vicinity of francium. Francium has a half-life of just 22 minutes and is the second rarest naturally occurring element (astatine is the rarest). It is doubtful anyone has actually reacted francium with water, but if it behaves as expected, it would certainly be a spectacle. In general, but moreso for the alkali metals at the bottom of the group (with the largest atomic radii and the lowest first ionization energies), the reaction is highly exothermic, partly because the reaction produces a strong base whose total dissociation in water is highly exothermic. The reaction also produces energy in the form of light and H2(g). When added to water, the interior of a piece of sodium metal, for example, will melt before it is consumed due to the high temperature produced by the reaction (the melting point of Na is ~800°C). In addition, the high heat ignites the flammable H2(gg) that is produced.
3. (B) The location of B on the table is in the halogens, specifically fluorine. Fluorine does not have the highest ionization energy of all the elements, just the elements we’ve been given to choose from in this question. Helium is the element with the highest first ionization energy. First ionization energy is the minimum amount of energy required to ionize a ground state, gaseous atom by removing an electron. The result is a cation. In general, the first ionization energy is low for metals and high for nonmetals. (See the figure in Questions 21 and 22 to compare the first ionization energies of elements 1–20.)
4. (B) The location of B on the table is in the vicinity of the halogens, specifically fluorine. Fluorine is the element with the highest electronegativity. Electronegativity is a measure of an atom’s ability to attract electrons to itself while in a bond (within a molecule). Because the measurement of electronegativity of an atom relies on the atom being in a bond, the noble gases He, Ne, and Ar do not have measured values for electronegativity. The ionization energies of the valence electrons in Kr, Xe, and Rn are sufficiently low, allowing these noble gases to form covalent bonds with other atoms (mostly those with a high electronegativity, like F, whose electron-attracting abilities are strong enough to force the Kr, Xe, or Rn atoms to share their electrons) and therefore have their electronegativities assessed.
5. (C) Electron affinity is the measure of the energy change that occurs when an electron is added to a ground state, gaseous atom. Atoms that have a high affinity, or attraction, for electrons have very negative electron affinities. The periodic trend for electron affinity is generally correlated with electronegativity, so regions B and C are the top contenders for answer choices. However, there are some important differences. All the noble gases have measured electron affinity (the lightest three have no values for electronegativity, see Answer 4) and chlorine, not fluorine, has the highest electron affinity. Electron affinity doesn’t change significantly within a group, and it can be thought of as the reverse ionization energy of an atom’s –1 anion. For example, the energy change to remove an electron from Cl− (to produce Cl) is 349 kJ mol−1 (endothermic), and the energy change to add an electron to Cl to produce Cl− is –349 kJ mol−1 (exothermic).
6. (E) Location E on the periodic table is in the vicinity of francium, which might have the largest atomic radius if it were known (with a half-life of just 22 minutes, it doesn’t exist long enough to do the necessary measurements). Atoms don’t have a sharp, well-defined edge, so their bonding atomic radius is used to infer the radius of an individual atom. The atomic radius is measured while the atom is in a bond with another atom of the same kind. The distance between the two nuclei is measured and then divided in half. What we do know is that cesium has the largest atomic radius of the elements measured so far. (See Answer 10 for an explanation of the periodic trend regarding atomic radius.)
7. (E) Metallic character is not something that is specifically measured. It is a set of properties given to metals, but the properties are due to one of the most basic properties of metals—their readiness to lose electrons. This is due to metallic bonding, which can be thought of as the most “sharing” form of bonding. Metals are lattices of positively charged ions that are bathing in a sea of electrons. These electrons are highly mobile and account for nearly all the properties of metals, especially their electrical conductivity. The weak pull on the valence electrons by the nucleus allows them to be pulled off easily, resulting in the low ionization energies and the relatively strong tendency of metals to lose electrons and take on (almost) exclusively positive oxidation states. Metals with the highest metallic character can be considered as those having the lowest ionization energies, though this is a bit of a simplification (but it will work for the AP Chemistry exam).
8. (C) The atomic mass of bromine is almost 80. The two isotopes are of masses 79 and 81. The average of these two numbers is 80, and that implies the masses were equally weighted in the calculation. Remember, different (naturally occurring) isotopes of atoms exist in different quantities. These are accounted for in the atomic mass calculation according to their percent natural occurrence. Remember, the atomic mass is the weighted average of all the naturally occurring isotopes of an element. (Questions 9 is similar.)
9. (C) The three isotopes of Sr are 86, 87, and 88. If they occurred in equal numbers, their atomic mass would be the average of their mass numbers, 87. Since the mass number is greater than the average, the isotopes of higher mass must be present in greater quantities. Additionally, because the atomic mass is closer to 88 than 87, we can predict the occurrence of the 88 isotope as the highest. Although (E) is mostly true—the natural occurrence of isotopes 86 and 87 are 9.9 percent and 7.0 percent, respectively, and that cannot be established from the information given in the question. (Questions 8 is similar.)
10. (D) Atomic radius decreases from left to right along a period. This is due to the shielding effect of the core electrons (this doesn’t apply to atoms with one electron, such as hydrogen, or a He+ ion). All the elements in a particular period have the same configuration of core electrons. These electrons shield the valence electrons from the pull of the nucleus. The effective nuclear charge is calculated Zeff = Z – S (Zeff is the effective nuclear charge; Z is the atomic number, a.k.a. the number of positive charges in the nucleus; and S is the number of nonvalence, or core, electrons). The Zeff for all the atoms in a period gets larger as the number of positive charges in the nucleus increases, but the number of nonvalence (core) electrons doesn’t. An increased effective nuclear charge means the valence electrons feel a greater pull from the nucleus, and can thus be found closer to the nucleus than the electrons in atoms with lower values of Zeff. (See Answer 6 for an explanation of how atomic radius is measured.)
11. (C) The alkali metals form strong bases when they react with water, not strong acids. (See Answer 2 for a description of the reaction of alkali metals with water.)
A general strategy for except questions: The except questions are tricky, even if what they are asking is not. Our brain doesn’t think in the negative, so a good habit to get into is to circle the word except in the question to remind us that we are looking for a false statement, then treat each answer choice as either true or false, marking each choice as we go. At the end of choice (E), we choose the false one as our answer.
12. (D) This question is asking if we know that ground state elements in the same group have similar properties. Phosphorus and astatine are both in group 15 (5A) so their valence shell electron configurations are both s2p3, conferring on them similar chemical reactivities. Sulfur, selenium, and oxygen are in group 16 (s2p4), while silicon is a group 14 semimetal (metalloid) with valence shell electron configuration of s2p2.
13. (B) This has a simple mathematical solution—take the atomic number (which will tell us the number of electrons in a neutral atom) of the element and add the absolute value of the negative oxidation states (more electrons) and subtract the absolute value of the positive oxidation states. F− (9+1) and Na+ (11–1) both have 10 electrons and are therefore isoelectronic.
We can also arrive at this answer by finding one of the elements in each pair on the periodic table and moving one element to the right for each negative charge and one to the left for each positive charge. If the two elements we are comparing lead us to the same element once we’ve accounted for their oxidation state, then they’ve got the same number of electrons. For Na+ and F−, this element would be neon. The same number and configuration of electrons does not correlate with similar chemical reactivity in ions. F− and Na+ are like neon in that they have a full valence shell and both are more stable and less reactive than in their ground state, but they are charged and therefore behave like ions. Their ionic radius also differs due to their different nuclear charge (see second paragraph of Answer 15).
14. (D) See Answer 13, keeping in mind that the iodide ion has 54 electrons.
15. (C) Ionic radius is not the same as atomic radius (described in Answer 6). In an ion, the number of electrons does not equal the number of protons. Atoms become ions because they gain or lose electrons (not protons), so ions that are positively charged will be smaller than what they are when in their ground state (same effective nuclear charge pulling on fewer electrons), whereas negatively charged ions will be larger when they are in their ground state (same effective nuclear charge pulling on more electrons).
For isoelectronic ions (ions with the same number of electrons), the ionic radius decreases with increasing nuclear charge. For example, O2–> F− > Na+ > Mg2+ > Al3+. This is because the same number of electrons are being pulled by an increasing number of protons. (See Answer 10 for an explanation of how size is affected by shielding.) For atoms of the same charge (and in the same group), the size of ions increases as you go down the group. Keep in mind that ionic size is an important determinant of lattice energy (see Answer 52 for a description of the factors that affect lattice energy).
16. (D) The first ionization energies of Kr, Xe, and Rn are sufficiently low to allow these noble gases to form covalent bonds with other atoms. Krypton difluoride, KrF2, was the first compound of krypton discovered. Xenon can form compounds with oxygen (XeO3 and XeO4) and fluorine (XeF4 and XeF6). Radon appears to form compounds with fluorine (RnF2). Notice that oxygen and fluorine are highly electronegative atoms. It is their strong electron attracting abilities that force Kr, Xe, and Rn atoms to share their electrons and form covalent bonds. (See Answer 11 for an except question strategy.)
17. (B) The operative word in this question is diatomic. The noble gases are monatomic and so any answer choice that contains a group 18 gas is incorrect. With the exception of astatine, which is more metallic than the rest of the elements in the group, all of the halogens (group 17) are diatomic in their standard states, but only F2 and Cl2 are gases. Br2 is a liquid and I2 is a solid.
18. (C) The actual pattern of atomic size is not as regular as our general trend describing it. The transition elements present some exceptions. For example, the atomic radius of the manganese group (7) and the copper group (11) have larger atomic radii than those to the right and left of those elements. But for elements with only s and p outer elections, the atomic size decreases only from left to right.
19. (D) Ionization energy is an indicator of effective nuclear charge. (See Answer 10 for an explanation of effective nuclear charge and the figure accompanying Questions 21 and 22 for a graph of ionization energies.) The other choices are incorrect because (A) has nothing to do with effective nuclear charge, (B) and (C) are false, and (E) states the opposite effect of shielding on ionization energy. Less shielding = higher ionization energy. (Because the effective nuclear charge is larger with less shielding, the nucleus pulls more strongly on the electrons. This is evident because more energy is required to remove the electron.)
20. (D) The table lists successive ionization energies, the minimum energy requirements for the further ionization of an element (by removal of successive electrons). From the table, we see that 786 kJ of energy per mol of silicon is required to remove the first electron (a p2 electron), leaving Si+. Removing another electron (the p1) requires an additional 1,577 kJ for per mol Si+. The trick to answering this kind of question is to find a very large “jump” in ionization energies. For silicon, it’s between the fourth and fifth ionizations. This indicates that the fifth ionization energy is “digging into” the core electrons because all the valence electrons have been removed. Now we know we’re basically looking for an element with four valence electrons. The other elements in group 14 will show a similar trend in successive ionization energies, but the absolute numbers will, of course, vary from lower than those for Si (Ge, Sn, Pb) and higher for C.
21. (D) Elements of atomic numbers 2, 10, and 18 are He, Ne, and Ar, respectively. These noble gases have the highest first ionization energies. The large drop in ionization energy is mainly because the elements of atomic number 3 (Li), 11 (Na), and 19 (K) have s1 electrons that are far from the nucleus. It is both this large radius and the lower effective nuclear charge (which is one of the reasons for the large radius) that make the energy requirements for removal of this electron so low. Generally, the size of the atom indicates the strength of the nucleus’ pull on the electrons.
We need to be careful, however, as there is another way to think about this: The force of an electric field produced by a charged particle is inversely related to the square of the distance. In other words, double the distance, and the force decreases by one-fourth. The effective nuclear charge is not the only determinant of an atom’s size; for larger atoms, we must also consider that the distance between an electron and the nucleus will significantly affect the force of the pull experienced by the electron.
Electron affinity is a measure of the energy change that occurs when an electron is added to a ground state, gaseous atom. Atoms 2, 10, and 18 don’t have a high electron affinity because they have full valence shells and much energy must be added to overcome the repulsion of the electrons already in the atom (like charges repel). Atoms that have a high affinity, or attraction, for electrons have very negative (exothermic) electron affinities. The periodic trend for electron affinity in generally correlated with electronegativity, but with some important differences: (1) All the noble gases have measured electron affinity (though the lightest three have no values for electronegativity) and chlorine, not fluorine, has the highest electron affinity. (2) Electron affinity doesn’t change much within a group. (3) Electron affinity can be imagined as the reverse ionization energy of an atom’s –1 anion. For example, the energy change to remove an electron from Cl− (to produce Cl) is 349 kJ mol−1, and the energy change to add an electron to Cl to produce Cl− is –349 kJ mol−1. (See Answer 3 for more on first ionization energy.)
22. (D) Statements I and II are correct. Statement III is not entirely correct because filled orbitals are not necessarily more stable. For example, the drop between elements 7 and 8 is due to the repulsion of the second electron in the px orbital. Having one electron in each px orbital is more stable than having two electrons in one orbital and one in the other 2 (the py and pz orbitals). The information we need to answer the question is all in the graph, even if we don’t know why. All we need to do is compare the ionization energy with the electron configuration using our periodic table.
23. (C) See Answers 243 and for strategies to compare atomic and ionic radii.
24. (B) We need to look for electron configurations that have electrons missing from lower energy subshells. We need to be careful, however, because the people who write the AP Chemistry exam often list 3d before 4s, and so it looks like the 4s is the highest energy subshell if we’re not paying attention. In choice (B), the 2p subshell has only five electrons instead of six, and yet the 3s subshell is filled. This indicates that a 2p electron jumped into the 3s orbital (which was already occupied by one electron) by absorbing energy.
25. (A) Gallium is a group 13 element, meaning its one valence electron is in the px orbital.
26. (C) Carbon has two p electrons, each of which is unpaired in their respective px and py orbitals.
27. (C) We need to make sure we don’t confuse energy levels (n = 1, 2, 3, etc.) with the s, p, d, and f sublevels (subshells). Carbon has two p electrons, each of which is unpaired in their respective 2px and 2py orbitals.
28. (B) Technetium has no stable isotopes and is the atom of lowest atomic number for which that is true. Nearly all Tc is produced synthetically. Naturally occurring Tc is produced by fission in uranium or by neutron capture by molybdenum.
29. (C) Sodium, and all the group 1 alkali metals, are highly reactive in their ground state. In particular, they react with water (even in the atmosphere, which is why alkali metals are stored in oil) to form H2 gas and a strong base (NaOH in this case). (See Answer 2 for a more detailed description about the reaction of the alkali metals with water.)
30. (A) Helium. Because of the small radius, helium’s complete and stable valence shell of electrons, and its high effective nuclear charge, He has the highest first ionization energy of all the elements.
31. (D) We are looking for an atom with an incomplete lower subshell or orbital. Atom D, sodium, has two electrons in its 3s subshell, but only one electron in the 2s subshell.
32. (B) Boron is an exception to the octet rule. The mnemonic “B is happy with 3” reminds us that boron can form compounds with just three bonds joining it to the other atoms. In these compounds (like BH3), the boron atom has no lone electrons, so both the electron and molecular geometry are trigonal planar. (See Answer 62 for more on boron.)
33. (E) Nitrogen. Only count the electrons in the s and p orbitals of the highest (but same) energy level (n) as valence electrons.
34. (E) Nitrogen. The standard state form of nitrogen, N2, makes up about 78 percent (mol fraction) of the earth’s atmosphere.
35. (A) Helium. Atoms form compounds to complete their valence shell. All the noble gases have a complete valence shell, which is why they are monatomic gases under standard conditions.
36. (A) An excited hydrogen atom. We are looking for an atom with an incomplete lower subshell or orbital. Atom A has an electron in its 2s subshell, but not the 1s orbital.
37. (E) Cobalt. The cations of transition elements produce colored compounds and solutions, so we are looking for elements in which the electrons of highest energy level (which may not be the highest in value) are in d orbitals. Cobalt produces magenta or blue solutions depending on its oxidation state (II or III, respectively).
38. (C) Neon. An unreactive atom is one in which the valence shell of the atom is filled, in other words, a noble gas.
39. (D) Sodium. Any of the group 1 metals react violently with water according to the following reaction:
Na(s) + 2 H2O(l) → NaOH(aq) + H2(g)
The reaction produces a strong base whose total dissociation in water is extremely exothermic and the high temperature it produces ignites the flammable H2(g) that is produced. (See Answer 2 for more on the reaction of alkali metals with water.)
40. (D) Sodium. The atom with the highest second ionization energy is the one in which removing the first electron leaves a full valence shell behind. Choice (D), sodium, has one 3s electron. Removing that electron leaves the neon electron configuration behind (but with a higher effective nuclear charge, so the second ionization energy of Na is higher than the first ionization energy of neon).
41. (E) Cobalt. (See Answer 37.)
42. (D) Sodium. The alkali metals are highly reactive due to their low first ionization energies.
43. (B) Rutherford bombarded a thin sheet of gold foil with alpha particles (He2+, helium nuclei). Most of the He2+ passed straight through the foil, indicating that the atoms making up the foil were mostly empty space. Some of the He2+ particles were deflected from their paths, but a few actually backscattered. These deflected and backscattered He2+ particles suggested that the positive charges of an atom were concentrated into a small volume, hence the great repulsion when the He2+approached. Bohr conceived of the energy levels of electrons. Choice (C) is not true, and several scientists contributed to the fact stated in choice (D).
44. (E) With the exception of astatine, which is more metallic than the rest of the elements in the group, all of the halogens (group 17) are diatomic in their standard states. Only F2 and Cl2 are gases. Br2 is a liquid and I2 is a solid. Astatine is the rarest of the naturally occurring elements, and is therefore not usually considered with the rest of the halogens, so we don’t use astatine to make exceptions for the halogen group.
45. (C) Metals practically never take on negative oxidation states. Nonmetals can take on negative or positive oxidation states. Compared to the other elements, the halogens have high electron affinities and most often take on a –1 oxidation state (fluorine always takes on a –1 oxidation state).
46. (A) A monovalent cation is a +1 cation. The alkali metals have 1 valence electron and low first ionization energies. They exclusively form +1 cations.
47. (B) First ionization energy is the minimum amount of energy required to ionize a ground state, gaseous atom by removing an electron. The result is a cation. In general, the first ionization energy is low for metals and high for nonmetals. Helium is the element with the highest first ionization energy. (See the figure accompanying Questions 21 and 22 to compare the first ionization energies of elements 1–20).
48. (E) All naturally occurring atoms with atomic number 84 or above (polonium and higher) are radioactive. The naturally occurring actinides have atomic numbers 89–92 (actinium, thorium, protactinium, and uranium, respectively).
49. (B) Oxidation is loss of electrons (the mnemonic OIL RIG is helpful: Oxidation Is Loss of electrons, Reduction Is Gain of electrons). Generally, the first ionization energy of an atom indicates how difficult an atom is to oxidize. The noble gases have the highest first ionization energies and are the most difficult to oxidize, mostly because the valence electrons in the noble gases experience the highest effective nuclear charge in their respective periods.
50. (E) Substance 1 contains Ca2+ and Substance 2 contains Cu2+. Although we should be familiar with the flame test, the real clues to the question are in the solutions. The alkali and alkali earth metals produce colorless solutions and transition metals produce colored solutions. It’s worth memorizing that Cu2+ produces a blue solution (use the mnemonic “Copper 2 in water blue”).
Chapter 2: Chemical Bonding
51. (D) Ionic character refers to a place on the continuum of bond character. On one end of this continuum is covalent character. This is the perfectly equal sharing of electrons between atoms. This occurs only when two of the same atoms are bonded together, F2, for example. Covalent character begins to decrease as the sharing of the electrons becomes less equal. This can be predicted by comparing the electronegativities of the atoms involved in the bond. The greater the difference in electronegativity between the two atoms, the less equal the sharing of electrons is until the difference gets so large, that sharing is no longer an option. On the opposite side of the bond character continuum is ionic character. An atom with a very low electronegativity, like Cs, doesn’t exert a strong pull on its valence electron, the one involved in the bond. Fluorine has the highest electronegativity and pulls very strongly on the electrons in the bond it is a part of. If cesium is not strongly attached to its electron, fluorine is happy to take it. So Cs loses an electron to F, becoming Cs+ and F−, respectively. Now, the opposite charges on these two ions will cause them to attract each other very strongly in a bond of high ionic character.
The bottom line is that the greater the difference in the electronegativity between the two atoms, the more ionic character the bond, or the compound, has. The compounds in choices (B), (C), and (E) are all covalent compounds. Ionic compounds are typically (though not always) a metal (low electronegativity) and a nonmetal (high electronegativity). Silicon is a semimetal and aluminum is a metal. The difference in electronegativity between Al and F is about 2.4, whereas the difference in electronegativity between Si and O is about 1.5. (This still indicates some ionic character. Some people draw the line for an ionic bond as an electronegativity difference of about 2, though that number varies and can be as low as 1.7.) However, we should recognize SiO2 (silicon dioxide, also known as quartz) as a network solid, a solid in which all of the atoms are covalently bonded in a continuous network.
52. (E) Lattice energy is the minimum energy required to completely separate the ions (to gaseous form) in an ionic solid. Ionics that contain ions with the smallest radii and largest charge will have the largest lattice energy (the configuration of the ions is also a consideration, but we won’t be asked about lattice energy at that level of detail on the AP Chemistry exam). Larger charges create a greater force of attraction. For smaller ions remember that the force of the electric field produced by a charged particle is inversely related to the square of the distance from that particle. Ions with smaller radii will be closer to the ions they are attracting and will thus exert a greater force on them, so the lattice energies of the answer choices are arranged from highest lattice energy to lowest. Since chlorine is the common anion to all of them, only the size and charge of the cation is needed to answer the question.
![]()
53. (E) Only covalent compounds use prefixes such as di- and tri-. Since any binary compound containing a metal and a nonmetal is ionic, we can eliminate choices (A), (B), and (C). The key in naming this ionic is to correctly name the anion, N3–. Because it is an anion we can eliminate choice (C) (again). Since there are no oxygen atoms involved, its name can’t be something that ends in -ate (or -ite). The -ide suffix is a general suffix for a monatomic anion of any magnitude of charge.
54. (C) Bond order (BO) is the number of chemical bonds between a pair of atoms. It is part of molecular orbital (MO) theory, another model of bonding. (Lewis-dot structures and VSEPR are other systems used to model bonding and compound structures.)

The bond order does not have to be a whole number. We don’t have to work out molecular orbitals to answer a question like this. If you do not remember the formula for bond order BO = ½ (number of bonding electrons – number of antibonding electrons), there’s a much simpler way. First, we draw the Lewis structure. If there’s resonance, we will likely have a fractional bond order. In a molecule of O3, there are 18 valence electrons to account for. Either of the structures below could represent ozone.
![]()
But the real ozone molecule has equal bond lengths and strengths between the oxygen atoms.
![]()
We can think of each oxygen as being bonded to another oxygen by 1½ bonds. The pi electrons (from the unhybridized p orbitals) are shared between all the oxygen atoms instead of localizing between the two atoms directly involved with the bond.
The shortcut for calculating fractional bond orders is to take the total number of bonds in one of the Lewis structures (in this case, three) and divide it by the minimum number of single bonds that would connect the atoms in question (in this case, two). For ozone, 3 ÷ 2 = 1.5.
55. (A) N2 has a triple bond. (See Answer 54 for an explanation of bond order.)
56. (C) The ending -ide tells us that we are not dealing with an oxygen containing anions (which end in -ate or -ite, like phosphate, ![]() ), just a lone P with whatever negative oxidation state it typically carries (–3). Since Cl− has a –1 charge, we know that X carries a +2 oxidation state. Then we assign the numerical value of the oxidation state of the cation as the subscript of the anion and vice versa:
), just a lone P with whatever negative oxidation state it typically carries (–3). Since Cl− has a –1 charge, we know that X carries a +2 oxidation state. Then we assign the numerical value of the oxidation state of the cation as the subscript of the anion and vice versa:
![]()
57. (B) For diamond to become graphite, covalent bonds must be broken. Graphite is a network solid composed of sheets of carbon stacked on top of one another. Within each sheet (called graphene), the carbon atoms are covalently bonded to three other carbon atoms. The sheets are not covalently bonded to each other, however. London dispersion forces keep the sheets together in stacks.
Diamond is a network solid, too, but one in which all the carbon atoms are bonded to four other carbon atoms, so every carbon atom is covalently linked to its partners. (See Answer 94 for a comparison of the structures of diamond and graphite.)
Choices (A) and (C) are phase changes, while (D) and (E) are ionic compounds dissolving in solution.
58. (D) See Answer 57. The sublimation of carbon dioxide differs from that of graphite because there are only van der Waals forces holding the individual carbon dioxide molecules together in the solid, and only those forces are broken when CO2 sublimes.
59. (E) See Answers 243 and for descriptions of diamond structure and bonding.
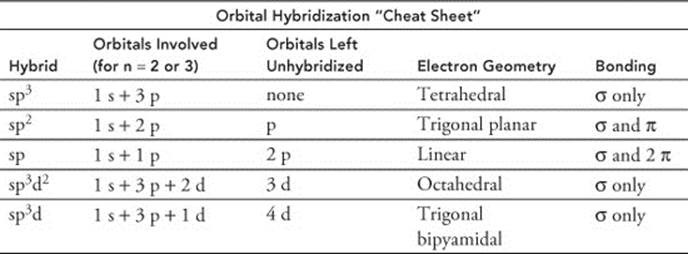
60. (C) The nitrogen in ammonia has a tetrahedral electron geometry. (See the orbital hybridization cheat sheet below Answer 59.) Since there are only three hydrogen atoms bonded to it, a single unbonded pair of electrons remains on the nitrogen atom, making the molecular geometry of ammonia trigonal pyramidal. This asymmetry greatly increases its polarity.
61. (A) Compounds with trigonal bipyramidal and octahedral electron geometries have orbital hybrids that contain one or two d orbitals, respectively. Molecules with trigonal bipyramidal molecular geometry have five atoms bound to their central atom. (Though not all molecules with five atoms bound to a central atom are trigonal bipyramidal. BrF5, for example, is square pyramidal.) Whereas compounds with octahedral molecular geometry have six atoms bound to their central atom. (See the orbital hybridization cheat sheet below Answer 59.)
Phosphorus and sulfur are important exceptions to the octet rule (and anything after chlorine on the periodic table may, but doesn’t have to, obey the octet rule). Phosphorus can have up to five electron domains (easy to remember because the “ph” in phosphorous has the f sound, like five) and sulfur can form up to six. Boron (see Answer 62) and hydrogen are two other exceptions.
62. (B) Boron is an important exception to the octet rule. We can remember the mnemonic device “B is happy with 3” because boron can form three bonds with other atoms without an unbonded electron pair on the boron, so molecules like BH3 and BF3 are trigonal planar in both electron and molecular geometry. (See the orbital hybridization cheat sheet below Answer 59.) Boron can form four bonds, but it requires one of the atoms to contribute both electrons to the bond without an electron contribution from boron (forming a coordinate covalent bond). (See Answer 334 for a description of coordinate covalent bonds.)
63. (E) The S–O bonds in sulfur dioxide have a bond order of 1.5. The sulfur atom is sp2 hybridized, but is bonded to only two other atoms, so the third hybrid orbital has a lone pair of unbonded electrons, repelling the oxygen atoms. Remember that electron domains for nonbonding electron pairs exert a greater force on neighboring electron domains than bonded electron domains. (See the orbital hybridization cheat sheet below Answer 59, and see Answer 54 for an explanation of bond order and resonance. Answer 84 explains the effect of unbonded electron pairs on bond angles.)
64. (D) The structure of carbon dioxide is linear: O=C=O. Each oxygen atom has two unbonded electron pairs. The C=O bonds are polar, but the symmetry of the molecule make it nonpolar. (See the orbital hybridization cheat sheet below Answer 59.)
65. (C) See Answer 60.
66. (C) Dipoles occur in molecules due to a nonuniform distribution of charges in the molecule. Typically, this occurs because the electron density is not equally shared between atoms. The O—H bonds in water are very polar. The electron density is greater around the oxygen atom compared with the hydrogen atom. The oxygen atom also has two unbonded pairs of electrons, which makes the water molecule bent. This makes the dipole moment of water 1.85 debye.
67. (B) Pi (π) bonds are covalent bonds involving the overlap of the two lobes of an unhybridized p orbital. The electron overlap occurs above and below the plane of the nuclei of the two atoms involved, but does not occur between the two nuclei (as in a sigma, σ, bond). Only double and triple bonds involve π bonds. All bond orders contain a σ bond. A single bond is simply a σ bond, a double bond consists of one σ bond and one π bond, and a triple bond consists of one σ and two π bonds. The molecule with the greatest number of π bonds is the one with the most double and triple bonds. C6H6, benzene, has three double bonds (although the bond order of the C—C bond in benzene is really 1.5 due to resonance and delocalized π electrons, see Answer 67 for an explanation of the bond order and resonance in benzene).
68. (B) A sigma (σ) bond is one in which the region of electron overlap between the atoms in the bond is between, and in the same plane as, the two nuclei. All bonds contain one σ bond, but double and triple bonds also contain an addition one or two pi (π) bonds, respectively. (See Answer 67 for a description of π bonds.)
69. (D) An atom that has sp hybridization will have two sp hybrid orbitals and two unhybridized p orbitals. A molecule with an sp hybridized central atom will most likely be linear, at least with respect to that part of the molecule (see the orbital hybridization cheat sheet below Answer 59). The unhybridized p orbitals don’t have to contain electrons, but if they do, they are likely to be involved with pi bonds. Since we have two unhybridized p orbitals, we can form two pi bonds, either two double bonds, or one triple bond. The carbon in CO2 is sp hybridized, which allows it to form two double bonds with each oxygen atom. (Answers 67 and 68 describe pi and sigma bonds, respectively.)
70. (E) CH2O is the empirical formula for a monosaccharide, but it also the molecular formula for formaldehyde (its systematic name is methanal). Because there is exactly one double bond (the carbonyl carbon and the oxygen), there is exactly one pi bond. (See Answer 67 for a description of pi bonds.)
71. (D) Hydrogen fluoride (HF) has the largest dipole moment of the molecules listed because the electronegativity difference between two atoms (H and F) is the largest. PH3 is a polar compound, but its dipole moment is less (0.58 μ, or debye, a coulomb meter) whereas the dipole moment of HF is a whopping 1.91 μ. The dipole moment is calculated as the product of the magnitude of the charges (or partial charges) and the distance between them. Values range from about 0–11 μ.
72. (A) Carbon monoxide, CO, has a triple bond. Double and triple bonds contain π bonds. A double bond consists of one σ (sigma) and one π bond, whereas a triple bond contains one σ and two π bonds. CO2, with its two double bonds, would have also been correct had it been an answer choice. (Answers 67 and 68 describe pi and sigma bonds, respectively.)
73. (E) A combustion reaction is a self-propagating exothermic reaction that combines oxygen with a substance and produces an oxide of the element.
74. (C) PH3 (phosphane, also called phosphine) has a tetrahedral electron geometry, but since only three hydrogen atoms are bound, there is a single unbonded pair of electrons on the phosphorous atom, making it trigonal pyramidal in molecular geometry. (See the orbital hybridization cheat sheet below Answer 59.)
75. (B) All single bonds are sigma bonds, as well as one of the bonds in a double bond and one of the bonds in a triple bond. C2H4 has six atoms, so it’s a good place to start looking for the most sigma bonds. In fact, it has five: one of each of the C—H bonds (four in all) plus one of the two bonds in the double bond between the carbons. (Answers 67 and 68 describe pi and sigma bonds, respectively.)
76. (E) Allotropes are pure forms of the same element with different structures. Atmospheric oxygen (O2) and ozone (O3) are well known allotropes of oxygen, as graphite and diamond are famous carbon allotropes.
77. (B) The carbon in CO2 is double bonded to each of the oxygen atoms.
78. (C) When looking for the compound with the greatest dipole moment, look for highly polar bonds. The H–O bond in water is the most polar bond of the compounds listed. The electronegativity difference between H–O is larger than H–N, so its dipole moment is greater. (See Answer 71 for an explanation of how dipole moments are calculated).
79. (D) See Answer 60.
80. (E) The electron geometry around the nitrogen atom in NH3 is tetrahedral (see the orbital hybridization cheat sheet below Answer 59), but because there are only three atoms bound to the nitrogen, there remains an unbonded pair of electrons. Therefore, the molecular geometry of NH3 is trigonal pyramidal. (See Answer 84 for an explanation of the effect of unbonded electron pairs on molecular geometry.)
81. (C) Ethane is an alkane (a saturated hydrocarbon) with the formula C2H6. Both carbons are sp3 hybridized. (See the orbital hybridization cheat sheet below Answer 59.)
82. (E) Hexene is an alkene with the formula C6H12. It has one C=C double bond. The carbons not involved in the double bond are sp3 hybridized. The two carbons involved in the double bond are sp2 hybridized. (See the orbital hybridization cheat sheet below Answer 59.)
83. (D) Butyne is an alkyne with the formula C4H6. It has one C≡C triple bond. The carbons not involved in the triple bond are sp3 hybridized. The two carbons involved in the triple bond are sp hybridized. (See the orbital hybridization cheat sheet below Answer 59.)
84. (C) Electron domains for nonbonding electron pairs exert a greater force on neighboring electron domains than bonded electron domains. CCl4 has both tetrahedral electron geometry and molecular geometry (see the orbital hybridization cheat sheet below Answer 59). When the molecular and electron geometries are the same, each electron domain has an atom bound to it. The 109.5° bond angle agrees perfectly with the angles representing the four corners of a tetrahedron.
The PCl3 molecule has a tetrahedral electron geometry but a trigonal pyramidal molecular geometry because there is one pair of unbonded electrons on the central phosphorous atom. The bond angles are less than they are for a tetrahedral—approximately 107° (although some have reported bond angles of around 100°, probably due to the further repulsion between the unbonded pair and the chlorine atoms).
Water has tetrahedral electron geometry but is a bent molecule because there are two unbonded electron pairs. The bond angles in the bent water molecule are 104.5°.
85. (C) The difference in electronegativity between carbon and oxygen is about 0.9 so the two C=O bonds in CO2 are polar. However, CO2 is linear and, therefore, the dipole moments of each of the bonds are 180° relative to each other, cancelling each other out and making CO2 a nonpolar molecule even though it has polar bonds.
Choices (A) and (B) can be eliminated right away because they are molecules consisting of only one kind of atom, so the bond has to be nonpolar since there is no electronegativity difference between them. C—H bonds are not very polar. The electronegativity difference between carbon and hydrogen is about 0.35. The molecule in choice (E), difluoromethane, has two polar bonds. The electronegativity difference between carbon and fluorine is about 1.4, but the electrons are not distributed evenly around the molecule (of course they are moving but they still form dipoles due to the presence of atoms with greater electron-drawing power in the molecules). This makes CH2F2 a polar molecule with polar bonds.
86. (C) This question is most easily answered by knowing the formula for the alkanes and the aldehydes and carboxylic acid functional groups. The first three compounds are all hydrocarbons. The formula for the alkanes (the series of hydrocarbons with only C—C single bonds in which every carbon is completely saturated with hydrogen) is CnH2n+2. Choice (C) is the only hydrocarbon that fits into the formula. Choice (D) is acetyladehyde. An aldehyde group is a carbon double bonded to an oxygen (C=O) that occurs on the first (or last) carbon of a compound. We can recognize an aldehydes group in a chemical formula by the appearance of CHO. Choice (E) is propanic acid. The COOH in the chemical formula indicates the presence of a carboxyl group (which contains an oxygen double bonded to a carbon, and a hydroxyl group bonded to the same carbon).
87. (D) The PCl5 molecule consists of one phosphorus atom bonded to five chlorine atoms with no unbonded electron pairs around the phosphorus (remember the mnemonic: “Phosphorus can form Five” bonds). There are five sp3d hybrid orbitals (the “averaging” of an s, three p orbitals, and one d orbital for a total of five hybrids). (See the orbital hybridization cheat sheet below Answer 59.)
88. (C) The least polar bond will contain the two atoms with the most similar electronegativities, fluorine and oxygen. The smaller the electronegativity difference between the two atoms in the bond, the more uniformly distributed the electron cloud is shared between them.
89. (A) Ozone has a bond order of 1.5, which means that instead of one single and one double bond, the central oxygen is bonded to the two outer oxygen atoms by a bond of intermediate strength and length. (See Answer 54 for an explanation of bond order and resonance in ozone.)
90. (D) Dipoles occur in molecules due to nonuniform distribution of charges in the molecule. Typically, this occurs because the electron density is not equally shared between atoms. The N—H bonds in ammonia are very polar, as the electron density is greater around the nitrogen atom compared with the hydrogen atom. The nitrogen atom also has an unbonded pair of electrons, which makes the ammonia molecule trigonal pyramidal in shape. The dipole moment of ammonia is 1.47 debye.
Chapter 3: States of Matter
91. (A) Gold (Au) is a metal, a lattice of cations bathing in a sea of electrons. (See Answer 92 to contrast metal and ionic lattices.)
92. (B) MgCl2 is an ionic lattice that contains both cations and anions. An ionic lattice has very different properties compared to the lattice in metals. A lattice describes a structure. The lattices in ionic compounds have roughly the same characteristics—alternating positive and negative ions—though the actual patterns of the lattices vary. Ionic lattices are very strong and rigid. They are not malleable or ductile, and they are poor conductors of heat and electricity because, unlike metals, the charges in ionic lattices are not free to roam. In other words, their charges are not mobile. The positive and negative ions are perfectly positioned to have maximum stability. Except for the ever-present vibration of atoms (at temperatures above 0 K), there’s nothing moving in the lattice. The lattice structure of a metal, however, has only positive charges. Like an ionic lattice, the cations in a metal are positioned very regularly, but they are not as rigid. Metals are malleable and ductile partly because their electrons are free to roam, particularly in a wire in which a current is applied, but mostly because the metal’s cations are able to take new positions relative to each other in the lattice (when a stress is applied) without breaking the metallic bonds.
93. (E) Carbon dioxide exists as individual molecules. The carbon is double bonded (a double bond consists of one σ [sigma] and one π [pi] bond; see Answers 243 and for descriptions of pi and sigma bonds, respectively) to the carbon atom (sp hybridization on the C; see the orbital hybridization cheat sheet below Answer 59). Carbon dioxide forms a solid only under high pressure and very low temperatures mainly due to slight dipoles between C and O atoms (but the symmetry of the molecule negates the polarity of the bonds) and London dispersion forces. These two types of intermolecular forces of attraction hold carbon dioxide molecules together in a solid. (See the phase diagram for carbon dioxide above Questions 99.)
94. (D) Graphite is a network solid (like quartz and diamond) that is composed of sheets of carbon. Within a sheet (called graphene), the carbon atoms are covalently bonded to three other carbon atoms, so the hybridization of carbon in graphite is sp2 (see the orbital hybridization cheat sheet below Answer 59). The electrons in the unhybridized p orbitals are delocalized; they spread out over several carbon atoms, creating a structure that can exert fairly strong London dispersion forces. This is what allows all the individual sheets to stick together. Graphite is brittle because even though the dispersion forces created by the pi electrons are strong, they are weak relative to covalent and ionic bonding, which hold most solids together under standard conditions. Graphite is remarkable in that it is a nonmetal solid that conducts electricity (due to the delocalized electrons, which are mobile). Like a metal, graphite has luster, but like a nonmetal, it’s not malleable. It is soft and flexible, but inelastic (it doesn’t reform after being deformed). Graphite and diamond are allotropes, pure forms of the same element with different structures. (See Answer 95 to compare with diamond.)
95. (C) Diamond is a network solid (like quartz and graphite). In diamond, all the carbon atoms are bonded to four other carbon atoms, so every carbon atom is covalently linked to its partner. The carbon atoms are sp3 hybridized (see the orbital hybridization cheat sheet below Answer 59), so there are no delocalized pi electrons. Diamond and graphite are allotropes, pure forms of the same element with different structures. (See Answer 94 to compare with graphite.)
96. The normal boiling point is the point at which a liquid turns to a gas at an atmospheric pressure of 1 atm. On this graph, it is the point on the line between gas and liquid phases that would meet with a line drawn perpendicular to the y-axis at 1 atm. A liquid (in an open container) boils when the vapor pressure above the liquid reaches the pressure atmosphere. Since the boiling point is determined in part by the atmospheric pressure, the normal boiling point(at 1 atm), is the boiling point at 1 atm.
97. The solid area of the graph meets directly with the gas area of the graph at low temperatures and pressures, to the left of the triple point.
98. (E) Typically, high pressure favors the formation of a solid. The negative slope of the line in the phase diagram indicates that for this compound, a decreased temperature is needed for the solid to form at higher pressures. This suggests that the solid form of this compound is less dense than the liquid form. Water is a compound whose solid is almost always less dense than the liquid, though it actually depends on the way the crystals form (there are high and very high density forms of amorphous ice, but it’s highly unlikely we’ll be asked about them for the AP Chemistry exam).
The graph does not support choice (E) because the area for solids extends well below 1 atm. The line indicates equilibrium between the two phases, but the area for solids shows that the lower the temperature, the less pressure needed. A lower temperature is needed for a high pressure solidification, but a low temperature solidification (say, –100°C) requires very little pressure. (See Answer 11 for an except question strategy.)
99. (E) At a constant pressure of 1 atm, the solid CO2 sublimes directly into a gas without going through the liquid phase. To liquefy, CO2 requires a pressure greater than 5 atm. At temperatures below –56°C approximately, CO2does not liquefy at all.
100. (B) See Answer 99.
101. (D) We should immediately recognize SiO2 as a network solid (silicon dioxide, also known as quartz). Network solids, like diamond and graphite, have high melting points. SiO2 is not a molecular formula, it is an empirical formula that represents the ratio of Si to O atoms in the compound. The melting point of SiO2 is ~1,600–1,725°C. H2S and C5H12 are both gases under standard conditions. I2 and S8 are solids but their melting points are low, relative to SiO2, at ~114°C and 115°C, respectively.
102. (D) This information is obtained directly from the graph. One kilogram of water absorbed approximately 2,300 kJ of energy to vaporize.
103. (B) Water is different in that the density of the liquid is greater than that of the solid (why ice floats). Each water molecule in solid water (ice) is connected to four other water molecules by hydrogen bonds. This spreads the molecules further apart than when they are in a liquid, where each water molecule is hydrogen bonded to two or three other water molecules at a time.
104. (C) During fusion (melting), the temperature of water doesn’t change. Use the formula q = mHfus, solving for Hfus. There are approximately 350 kJ of heat absorbed by 1 kg of water during the interval where the water is at 0°C (the temperature, or average kinetic energy, of a substance does not change while it is undergoing a phase change, see Answer 109 for an explanation).
105. (D) The 1.0 kg water absorbed approximately 2,300 kJ to vaporize.
106. (A) There is a temperature change, so we use the formula q = mcΔT and solve for c, the specific heat ∴ c = q/mΔT = 100 kJ/(1.0 kg)(50°C) = ~2.0 kJ kg−1°C−1.
107. (E) The heat of vaporization is much higher than the heat of fusion. The table below shows that an average of 1.5 mol of hydrogen bonds per mol of water are broken during fusion (4 mol H bonds per mol ice – average of 2.5 mol H bonds per mol water = 1.5 mol H bonds broken during fusion), whereas an average of 2.5 mol of hydrogen bonds are broken (per mol of water) during vaporization. Water molecules do move closer together during fusion and do move farther apart during vaporization, but this fact alone does not explain the difference in energy requirements between fusion and vaporization.

108. (B) With the information in the table above, the enthalpy of hydrogen bond formation can be calculated (it will have the same magnitude but opposite sign of the enthalpy of breaking hydrogen bonds).
Water is not forming from H2 and O2 gas, it is simply changing state, so we cannot calculate the enthalpy of formation from the data. Superheated steam is not represented in the graph so we have no data to use in a calculation. There is no time component, so we do not know the rate at which heat is being added, and there is no volume data with which to calculate density (density = mass/volume).
109. (D) The absolute temperature of a substance is proportional to the average kinetic energy (KE) of the particles in the substance. It is helpful to replace the word temperature with “average kinetic energy” when solving chemistry problems. If the temperature is increasing, the average KE of the particles is increasing. During phase changes, the temperature remains constant. Therefore, there is no change in KE. The heat added is used to change (increase) the potential energy (PE) of the particles, which are changing positions relative to each other during phase changes. The definition of zero entropy is a perfect, pure crystalline solid at 0 K (it’s also the third law of thermodynamics). Any deviation from 0 K and/or a pure, perfect crystalline solid indicates that entropy is increasing. An increase in temperature typically increases the entropy (the units of which are J mol−1 K−1). (See Answer 11 for an except question strategy.)
110. (A) Diethyl ether. A high vapor pressure indicates the particles of the liquid are not strongly attracted to each other since a low temperature (average kinetic energy) allows them to escape from solution.
111. (D) Only substance D and E can form hydrogen bonds (because of their O–H groups) but E (methanol) has a higher vapor pressure so its intermolecular forces must be weaker. All molecules exhibit London dispersion forces, but these are very weak relative to hydrogen bonds and dipole–dipole attractions, so they are not considered significant in polar molecules. The strength of London dispersion forces increases with the number of electrons, which is typically proportional to molar mass. Particles with a high molar mass (and therefore lots of electrons) will exhibit stronger London dispersion forces. Because substance D, ethanol, is larger than methanol, it exhibits greater dispersion forces and therefore has a lower vapor pressure.
112. (C) The nonpolar compounds in the table are A (diethyl ether), B (carbon disulfide) and C (carbon tetrachloride). A volatile compound readily evaporates, will have a high vapor pressure, and have weak intermolecular forces of attraction.
113. (D) Ionic compounds that contain ions with the smallest radii and largest charge will have the highest melting points (and largest lattice energies, see Answer 52 for an explanation of lattice energy). The configuration of the ions within the lattice is also a consideration, but we don’t need to be concerned with that level of detail for the AP Chemistry exam. Larger charges on the ion create a greater force of attraction, increasing the melting point. The force of the electric field produced by a charged particle is inversely related to the square of the distance from that particle, so ions with smaller radii will be closer to the ions they are attracting and will thus exert a greater force on them, thereby increasing the melting point.
114. (E) Atmospheric pressure is the column of air above a particular area. The higher the altitude, the shorter the column of air, so the lower the pressure. The pressure drop with increasing altitude is fairly linear until about 10 km above sea level, after which it drops precipitously. Therefore, the column of air is densest closest to sea level (since the weight of the column of air above the air closest to the ground is greatest, pressing all the particles closer together). The column of air above an open container pushes down on the particles in the container that are trying to escape. The greater the pressure, the greater the escape velocity required by the particles, therefore, the higher the temperature (average kinetic energy, KE) required for escape (KE = ½ mv2, where m = mass and v = velocity). Choice (A) is not correct because at the same temperature, the particles of any two substances have the same average kinetic energy.
115. (C) See Answer 114.
116. (D) Carbon dioxide exists as individual molecules. The carbon is double bonded to each of the carbon atoms in a linear molecule. Carbon dioxide forms a solid only under high pressure and very low temperatures mainly due to London dispersion forces and the slight polarity of the C and O bond (but the symmetry of the molecule mostly negates the polarity of the bonds). These two types of intermolecular forces of attraction hold carbon dioxide molecules together in a solid. Remember that phase changes are physical, not chemical, meaning that only intermolecular forces of attraction (IMFs, also called van der Waals forces) are being formed or broken.
Although deposition (and solidification) typically requires nucleation sites, that choice is not the best answer because it doesn’t address the changes in the attractive forces. (See the phase diagram for carbon dioxide above Questions 99.)
117. (A) Phase changes are physical changes, not chemical changes, so covalent and ionic bonds are not broken or formed. Only van der Waals forces (intermolecular forces of attraction, or IMFs) are being formed or broken. The density of liquid water is greater than that of solid water. Water, unlike most substances, is less dense as a solid because there are four hydrogen bonds per water molecule in ice (compared to the two to three hydrogen bonds per water molecule in liquid water) that cause the water molecules to spread farther apart from each other, forming a well-organized crystal. Because fewer molecules of water are present per volume of water in ice, the density is lower (so the solid form floats in its liquid form). (See the phase diagram for water above Questions 96, and see Answer 11 for an except question strategy.)
118. (A) The strength of London dispersion forces correlates with molar mass, but only because the number of electrons is correlated with molar mass. Helium atoms have only two electrons, so they cannot form a strong temporary dipole. Xenon atoms have 54 electrons, so they can form significant dipoles (at low temperatures). The importance of the trend in electron number and London dispersion strength is reflected in the different boiling points of He and Xe. Helium has the lowest boiling point of the elements, –269°C (a mere 4 K), while the boiling point of Xe is –108°C (165 K).
119. (B) All of the elements are nonpolar, so the only IMF to consider is London dispersion. Br2 has the highest molar mass (and the greatest number of electrons) of the choices listed, therefore it has the strongest dispersion forces. Br2 is also the only liquid among gases (under standard conditions), so that fact alone would indicate the highest boiling point (since the rest boiled at temperatures below 25°C if they are gases at room temperature). (See Answers 243 and for more on the relationship between London dispersion forces, molar mass, and number of electrons.)
120. (E) The question basically is asking us what happens during melting, when a substance is at its melting point and has already started but has not yet melted completely. The heating curve of water is shown above Questions 102. Melting occurs at 0°C and evaporation occurs at 100°C. The temperature does not change during the phase changes even though the water is still absorbing heat. These temperature plateaus are due to an increased potential energy of the substance changing phases. Temperature is the average kinetic energy of the particles in a substance, so if the temperature is not changing, neither is the average kinetic energy. Covalent bonds are typically not broken during melting, which is a physical, not a chemical, change. The volume of a substance often increases with melting, since the solid form of most substances is denser than the liquid form. Water, however, is an important exception. (See Answer 109 for a comparison of kinetic and potential energy changes that occur during heating and phase changes and Answer 117 for a comparison of the densities of liquid and solid water.)
121. (B) The gas with the greatest mass will have the greatest density since at the same temperature and pressure, equal volumes of gas contain the same number of gas particles. One mol Xe at STP occupies 22.4 L and has a mass of 131 g (131 g/22.4 L = 5.85 g/L). Helium, on the other hand, has a density of 0.18 g/L (4 g per mole/22.4 L) at STP. As long as we compare the same volumes at the same temperature and pressure, we only have to compare molar masses to compare densities.
122. (E) The absolute (Kelvin) temperature of a substance is directly proportional to the average kinetic energy (KE) of its particles. The equation for KE is ½ mv2. Since the molar mass of compound is an intrinsic property, it remains constant. Only the velocity of the particles changes when the kinetic energy changes. Mass and velocity are both proportional to KE, but inversely related to each other. A more massive gas will move slower than a lighter one at the same temperature. The molar mass of N2 is 28 g mol−1, so any gas of similar molar mass (CO) will have a similar velocity under the same conditions. (See the Answer 123 for the formula to calculate a ratio of gas speeds at the same temperature.)
123. (A) Effusion is the diffusion of a gas through a tiny hole or opening. The faster a gas particle can move, the more quickly it effuses (and diffuses). The speed of a particle of gas is related to its kinetic energy and molar mass.
Under the same conditions, the least massive gas (He, in this case) will effuse the fastest and the most massive gas (Xe, in this case) will effuse the slowest.
Absolute temperature is directly proportional to the average kinetic energy (KE) of the particles in a substance. KE = ½ mv2. For the same KE, ![]() , or, we can say that the velocity is inversely proportional to the square root of the molar mass. Graham’s law of effusion allows us to calculate the relative speeds of two different gases at the same temperature. To compare Xe and He:
, or, we can say that the velocity is inversely proportional to the square root of the molar mass. Graham’s law of effusion allows us to calculate the relative speeds of two different gases at the same temperature. To compare Xe and He:
![]()
That means that the rate of effusion of helium is 5.7 times greater than that of xenon. (See Answer 237 for another derivation of Graham’s law of effusion.)
124. (B) See Answer 123.
125. (A) Helium, which has the fewest IMFs, will require high pressures and low temperatures to liquefy. It has the lowest boiling point of all the elements, 4 K. (See Answer 118 for a comparison of the boiling points of the noble gases.)
126. (B) Since all the gases listed are nonpolar, the only significant IMF they can form is London dispersion. The gas with the greatest number of electrons (the most massive gas) will generate the greatest London dispersion forces under the same conditions. Helium, then, will have the lowest boiling point and require the most pressure to condense into a liquid. (See Answer 118 for more on the relationship between London dispersion forces, molar mass, and number of electrons.) Helium has the lowest boiling point of the elements, –269°C at 1 atm (a mere 4 K).
127. (B) The highest condensation temperature is also the highest boiling point, so we are looking for the gas capable of forming the strongest IMFs. A pressure of 10 atm is very high (for comparison, the air pressure in car tires is about 2 atm), but high pressure makes IMFs more likely to form, and therefore helps the gas to condense. The boiling point of Xe is –108°C at 1 atm (165 K). (See Answer 118 for more on the relationship between London dispersion forces, molar mass and number of electrons.)
128. (D) An ideal gas is an imaginary gas whose behavior with regards to temperature, pressure, and volume is completely described by the ideal gas equation, PV = nRT (it may be helpful to remember the name “pivnert”). Actual measurements of T, P, and V of real gases vary (very) slightly from the predictions made by the ideal gas law. These real gases deviate mainly because of the forces of attraction and repulsion between the particles. Low pressures maintain a low density of the gas, so particles are far enough apart that attractive and repulsive forces aren’t felt, and high temperatures overcome forces of attraction and repulsion. With these forces of attraction and repulsion minimized, real gases behave quite ideally.
129. (D) The two gases are at the same temperature and pressure, so equal volumes of the two gases will contain the same number of particles, but there are more CO2 molecules (and therefore a larger volume) than O2 molecules. CO2 is more massive than O2, and there are a greater number of particles, so the masses of the two gases are certainly not the same. The density of two gases at the same temperature and pressure can be compared simply by comparing their molar masses. CO2 is more massive than O2, at 298 K and 1 atm, the CO2 gas is denser. If they are at the same temperature, their average KE is the same, but CO2 is more massive and so the average velocity of the particles is less than that of the O2 particles. Two gases at the same temperature will have the same average kinetic energy, but the more massive gas will have, on average, slower moving particles. (See Answer 123 for an explanation of KE and average molecular, as well as Graham’s law, a simple formula to calculate the relative speeds of two gases.)
130. (C) Two gases at the same temperature will have the same average kinetic energy, but the larger gas will have, on average, slower moving particles. Since these two gases are equally massive (44 g mol−1), their particles will have the same average speed. The N2O(g) sample has more moles so it has more particles, and it will occupy a greater volume than CO2 at the same temperature and pressure. Notice density was not given as a choice. Gases of the same molar mass at the same temperature and pressure have the same densities. (See related Question and Answer 129.)
131. (D) This is a simple conversion. Thirty-two grams of O2 is one mole of O2. At 1 atm and 298 K, this number of oxygen molecules would occupy 22.4 L, but the pressure is four times. However, temperature and pressure are directly proportional, so a fourfold increase in pressure would be accompanied by a fourfold increase in temperature (298 × 4 = 950).
We will arrive at the same answer using the ideal gas law (PV = nRT, known as pivnert). Or, since we know there is 1.0 mole of gas (and at 298 K and 1 atm, it will occupy 22.4 L), we can use Gay–Lussac’s law: P1/T1 = P2/T2, 1 atm/298 K = 4 atm/T2 ∴ T2 = 950 K. Remember to only use the Kelvin (absolute) temperature scale when dealing with gases because there are no negative numbers.
132. (B) Rearranging PV = nRT for P gives P = nRT/V. Choice (E) is incorrect because the numerical value of the gas constant depends on the units. The number 0.0821 is used when P is measured in atm. The value 8.314 is used when P is given in kPa (kilopascals). Remember to only use the Kelvin (absolute) temperature scale when dealing with gases (because there are no negative numbers).
133. (B) The total initial pressure in the container is the sum of the partial pressures of all the gases present (Dalton’s law of partial pressures), so the total Pinitial = 1.2 + 3.8 = 5 atm. Since the pressure is proportional only to number of particles at a given volume and temperature, we know that there are three times more H2 molecules than N2 molecules (1.2 × 3 = 3.8). This ratio of H2 to N2 is exactly the same as the ratio of coefficients in the balanced equation, so this is not complicated. When the partial pressure of N2 falls to 0.9 atm, that means one-fourth of the N2 has been consumed by the reaction (0.3 is 25 percent of 1.2). If two NH3 are formed for every N2 molecule that is consumed, then 0.6 atm (0.3 × 2) of NH3 will be formed. The important thing to remember is that the partial pressures of the gases in a mixture tells us the relative number of particles of each gas in the mixture.
134. (E) Sulfur dioxide, SO2, has bonds of high polarity and has a bent molecular geometry, making it very polar and therefore subject to dipole–dipole IMFs and of course, London dispersion forces. Ideal gases are the imaginary gases in which the particles experience no forces of attraction or repulsion. (See Answer 128 for a definition of ideal gases and the conditions under which real gases behave most like ideal gases.)
135. (D) See Answer 128.
136. (B) Dalton’s law of partial pressure states that the pressure exerted by a specific gas within a mixture is proportional to the mole fraction of that gas. The trick to answering this question is realizing that if the equal masses of neon and argon are in the container, then twice the number of neon atoms are present (since the molar mass of argon is twice that of neon). Since we only need to be concerned about mol fraction and no other information is given, let’s simply assume we have 1 mole Ar (40 g) and 2 moles Ne (40 g). That means there’s a total of 3 mol of gas, of which one-third are Ar atoms and two-thirds are Ne atoms. If the total pressure is 1.2 atm, one-third of that pressure, or 0.4 atm, is due to Ar and 0.8 atm is due to Ne. It doesn’t matter what actual number of mole we use, only the ratio between Ar and Ne. As long as there are twice as many Ne atoms as Ar atoms in our calculations, our answer will be the same (and correct).
137. (E) Dalton’s law of partial pressure states that the pressure exerted by a specific gas within a mixture is proportional to the mole fraction of that gas. The total number of moles of gas is 0.5 + 1 + 1 = 2.5 mol. The pressure exerted by each mole of gas = 750 mmHg/2.5 mol = 300 mmHg per mole gas. If a half mole of SO2(g) is present, then half of 300 mmHg, or 150 mmHg, of pressure is exerted. We will arrive at the same answer by calculating the mole fraction of SO2(g) (0.5 mol/2.5 mol = 0.2) and multiplying it by the total pressure (0.2 × 750 mmHg = 150 mmHg).
138. (D) A 2-L container will hold approximately 0.1 mole of gas at STP. The molar mass of O2 is 32 g mol−1 ∴ 0.1 mol = ~3 g.
139. (C) The 2-L flask holds a total of three mole gases at a pressure of 800 mmHg. Dalton’s law of partial pressure states that the pressure exerted by a specific gas within a mixture is proportional to the mole fraction of that gas. The pressure of each gas can be calculated as follows. We can easily check our work because the sum of the partial pressures should equal the total pressure.
140. (D) This is a Graham’s law of effusion problem. The two gases are released at opposite sides of a tube and if they diffused at the same rate, they would meet in the middle. The speed at which a gas diffuses at a given temperature is inversely proportional to the square root of its molar mass (see Answer 123 for a derivation of Graham’s law). The larger mass of HCl makes it diffuse more slowly than NH3 by the following equation rate NH3/rate HCl= ![]() diffuses 1.5 times as quickly, therefore in the same period of time, NH3 will travel 1.5 times as much distance.
diffuses 1.5 times as quickly, therefore in the same period of time, NH3 will travel 1.5 times as much distance.
If distance HCl travels = x, the distance NH3 travels = 1.5x.
Total distance = 1x + 1.5x = 2.5x = 100 cm ∴ x = 40 cm and 1.5x = 60 cm.
141. (D) A 2-L container will hold approximately 0.1 mole gas at STP. The molar mass of Cl2 is 71 g mol−1 ∴ 0.1 mole = ~7 g (Questions 138 is similar).
142. (A) This question is asking us to identify the gas that is least soluble in water, and therefore if collected above water, will produce a high yield because the least amount will be dissolved into the water (and therefore not collected).
143. (D) It is sometimes useful to imagine the atmosphere as a very thin, light fluid. The rubber duck in a bathtub floats because the average density of the duck is less than the density of the water as is the average density of a luxury ocean liner. A submarine, on the other hand, can change its average density to sink or float.
144. (D) The term constant temperature is our clue that the average kinetic energy of the particles will be the same. Although the speed of the particles is related to their kinetic energy, so is their mass. At the same temperature, more massive gases will move with slower speed. Since all these particles are the same, their speed remains the same at the same temperature.
145. (A) The speed at which a gas diffuses (or effuses through a tiny hole) is inversely proportional to the square root of its molar mass (see Answer 123 for a derivation of Graham’s law). Therefore, Ar will effuse out of the container the fastest, leaving the least number of Ar particles behind, and therefore exerting the least partial pressure. Kr is of intermediate mass between Ar and Xe and so will effuse at an intermediate speed. Kr will effuse the slowest leaving the greatest number of particles in the container and therefore having the highest partial pressure.
146. (B) The speed at which a gas diffuses (or effuses through a tiny hole) is inversely proportional to the square root of its molar mass (see Answer 123 for a derivation of Graham’s law). The lightest gas in the list is H2, so at the same temperature (average kinetic energy), H2 will move the fastest.
147. (A) The sodium carbonate reacts with hydrochloric acid according to the equation:
Na2CO3(aq) + 2 HCl(aq) → 2 NaCl(aq) + CO2(g) + H2O(l)
0.250 L HCl × 2.5 mol/L = 0.63 mole HCl
10.6 g Na2CO3 × 1 mol/106 g = 0.10 mole Na2CO3
Remember to check for limiting reagents if the amounts of two reactants are given. A simple check is to take the number of moles of each reactant and divide it by its stoichiometric coefficient in the balanced equation.
0.63 mole HCl ÷ 2 ÷ 0.325
0.10 mole Na2CO3 ÷ 1 = 0.10 (limiting reactant)
Since there is a 1:1 ratio between Na2CO3 consumption and CO2 formation, 0.11 mole CO2 is the theoretical yield.
148. (A) Carbon dioxide, CO2(g), is relatively nonpolar, so not a great deal of it dissolves, but a small fraction does (from about 3.5 g CO2 per kg water at 0°C to 0.5 g CO2 per kg water at 60°C at sea level). Importantly, CO2 reacts with water to form carbonic acid, a weak acid, according to the equation.
![]()
(Our lives depend on this reaction. CO2 is transported in the blood mainly as HCO3−, and the regulation of our breathing relies on it. The reaction occurs in red blood cells with the help of the enzyme carbonic anhydrase.)
149. (D) Any gas collected over water will be a mixture of at least two gases, the gas (or gases) produced in the reaction and water vapor from the eudiometer. Just remember Dalton’s law of partial pressures, the total pressure of a mixture of gases is the sum of the partial pressures of its constituent gases. The partial pressure of each gas is determined solely by its mole fraction in the mixture.
Because we don’t know the mole fraction of either gas, we determine it by the pressure. We start with the partial pressure of the water and we use a handy fact: The vapor pressure of water is determined solely by the temperature. If the vapor pressure of water at 22°C is about 20 mmHg (this information would be provided), we can then deduce the pressure of the gas in our mixture.
The total pressure of the gas in the eudiometer is the atmospheric pressure in the lab, which was given as:
760 – 20 = 740 mmHg of CO2(g). Then we use the ideal gas law, PV = nRT, to calculate the number of moles of CO2.
150. (D) First start by calculating the number of moles of each gas:
1.6 g He = 0.4 mole, 4 g Ar = 0.1 mole, 26 g Xe = 0.2 mole
Total number of moles = 0.7
P = 2.1 atm ∴ 0.3 atm per 0.1 mole of gas
0.2 mole Xe ∴ 0.6 atm of pressure
We can also find the mole fraction of Xe and multiplying it by the total pressure:
0.2 mol Xe/0.7 total mol = 0.29 = ~0.3
0.3 × 2.1 atm = 0.63 = ~0.6 atm
Chapter 4: Solutions
151. (A) Raoult’s law states that the vapor pressure of a solution of a nonvolatile solute is equal to product of the vapor pressure of the pure solvent and its mole fraction. A solution will have a lower vapor pressure than the pure solvent and therefore a higher boiling point because a liquid or solution boils when the vapor pressure above the liquid reaches the pressure of the atmosphere. Solutes will also depress the freezing point and increase the osmotic pressure in direct proportion to their concentration. (See Answer 459 for the formula to calculate the DT of boiling points or freezing points of solutions.)
152. (A) Most substances undergo a change in density (and volume) with a change in temperature. Mass, however, doesn’t change with temperature. Molality (m) is the concentration expressed in mole per kilogram solvent, a unit of mass. Molarity (M) is a unit of concentration expressed in mole per liter, a volume.
153. (C) We need to convert mL to L to solve for number of mole from molarity, mol/L.
0.125 × (0.2 mol/L) = 0.025 mol CuSO4. 5 H2O.
0.025 mol × (250 g/mol) = 6.25 g
154. (B) This is a dilution problem. Use M1V1 = M2V2
MHClVHCl = M0.8MsolV0.8Msol
(20) (5) = (0.8) (V0.8Msol)
V0.8Msol = 125 mL
But this is the final volume of the solution. The question asked how much distilled water must be added. We must subtract the 20 mL of 5 M HCl from the final volume to calculate the volume of water added:
125 mlFinalSol – 20 mLHCl = 105 mLwater
155. (E) The lead nitrate and sodium chloride react according to the equation:
Pb(NO3)2 + 2 NaCl → PbCl2(s) + 2 NaNO3(aq)
0.100 L Pb(NO3)2 × (0.2 mol/ L) = 0.02 mol Pb2
0.100 L NaCl (0.3 mol/L) = 0.03 mol mol Cl−
Since two Cl− are needed for each Pb2+, Cl− is our limiting reactant (remember the simple check: divide the number of mole of each reactant by its stoichiometric coefficient in the balanced equation, the smallest quotient is the limiting reactant) and we’ll have excess Pb2+ ions in solution.
0.03 mole Cl− × (1 mol Pb2+/2 mol Cl) = 0.015 mole of Pb2+, ions precipitated out with the chloride ions
0.02 mole Pb+2 – 0.015 mole Pb+2 precipitated = 0.005 mole Pb2+
0.005 mole Pb2+ in a final volume (don’t forget to add the volumes of both solutions) of 200 mL (or 0.2 L) = 0.025 M.
156. (E) The two solutions were prepared with the same number of moles of their respective compounds, so the determinant of electrical conductivity is the concentration of ions of their solutions. A particle that does not dissociate into ions when dissolved does not conduct electricity. The higher the ion concentration of a solution, the greater its electrical conductivity. Ions (or charges) must be mobile in order to conduct electricity, so solid salts do not conduct electricity (but molten salts do). Soluble salts and strong acids and bases are the best electrolytes because they completely dissociate in water, producing at least 2 mole ions per mole compound.
157. (C) Since we’re given a percent and not an absolute mass, assume a 100 g sample of solution ∴ 66% C2H4O 66 g C2H4O = 1.5 moles.
100 g of sample – 66 g C2H4O 34 g of water, or about 1.9 (round to 2) mole water. Total moles = 3 ∴ 1.5 mol ethanol/3 mol total 50%.
158. (A) There are 2 moles of ethanol in a total of 10 mol solution (20 percent).
144 g H2O @ (18 g mol−1) = 8 moles
92 g ethanol @ (46 g mol−1) = 2 moles
159. (D) 29 g NaCl @ (58 g mol−1) = 0.5 mole NaCl
0.5 mol NaCl/0.2 kg solvent = 2.5 m solution
160. (D) Phosphates are not particularly soluble. All nitrate and ammonium salts are soluble, as well as all group 1 salts. Acetate is also very soluble.
161. (C) The addition of HF, a weak acid, will lower the pH by increasing the H+ concentration in the solution. The increased H+ concentration will shift the equilibrium of the BaF2 to favor its dissociation (and therefore its solubility) by providing the F− already in the solution with more H+ ions to bind to form HF. F− is a good conjugate base, so it will take up many of the H+ ions. The H+ and F− ions in the acid are already at equilibrium, so adding them to the BaF2 solution will not increase the F− concentration when it is added, as much as it will increase the H+ concentration. This is because the F− that is being added will bond to the H+ ions to form HF, further lowering the F− concentration, and further pulling the solubility equilibrium to the right, favoring dissociation and increased solubility. By the time the two solutions have completely mixed and a new equilibrium is achieved, the final F−concentration is lower, allowing more Ba2+ ions to be dissolved.
162. (D) The formation of hydrogen bonds is exothermic.
163. (A) Assume 100 mL of each liquid.
100 mL ethano × 0.79 g/mL = 79 g ethanol @ 46 g mol−1 = 1.7 moles
100 mL water × 1.0 g/mL = 100 g H2O @ 18 g mol−1 = 5.5 moles
Total mol solution = 1.7 + 5.5 = 7.2 moles
1.7 mol ethanol/7.2 mol total = 0.24
164. (A) Different proportions of solute and solvent can produce different enthalpy changes, but the solvation of ethanol and water is unusual in that it starts out exothermic at low concentrations of water, changes to endothermic in the mid-range, and then reverts back to exothermic at high concentrations of water. Solutions are complex, but we can still arrive at a fairly simple but logical interpretation of this situation. If a particular solvation process is exothermic, then more (or “stronger”) intermolecular forces of attraction (IMFs) are formed than broken (IMF formation is exothermic). If it is endothermic, then more (or stronger) IMFs are broken than formed (IMF “breakage” is endothermic).
165. (D) Any particle with electrons will exhibit some amount of London dispersion forces, so our answer must contain choice III. The name ethanol lets us know the compound is an alcohol, but the —OH group is obvious from the chemical formula given at the beginning of the question set. Water, of course, has two O–H bonds. Molecules with O—H, N—H, and F—H bonds can form hydrogen bonds. Hydrogen bonding is the strongest of the intermolecular forces of attraction (technically, hydrogen bonding is a special case of dipole–dipole attraction). The C—H bonds in ethanol are fairly nonpolar, so there are no dipole–dipole forces holding these two molecules together.
166. (C) First, we need to determine the number of moles of Cl− ions in the solution. There are 0.1 mole from KCl, 0.2 mole from CaCl2, and 0.3 mole from AlCl3 ∴ 0.6 mole Cl− in 1-L solution. Because each Pb2+ ion can precipitate 2 Cl− ions, 0.6 ÷ 2 = 0.3, the minimum number of moles of Pb2+ ions needed.
167. (B) The solubility of gases in water is greatest at high pressures and low temperatures.
168. (C) Water soluble salts are not soluble in nonpolar solvents, like CCl4. Salts dissolve by dissociating. Their ions form interactions with solvent molecules that have polar groups on them (ion-molecule attractions). A Na+, for example, will be attracted to the partially negatively charged oxygen atom in an O—H bond (of water, for example), whereas the Cl− ion will be attracted to the partially positively charged hydrogen atom of the O—H bond.
169. (C) We are looking for the compound whose solubility is drastically reduced by a reduction in temperature. If this data were presented in a graph, we’d be looking for the solubility curve with the steepest slope within the temperatures given in the question.
170. (B) The Ag+ is not soluble as a chloride or a sulfate, but it is soluble as a nitrate.
171. (A) The chloride from NaCl forms an insoluble precipitate with Ag+ (AgCl).
172. (E) First, we calculate the number of moles of NaOH (and OH−):
60 mL × 0.4 M = 24, but the unit M is mol L−1, so we need to move the decimal over three places to account for 1,000 mL per liter ∴ 0.024 mol NaOH (or, convert to L before doing the calculation) ∴ 0.024 mole OH− (only one OH−per NaOH).
Next we calculate the Ba (OH)2 (and OH−): 40 × 0.6 M = 0.024 mol BaOH2 ∴ 0.048 mole OH− (2 moles OH− per mole BaOH2).
There are a total of 0.072 moles OH− (0.024 + 0.048) in 100 mL (0.1 L, add the volumes) so the molarity is 0.72 M.
We can use a variation on M1V1 = M2V2 to solve this problem, but we need to remember that when mixing solutions, we must add their volumes.
M1V1 + M2V2 = M3V3
(0.06) (0.4) + 2 (0.04) (0.6) = M3 (0.1)
173. (C) The first solution, a mixture of CuCl2 and MgSO4, is totally soluble. That not only tells us that CuCl2 and MgSO4 are soluble, but the products of the double replacement reaction between them are also soluble. That would be CuSO4 and MgCl2.
When the student then mixes Al2(SO4)3 and CuF2, however, a precipitate forms. We assume Al2(SO4)3 and CuF2 are soluble because they were combined as solutions, but one of the products of the double replacement is obviously not soluble. AlF3 and CuSO4 are the products. We may have our solubility rules memorized, but for this particular question, we don’t need them. The first solution made by the student already tells us that CuSO4 is soluble, so we must conclude it’s the AlF3.
The important thing to remember for this question is about making sure the charges on our ions are correct so we can predict the formulas of the new compounds. The oxidation state of copper is obvious, all the alkali earth metals take on a +2 oxidation state, and all the halogens take on a –1 oxidation state, but we’d have to have memorized that Al always takes on a +3 oxidation state, and that the sulfate ion, SO42–, has a –2 charge.
A trick for remembering the oxidation states of Ag, Zn, and Al: they are connected diagonally on the periodic table, and as we progress from Ag to Zn to Al, the oxidation states are +1, +2, and +3. Many transition metals take on more than one oxidation state, but Ag and Zn are two important exceptions. So much so that the parentheses that indicate the oxidation states of transition metals in ionic compounds are not used for compounds with Ag and Zn.
174. (D) Ideal solutions are like ideal gases, they don’t exist. However, they are useful imaginary models for predicting the properties of real solutions (and gases). An ideal solution is one in which each of the particles in solution is subject to the same forces it would be in its pure state. In other words, the different molecules present in an ideal solution have no greater or lesser attractions for the other molecules in the solution as they do for their own kind. There are some assumptions about ideal solutions we should be familiar with: (1) The volume of the solution is purely the sum of the volumes combined. There is no expansion or contraction upon mixing. (2) The heat of solution is zero. It’s neither exothermic nor endothermic. (3) All the components of the solution obey Raoult’s law (the vapor pressure of each component of the solution is proportional to the mole fraction of that component).
The vapor pressure above an ideal solution is ideal, too—the total pressure is the sum of the partial pressures of the constituent gases (they obey Dalton’s law of partial pressures).
The pairs of liquids in choices (C) and (E) are not miscible so we can eliminate them immediately. Choice (A) is incorrect because HCl has a fairly exothermic dissolution (violates assumption No. 2) and it also dissociates into ions, which form ion-molecule attractions with water instead of hydrogen bonds (violates assumption No. 1). Finally, HCl is a gas under standard conditions and this is fairly volatile when in a solution. Choice (B) is tempting because it is an alcohol that can hydrogen bond with water, however the CH3CH2– group is nonpolar and would have a greater attraction for other CH3CH2OH molecules than it would for water. It’s a tough choice, but overall, the pairs of liquids in choice (D) are more similar than the pairs of liquids in choice (B).
175. (D) The most direct and efficient method to determine the molarity of the solution is to measure the mass and volume of a sample of the solution (or, the whole solution). We need two pieces of information to get the unit of molarity (M), mol and L. If a solution contains 10 percent hexane by mass, let’s assume we have a 100 g sample. That’s 10 g, or 0.12 mole, of hexane. The volume of the sample will then allow us to calculate molarity. To make sure we answer a question like this correctly, we can try imagining the situation. We’ll find with this problem that we must know the mass of the sample to calculate the number of moles of solute. Once we’ve brought in the assumption of a 100-gram sample, we’ve admitted that a mass is needed.
176. (A) The trick in this question is in the units of glucose—milligrams. The obvious mistake is assuming we have 2 moles of glucose: 360/180, but we really have 2/1,000 mole glucose, or 0.002 mole, dissolved in 200 mL of water, or 0.2 L. The final molarity of the solution is 0.002/0.2 = 0.01 M solution. Because glucose is very soluble and the solution is homogenous, any sized sample will have the same concentration.
177. (C) The solution with the highest boiling point will be the one with the greatest number of particles. A simple calculation is to multiply (m) × (i) (i = the van Hoff factor, the ratio of the number of particles a compound produces when dissolved versus the number of particles of compound added).
178. (C) To solve this problem, we must remember Raoult’s law: The vapor pressure of an ideal solution (see Answer 174 for an explanation of ideal solution) is dependent on the vapor pressure of each component and the mole fraction of the each component. Having 2 moles propylene glycol and 8 moles of water means that out of the 10 moles of total solution, 20 percent is propylene glycol and 80 percent is water. The effect of the solute (propylene glycol) is to lower the vapor pressure of the water because the solute is nonvola-tile, that is, it doesn’t readily vaporize. If the solution is 20 percent nonvolatile solute, then the vapor pressure of the solution will be 20 percent lower than the vapor pressure of pure water. 20 percent of 20 mmHg is 4 ∴ 20 – 4 = 16 mmHg.
179. (C) The precipitate that formed after the addition of HCl indicates that the solution contained an ion that was insoluble as a chloride. Ag+, Pb2+, and Hg2+ immediately spring to mind. Out of those, only Ag+ forms a soluble complex ion with NH3, Ag(NH3)2+. The ion that remained in solution must have been soluble in chloride but insoluble as a sulfate. Out of the ions that are not soluble as a sulfate, Ba2+, Ca2+ (not an answer choice), Pb2+, and Ag+, Ag+, and Pb2+ are not soluble as chlorides and would have already precipitated (as Ag+ did).
180. (B) All ammonium and nitrate salts are soluble, as are all the group 1 salts. That leaves BaCO3 as the insoluble compound.
Chapter 5: Chemical Reactions
181. (E) H2O is 18 g mol−1, so 180 g water is 10 moles of water, one order of magnitude greater than 1 mole (6.02 × 1023 molecules) ∴ 6.02 × 1024 molecules.
182. (B) Carbon dioxide (CO2) has a molar mass of 44 g mol−1, so 4.4 g = 0.1 mole (6.02 × 1022 CO2 molecules). Each CO2 molecule contains two oxygen atoms, so there are 0.2 mole oxygen atoms or (0.2) × (6.02 × 1023) = 1.2 × 1023 atoms of oxygen.
183. (A) Ribose has a molar mass of 150 g mol−1, so 1.5 g = 0.01 mole ribose (6.02 × 1023 ribose molecules). Each ribose has 10 hydrogen atoms, so there are 0.01 × 10 = 0.1 mole hydrogen atoms (6.02 × 1022 hydrogen atoms), one order of magnitude less than 1 mole (6.02 × 1023 molecules).
184. (E) The compound is Ti(CO)6, titanium hexacarbonyl.
Assume a 100-g sample to convert percent to number of grams. Then convert grams to moles and find the simplest, whole number mole ratio between them.
22.2 g Ti @ 48 g mol−1 = ~0.5 moles Ti ÷ 0.5 = 1
33.3 g C @ 12 g mol−1 = ~3 moles ÷ 0.5 = 6
44.4 g O @ 16 g mol−1 = ~3 moles ÷ 0.5 = 6
Remember to use the mass of atomic oxygen (16 g mol−1) when calculating its percent in a compound.
185. (A) Assume a 100 g sample, convert to moles and find the simplest, whole number mole ratio between them.
92 g C × (12 g mol−1) = ~8 moles
8 g H × (1 g mol−1) = ~8 moles
There is a 1:1 ratio of carbon-to-hydrogen atoms, so CH is the empirical formula.
186. (C) The molecular formula of a compound is a whole number multiple of its empirical formula. If we calculate the molar mass of each of the answer choices, CHO = 29 g mol−1, C2H3O = 43 g mol−1, CH2O = 30 g mol−1, and CH2O2 = 46 g mol−1. CH2O, at 30 g mol−1, is the only choice that 150 g mol−1 can divide into without a remainder (5 x, it is also the empirical formula for the monosaccharides, a class of carbohydrates). Choice (E) is not an empirical formula, it is the molecular formula of ribose (CH2O × 5 = C5H10O5).
187. (E) We’re already given the number of mole of each element, so we only need to find the simplest, whole number ratio between them.
0.2 mole Pd ÷ 0.2 = 1
0.8 mole ÷ 0.2 = 4
1.2 moles H ÷ 0.2 = 6
0.8 mole O ÷ 0.2 = 4 ∴ PdC4H6O4, but we can factor 2 from the subscripts on the C, H, and O (the nonmetals which are forming a complex around the Pd cation) to Pd(C2H3O2)2, palladium acetate. The formula Pd(C2H3O2)2 is much more structurally informative than PdC4H6O4 because it highlights the fact that there are two acetate ions attached to each Pd2+ ion.
188. (D) Assume a 100-g sample, convert to mole and find the simplest, whole number mole ratio between them.
38 g F @ 19 g mol−1 = 2 moles ÷ 0.5 = 4
62 g Xe @ 131 g mol−1 = 0.5 mole ÷ 0.5 = 1 ∴ XeF4
189. (B) If a hydrocarbon is 75 percent carbon, it must be 25 percent hydrogen (100% – 75%). Assume a 100-g sample, convert to mole and find the simplest, whole number molar ratio between them.
75 g C @ 12 g mol−1 = 6.25 moles ÷ 6.25 = 1
25 g H @ 1 g mol−1 = 25 moles ÷ 6.25 = 4 ∴ CH4
190. (B) The excess H2 tells us that this is not a limiting reactant problem. The molar mass of Cu2O is 143 g mol−1 but that piece of information is not needed to solve the problem. It is a red herring. What we want to know is that 0.05 mol Cu2O has 0.1 mol Cu, since there are 2 moles Cu atoms per mole of Cu2O. The molar mass of Cu is 63.5 g mol−1, so 0.1 mole weighs 6.35 g.
191. (D) A coordination complex consists of an atom or ion surrounded by an array of ions or molecules (called ligands). The central atom or ion is typically a metal (most often a transition metal) and the complex it creates is often charged, which is a clue for identifying the complex. In choice (D), Pt is the central metal and the chloride ions are its ligands.
192. (E) Chlorine has an oxidation state of 0 in Cl2, –1 in Cl− and +5 in ClO−.
193. (A) An acid and a base are the reactants of a neutralization reaction. A salt and water are the products.
194. (B) A precipitation reaction produces a solid (if the state of matter of the products is not specified, look for an insoluble compound).
195. (C) Combustion reactions consume O2 as a reactant and produce oxides.
196. (A) An acidic salt is formed when a strong acid reacts with a weak base. Weak bases form good conjugate acids, whereas strong acids form weak conjugate bases. For example, if Cl− was able to pick up H+ in solution, then HCl wouldn’t be a strong acid because it wouldn’t fully dissociate in solution. The Cl− would pick up H+ ions and an equilibrium between HCl and H+ + Cl− would be reached. The equilibrium of a strong acid/base ionization isn’t a true equilibrium. The reaction goes to completion. The concentrations of products and reactants don’t change, but only because the reverse reaction doesn’t occur, not because the forward and reverse reactions occur at equal rates. In fact, the concentration of reactant in strong acid or base dissociations is practically zero.
197. (E) Solid aluminum is not oxidized during this reaction. (See Answer 11 for an except question strategy.)
Important Note: Questions like this will typically not appear in the multiple choice section of the exam. The free response section, however, has a mandatory “Reactions” section that requires test takers to write out a balanced equation and answer a question about it.
198. (B) Sodium carbonate is not produced by the heating of sodium bicarbonate. (See important note following Answer 197.)
199. (A) The compound P4O3 does not form under the conditions in question. It is not a naturally occurring oxide of phosphorus. (See important note following Answer 197.)
200. (A) Choices (B) through (D) contain the spectator ions of the total ionic reaction. Choice (E) involves the formation of HI, a strong acid, which is not produced in this reaction. (See important note following Answer 197.)
201. (E) HCl(aq) and KOH(aq) = The exothermic neutralization would increase the temperature of the reaction vessel.
CaCO3(aq) and HF(aq) = Bubbling from the carbon dioxide gas would be observed.
Mg(s) and HI(aq) = Bubbling of the hydrogen gas would be observed.
Pb(NO3)2(aq) and NaCl(aq) form an insoluble precipitate (PbCl2).
The mixing of NH4NO3(aq) + HCl(aq) solutions is not very exciting. They are both very soluble in water and a double replacement switch produces no insoluble compounds. Nitrate (NO3−) and chloride (Cl−) are poor conjugate bases and will not pick up protons in solution to form HNO3 or HCl, two strong acids (see Answer 196 for an explanation of why strong acids form the weakest conjugate bases). Ammonium chloride is a completely soluble salt. (See Answer 11 for an except question strategy.)
202. (C) A combustion reaction is a vigorous, exothermic, self-sustaining reaction in which the substances combine to give off heat and light. The combustion reactions we are most familiar with use an oxidizing agent (like O2). Choice (E) is an oxidation, but the oxidizing agent in not represented, it is an electrochemical half-reaction.
203. (B) It’s the only reaction that produces an ion, ![]()
204. (E) Remember OIL RIG: Oxidation Is Loss of electrons, Reduction Is Gain of electrons.
205. (A) Carbonates form CO2 gas when acidified according to the equilibrium equation:
![]()
206. (D) When balancing the combustion of carbon-containing compounds, balance carbon atoms first, hydrogen atoms second, and oxygen atoms last (remember Mrs. Cho, she’ll help you balance combustion reactions).
![]()
207. (C) When balancing the combustion of carbon containing compounds, balance carbon atoms first, hydrogen atoms second, and oxygen atoms last (remember Mrs. Cho, she’ll help you balance combustion reactions).
![]()
208. (C) This reaction can be quickly balanced by noticing that the H2O on the left side of the arrow has the hydrogen and oxygen atoms that will be distributed of NH3 and OH−. On the right side, we can see that each water molecule breaks up into a hydrogen ion and a hydroxide ion, so we need 3 water molecules because the N from Li3N will need 3 hydrogen ions to form ammonia.
![]()
209. (B) To balance redox reactions, it is typically easiest to use oxidation states. We know that Cr3+(aq) needs 3 electrons to be reduced to Cr(s). The confusing part may be with the chlorine ions. Each ![]() needs to lose 1 electron but we need them to combine in pairs to produce Cl2(g), so we should immediately put a 2 near
needs to lose 1 electron but we need them to combine in pairs to produce Cl2(g), so we should immediately put a 2 near ![]() to remind us later that we accounted for chlorine’s electrons in pairs. We know we need 3 electrons to reduce chromium and 2 electrons to oxidize the chlorines. The least common multiple of 3 and 2 is 6 electrons, and we see that if we lost 6 electrons from 6 chlorine ions (or 3 pairs of chlorines), we’d be able to reduce 2 chromium ions.
to remind us later that we accounted for chlorine’s electrons in pairs. We know we need 3 electrons to reduce chromium and 2 electrons to oxidize the chlorines. The least common multiple of 3 and 2 is 6 electrons, and we see that if we lost 6 electrons from 6 chlorine ions (or 3 pairs of chlorines), we’d be able to reduce 2 chromium ions.
![]()
210. (D) When balancing an equation that contains a polyatomic ion, keep the ion together when possible. For example, the first thing we might balance is the magnesium. Once the 3 is in front of Mg(H2PO4)2(s), we are left with 6 H2PO4− ions. It’s easy to see that the phosphate from magnesium phosphate picked up a hydrogen ion from phosphoric acid (H3PO4), so we can work backward. There is a 2:1 ratio of phosphates between Mg3(PO4)2(s) and H3PO4(l), so the 6 we have in the products should be allocated accordingly. Six phosphates can be allocated to 4 phosphoric acid molecules and the other two come from one magnesium phosphate.
![]()
211. (D) Important note: A type of balancing that appears on the AP Chemistry exam is a redox reaction occurring in an acidic or basic solution. If a question like this appears in the multiple choice section, just balance as you normally would. If the number of each atom on the reactant side of the equation is identical to those in the products, nothing else is needed. However, if it doesn’t work, or the question appears in the free-response section (probably associated with an electrochemistry problem) and asks our work be shown, we should be familiar with the procedure.
To balance redox reactions that occur in acidic and neutral conditions: (1) Balance the atoms other than oxygen and hydrogen. (2) Balance oxygen atoms by adding water molecules. (3) Balance hydrogens by adding hydrogen ions. (4) Balance the charges by adding electrons.
![]()
212. (C) See important note in Answer 211. To balance redox reactions that occur in alkaline conditions: (1) Balance atoms other than oxygen and hydrogen. (2) Balance oxygen and hydrogen atoms at the same time. To balance oxygen, we can add hydroxide ions or water, but they both contain oxygen and hydrogen. However, there is a 1:1 hydrogen-to-oxygen ratio in OH− and the 2:1 hydrogen-to-oxygen ratio in H2O. To add a hydrogen atom, add a water molecule, the only way to be “one up” on hydrogen in the hydrogen-to-oxygen ratio.
![]()
213. (C) When given the amounts of both reactants in a problem, check for limiting reactants. A simple way to do this is to take the number of mole of each reactant and divide it by its stoichiometric coefficient in the balanced equation. In this case, 0.4 mole CS2 ÷ 1 = 0.4, and 1.20 moles O2 ÷ 3 = 0.4. Because their quotients are equal, there is no limiting reactant. The two reactants are available in the correct ratio. If there is no limiting reactant, use the reactant that has the easiest numbers to crunch. In this case, it is 0.4 mole CS2. One mole CS2 would produce 3 moles of products (1 mole of CO2 and 2 moles of SO2) so 0.4 mole CS 2 will produce 1.2 moles of products.
214. (D) Both the products are gases and the total number of them produced is 3, which also happens to be the number of moles of O2 required to produce them. So the number of moles of gas produced will be the same as the number of moles of O2 that reacted.
215. (A) There are 3 moles of gas produced for every mole CS2 reacted. If 33.6 L of gas formed at STP, then 1.5 moles of gas is produced (33.6 L × (1 mol gas/22.4 L)). The math is easy: 1.5 is half of 3, so 0.5 mole of CS2 reacted (or, 1.5 moles products × (1 mol CS2/3 mol products)).
216. (B) One hundred mL (0.1 L) of 0.6 M HCl contains 0.06 mole H− ions (0.1 L × (0.6 mol HCl/1 L)) which will get reduced to 0.03 mole H2 gas (Zn is above hydrogen in the activity series and will lose electrons to H+ ions in an acidic solution). Because the reaction occurs at STP, we don’t need to use a gas law, only the conversion factor of 1 mole gas = 22.4 L ∴ 0.03 mol × 22.4 L/mol = 0.672 L, or 672 mL.
For Questions 217 – 219:
(A) 4 SO3 only
(B) 2 SO2 and 2 SO3
(C) 3 SO2, 1 O2 and 2 SO3
(D) 3 SO2 and 2 O2
(E) 4 O2 and 5 SO3
217. (D) The reaction of 3 moles of SO2 with excess oxygen would produce 3 moles of SO3. Since 1 mole of O2 is needed for the reaction, and the question asks for 1 mole O2 in excess, we need to start with 2 moles of O2. Choice (D) is the only one that contains only reactants (see list above for the contents of each box), so we could have easily chosen the correct answer without even doing the stoichiometry.
218. (B) A simple way to check for a limiting reactant is to take the number of moles of each reactant provided in the question and divide it by its stoichiometric coefficient in the balanced equation: 4 mol SO2 ÷ 2 = 2 and 1 mol O2÷ 1 = 1 ∴ O2 is the limiting reactant, so we won’t get 4 moles of SO3, we’ll get as much SO3 as what 1 mole of O2 will produce with excess SO2 (2 moles). We started with 4 moles of SO2 and 2 moles were consumed to produce 2 moles of SO3, leaving 2 moles in excess. (See list above for the contents of each box.)
219. (E) A simple way to check for a limiting reactant is to take the number of moles of each reactant provided in the question and divide it by its stoichiometric coefficient in the balanced equation: 5 mol SO2 ÷ 2 = 2.5 and 6.5 mol O2 ÷ 1 = 6.5, so SO2 is our limiting reactant. With 5 moles of SO2, we can produce 5 moles of SO3 and will consume 2.5 moles of O2 in the process. That leaves us with 4 moles of O2 in excess. (See list above for the contents of each box).
220. (B) When we are presented with the quantities of two or more reactants in a problem, we check for a limiting reactant. A simple way to check for a limiting reactant is to take the number of moles of each reactant provided in the question and divide it by its stoichiometric coefficient in the balanced equation: 0.1 mol NH3 ÷ 4 = 0.025, 0.1 mol O2 ÷ 5 = 0.02 ∴ O2 is our limiting reactant. The maximum amount of NO that can be produced with 0.1 mole of O2 is 0.08 mole of NO (0.1 mol O2 × 4 mol NO/5 mol O2), or 2.4 g NO.
221. (E) The reaction of NH3 and O2 proceeds according to the equation:
![]()
The 4:5 molar ratio given in the question are the exact coefficients of the reactants in the balanced equation, which makes the problem fairly simple. Because the problem states that the reactants reacted 100 percent, only a ratio that reflects the same relationship as the stoichiometry in the balanced equation could be given.
The products, NO and H2O, will be present in a 4:6 ratio as given in the balanced equation. Remember, however, that these are mole ratios, not mass ratios. The molar mass of NO = 30 g mol−1 and the molar mass of H2O = 18 g mol−1. 4 moles NO = 120 g and 6 moles H2O = 108 g. 120 g + 108 g = 228 g, the exact yield of the reaction. If the numbers given in a problem don’t align perfectly with the balanced equation, use the mass stoichiometry instead. For example:
Now we can determine the percent mass of each product:
120 g NO/228 g total = ~53% NO ∴ 47% H2O
You must use the 228 g from the addition of 120 g NO + 108 g H2 from the equation to get the correct percentages. If we were given another mass of product, let’s say 72 g, we would take the 53 percent we arrived at by the method above and then apply it to the mass given in the problem: 53 percent of 72 g = ~38 g NO ∴ 34 g H2O.
222. (C) We use M1V1 = M2V2 ∴ (0.375) (400) = (x) (500) ∴ x = 0.3 M. Add volumes when combining solutions.
223. (E) Some questions ask us to set up a problem as opposed to actually calculating an answer. In this example, start by setting up the expression to convert 1.0 L of NO into moles of NO (1.0 L × 1 mol/22.4 L). Since we need 22.4 in the denominator, we can immediately eliminate choices (A) and (B). Next, arrange an expression that converts mol of NO produced into mol O2 consumed using the stoichiometric coefficients from the balanced equation (moles NO produced × 5 mol O2 consumed/4 mol NO produced = moles O2 consumed). We need the fraction 5/4, which leaves (E) as our only choice.
224. (B) When presented with the quantities of two or more reactants in a problem, check for a limiting reactant. A simple way to check for a limiting reactant is to take the number of moles of each reactant provided in the question and divide it by its stoichiometric coefficient in the balanced equation. 2.5 mol NH3 ÷ 4 > 2.5 mol O2 ÷ 5, therefore O2 is our limiting reactant and NH3 would remain in excess.
2.5 moles O2 available × 4 mol NH3 consumed/5 mol O2 consumed = 2 moles NH3 consumed
We started with 2.5 moles NH3 ∴ 0.5 mole remains.
225. (A) When presented with the quantities of two or more reactants in a problem, check for a limiting reactant. A simple way to check for a limiting reactant is to take the number of moles of each reactant provided in the question and divide it by its stoichiometric coefficient in the balanced equation: 6 moles KO2 ÷ 4 < 9 moles CO2 ÷ 2 ∴ KO2 is our limiting reactant.
6 mol KO2 × 3 mol O2/4 mol KO2 = 4.5 moles O2 produced
226. (A) When carrying out stoichiometry with gases under the same conditions (temperature and pressure), we can treat volumes like moles (at the same temperature and pressure, equal volumes of any gases have the same number of particles).
6 L O2 × 2 CO2/3 O2 = 4 L CO2
227. (A) Because we are given answers in L, it is easiest for us to convert CO2 to L first, and then use the gas stoichiometry shortcut described in Answer 226 (treat volumes like moles).
2.9 g CO2 × 1 mol/44 g × 22.4 L/1 mol = ~1.5 L CO2
1.5 L CO2 × 3 O2/2 CO2 = 2.25 L O2
228. (A) The net ionic equation for just about every neutralization reaction is H+ + OH− → H2O. Na+ and NO3− ions are (always) spectator ions. They remain in solution (in other words, they don’t react) for the entire reaction.
229. (B) An addition reaction is mainly limited to alkenes and alkynes. A typical addition reaction on the AP Chemistry exam will add a halogen to an alkene. Reaction (A) is a substitution reaction. A substitution reaction occurs when a functional group (or a hydrogen atom, as in this reaction) is replaced by another group (or element, often a halogen). These reactions mainly involve alkanes. Reaction (D) is saponification, the process that produces soap from fat and a strong base (typically NaOH, also called lye). The result is a soap of the carboxylate (in this case, it is a fatty acid, a long hydrocarbon chain with a carboxyl group at the end). Reaction (E) demonstrates photosynthesis.
230. (E) The reaction between carbon dioxide and water is worth memorizing.
![]()
Chapter 6: Thermodynamics
231. (B) The definition of lattice energy (kJ mol−1). (See Answer 52 for a description of the factors that determine lattice energy.)
232. (C) The definition of (Gibb’s) free energy (ΔG, kJ mol−1). (See Answer 239 for an explanation of Gibb’s free energy.)
233. (A) A definition of activation energy, Ea. (See Questions 234 for another definition of Ea and Answers 235, 241, and 251 for descriptions of the different aspects of activation energy.)
234. (A) Another definition of activation energy, Ea. (See Questions 233 for another definition of Ea and Answers 235, 241, and 251 for descriptions of the different aspects of activation energy.)
235. (A) Yet a third way to consider the activation energy, Ea, of a reaction (see Question 233 and 234) is that it reflects the sensitivity of the reaction rate to temperature changes. The Arrhenius equation quantifies the relationship between activation energy and the rate at which a reaction proceeds: Ea = RT ln (k/A), where R is the universal gas constant, T is the temperature, k is the rate constant, and A is the frequency factor for the reaction, a constant for a given reaction that represents an empirical relationship between temperature and the rate constant and has the units s−1. (See Answer 241 for an explanation of how the Arrhenius equation illuminates the relationship between Ea and Keq.)
236. (E) Substances undergoing phase changes don’t experience a change in temperature, an indication of the average kinetic energy of the particles. Instead, the potential energy of the particles changes as they change positions relative to one another.
237. (D) The formula for kinetic energy is ½ mv2. Graham’s law of effusion allows us to calculate the relative speeds of two different gases at the same temperature and is derived from the formula for kinetic energy, which can be rearranged to solve for the average velocity, v, of the particles. Because they are at the same temperature, the value for kinetic energy (which is proportional to but not equal to the absolute temperature) is the same for gases 1 and 2:
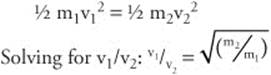
The velocity is inversely proportional to the square root of the molar mass. (See Answer 123 for an in-depth explanation of effusion.)
238. (B) The definition of zero entropy is a perfect, pure crystalline solid at 0 K (and the third law of thermodynamics).
239. (E) Gibb’s free energy is a thermodynamic state function given by the formula ΔG = ΔH – TΔS. It is a measure of how much useful (but nonmechanical) work can be done by a system (at a constant pressure and temperature). Systems in disequilibrium, that is, not at equilibrium, have the ability to do work as they move toward equilibrium. Once at equilibrium, no more work can be done. A useful form of the Gibb’s free energy equation, ΔG° = –RT ln Keq, allows the standard-state free energy change of a reaction to be calculated if the Keq is known. More importantly, it shows that the equilibrium established for a reaction is a function of the free energy change (for reactions in solution).
240. (A) The standard enthalpy of formation ![]() is the enthalpy change that accompanies the formation of one mole of a compound (or an element in its nonstandard state) from its standard-state elements. For example,
is the enthalpy change that accompanies the formation of one mole of a compound (or an element in its nonstandard state) from its standard-state elements. For example, ![]() mol−1 but the
mol−1 but the ![]() , which tells us that the formations of one mole of carbon dioxide gas from 1 mole of C(g) and 1 mole of O2(g) is exothermic and produces 393.5 kJ of heat.
, which tells us that the formations of one mole of carbon dioxide gas from 1 mole of C(g) and 1 mole of O2(g) is exothermic and produces 393.5 kJ of heat.
241. (D) The Arrhenius equation quantifies the relationship between the activation energy, Ea, and the rate at which the reaction proceeds: Ea = –RT ln (k/A) where R is the universal gas constant, T is the temperature (in K), k is the rate constant (from the rate law) for the reaction, and A is the frequency factor for a particular reaction. It is a quantity related to the frequency of collisions between particles that are correctly oriented to produce an effective collision. Any reaction with a positive activation energy (the vast majority) will experience an increased rate of reaction with increasing temperature. (See Answer 235 for an explanation of how Ea is measured, Answer 251 for a practical description of activation energy, and Answers 277 and 278 for further explanations of temperature, activation energy, and reaction rate.)
242. (D) An exothermic reaction that increases entropy will be spontaneous (exergonic) at all temperatures. In general, nature prefers moving toward a lower energy and more entropic state. (See table below.)
243. (A) Melting is an endothermic reaction (heat must be added) that increases entropy.
![]()
244. (B) Both deposition and condensation are exothermic processes that decrease entropy.
![]()
245. (D) Wood is mostly made of lignin and cellulose. Lignin is a complex chemical compound (its formula is C9H10O2, C10H12O3, C11H14O4) and cellulose is a large, linear polymer of glucose (C6H12O6)n. Their combustion with O2 produces CO2 and H2O as follows (for one glucose):
![]()
The combustion of wood (or petroleum products) is highly exothermic (which is why we use it for heat, light, and work) and produces six more moles of gas than it consumes, which results in increased entropy. In addition, the high heat produced is also highly entropic. Remember, the definition of zero entropy is a perfect, pure crystalline solid at 0 K (it’s also the third law of thermodynamics). Any deviation from 0 K and/or a pure, perfect crystalline solid indicates that entropy is increasing. An increase in temperature typically increases the entropy of the system. (See table below Answer 242.)
246. (C) A reaction that is both endothermic and results in decreased entropy will never be spontaneous at any temperature (like the formation of wood from CO2(g) and H2O(g). Plants do it all the time, but it takes a lot of energy (from the sun) to do it. In general, nature moves toward a state that is of lower energy and higher entropy. When an entropy reduction occurs in one process (organizing the chemistry lab), it does so by creating more entropy in the universe. (The heat created by our energy metabolism, for example. Our mitochondria are only ~40 percent efficient at converting food energy into cellular energy, the rest is lost as heat, the most entropic form of energy.) (See table below Answer 242.)
247. (E) The mixing of two gases at a constant temperature (an adiabatic process is one that occurs without heat transfer) must have a ΔH = 0. If no reaction occurs and the gases experience no forces of attraction or repulsion, then the entropy of the system must increase (ΔS > 0). The entropy of a system is proportional to the number of states a system can assume versus the number of states that are “right.” For example, imagine a strange deck of cards in which each card has a number from 1 through 52 and, all 52 are in numerical order. For simplicity, we will assume this is the only ordered state of the deck. If the cards are shuffled in any way, they are out of order, or disordered. It is easy to see that there are there are (52 – 1)! ways the cards are considered disordered and only one way they are considered ordered. If there were only two cards in the deck, there would be (2–1)!, or one way the cards could be disordered and one way they could be in order, so the more (different) components that make up a system, the more potential for disorder and entropy in the system.
248. (C) To calculate the ΔHrxn from ![]() values, remember a simple formula:
values, remember a simple formula:
![]()
Add the ΔH°f of the products and reactants separately, then subtract the value of the sum of the reactants from the sum of the products. Be careful with signs and make sure to multiply the ΔH°f of each substance by its stoichiometric coefficient.
249. (C) This is a Hess law problem. Since enthalpy is a state function (it only depends on final and initial states, a D sign is usually a clue), it doesn’t matter how we get there, only where we start and where we end up. (H, G, and Sas well as T, V, and P are all state functions, too.)
If we keep the conversion of graphite and oxygen to carbon dioxide, but “flip” the conversion of diamond and oxygen to carbon dioxide (and remember to reverse the sign of ΔH), the two equations cancel out oxygen (with a double slash) and carbon dioxide (with a single slash) and their sum leaves us with the conversion of graphite to diamond that we wanted:
250. (D) One way to think of entropy is as a measure of disorder in a system. The third law of thermodynamics states that the entropy of a pure, perfect crystal at 0 K is zero. Any change that takes a system further from that state is typically thought of as increasing entropy. This is mostly true, and is a good guide to predicting whether a particular change will increase or decrease entropy. For example, a mixture typically has more entropy than a pure substance.
Under certain circumstances, however, the entropy changes expected are not what is observed. In this situation, the addition of Ca2+ and Cl− ions decreases entropy because the water molecules end up becoming more ordered as they form hydration shells around the ions. The entropy change of a system is the sum of the entropy changes of its components, so even though the CaCl2 became less ordered as it dissolved, the magnitude of the entropy decrease of the water molecules was greater than magnitude of the entropy increase of CaCl2. (See Answer 247 for an explanation of why a mixture is expected to have greater entropy than a pure substance and for a different consideration of entropy.)
251. (D) We can think of the activation energy of a reaction as the energy needed for the formation of the transition state of the reaction. It is considered an energy barrier to the reactions progression. The spark of the lighter produces the heat needed to get the combustion going by supplying the energy needed by the reactants to form the transition state needed to form the products. The combustion of the butane is highly exergonic, so once the reaction starts, the energy it liberates supplies the activation energy for the other butane molecules in the lighter (which are sprayed into the atmosphere where they react with oxygen).
We probably don’t go to bed at night worrying that our chemistry textbook will turn into carbon dioxide and water while we sleep. The conditions at our desk don’t provide the activation energy needed to start the process. But if we applied the heat of the lighter flames to it (or, if we could get the spark to directly catch one of the pages), we could easily turn our book into carbon dioxide and water (not recommended until after the AP Chemistry exam). (See Answer 235 for an explanation of how Ea is measured, Answer 241 for the relationship between Ea and Keq, and Answers 243 and for further explanations of temperature, activation energy, and reaction rate.)
252. (D) (See Answer 250 for an explanation of entropy and Answers 243 and for tables of the entropy changes associated with phase changes.)
253. (C) Reaction (C) formed one kind of solid compound from a solid and a gas. (See Answer 250 for an explanation of entropy, and Answers 243 and for tables of the entropy changes associated with phase changes.)
254. (E) This is a stoichiometry problem. The tricky part is that the ΔHrxn is given in kJ mol−1 CH6N2, but the stoichiometric coefficient of CH6N2 in the reaction is 2. That means we don’t convert 6 moles of H2O(g) to H2O(l), we only need to convert 3 (because we’re asked per mole of CH6N2).
![]()
The negative sign indicates the condensation of water is exothermic (which we probably already knew), but that means we will actually get more energy out of the combustion of CH6N2, so we’ll want a ΔH value that is more negative
∴ –1,303 kJ mol−1 + (–132 kJ) = –1,435 kJ mol−1.
255. (A) If a reaction lowers the temperature of the container in which it occurs, the reaction is endothermic. It is absorbing heat from its environment; this is why it decreases in temperature (it is losing heat to the reaction). Most dissolutions have a +ΔS, they increase entropy. (See Answer 250 for an exception.)
256. (A) The units of ΔS are J mol−1 K−1, not kJ mol−1, like ΔG and ΔH. A spontaneous reaction has a –ΔG. If the reaction is spontaneous only at low temperatures, then we know that the reaction is exothermic and the entropy change is positive (see table below Answer 242).
ΔH – TΔS < 0
(–18,000 J mol−1) – (300) (ΔS) < 0
300 ΔS < 18,000 ∴ΔS < 60 J mol−1
257. (D) The double arrow tells us that the reaction is at equilibrium. At equilibrium, ΔG = 0. Using Gibbs free energy equation ΔG = ΔH – TΔS, if ΔG = 0 then ΔH = TΔS. For a phase change , the change in entropy is equal to the heat of fusion divided by the melting point temperature (in Kelvin).
258. (C) We live in a fairly stable environment thanks to activation energies. We probably don’t go to bed at night worrying that our bed will turn into carbon dioxide and water while we sleep. The conditions in our house don’t provide the activation energy needed to start the combustion process. But a bed in the middle of a forest fire would quickly be consumed and become a great mass (and volume) of gases. The higher the Ea of a given reaction, the more thermodynamically stable the reactants and the more energy they need to form the activated complex in their transition to product(s). We literally can sleep at night because of the stability activation energies provide.
259. (E) The short explanation (probably worth memorizing) is that all adiabatic, reversible process are isentropic (ΔS = 0, no entropy change). In an adiabatic process there is no heat flow between the system and its surroundings ∴Δheat = 0. If ΔS = Δheat/ΔT and Δheat = 0 ∴ S = 0. The entropy of the gas in a cylinder is increased when its temperature is increased and the entropy is decreased when its volume is reduced. When we compress the cylinder of gas without heat exchange, its volume is reduced while its temperature rises, so its entropy is unchanged.
260. (D) Bond energy is a measure of bond strength. It is the amount of heat required to break one mole of a particular bond. All bond energies are positive numbers, because breaking bonds is always endothermic. We use the formula Hrxn = (energy of bonds broken) – (energy of bonds formed) to determine the ΔHrxn using bond energy data. A simple mnemonic device to remember the formula is “B-FOR” (before): bonds broken are accounted for B-FOR bonds FORmed.
For the reaction 2 H2(g) + O2(g) → 2 H2O(l)
The bonds broken are:
2 moles H—H bonds @ 432 kJ mol−1 = 864 kJ
1 mole O=O bonds @ 494 kJ mol−1 = 439 kJ
Total energy needed to break bonds = 1,358 kJ
The bonds formed are:
4 moles O—H bonds @ 459 kJ mol−1 = 1,836 kJ
Total energy released by bond formation = 1,836 kJ
The formula already accounts for the exothermicity of bond formation with the “–” sign, so don’t change the signs; leave them positive.
ΔHrxn = (energy of bonds broken) – (energy of bonds formed)
ΔHrxn = (1,358 kJ) – (1,836) = –478 kJ per 2 moles H2O
∴ The formation of one mole of H2O is –239 kJ. Alternatively, we could have performed the calculations for 1 mole H–H bonds and ½ mole O2 bonds to form 1 mole H2O.
Break all bonds in the reactants and form all the bonds in the products unless the reaction mechanism is known with complete certainty. If a bond isn’t actually broken during the reaction, its formation will be included in the calculation, so the value of the bond energy of the formed bond will be subtracted from the bond energies of the broken bonds, canceling itself out (the magnitude of the energy change for breaking and forming a particular bond is the same, only the sign changes). For example, to break an H–H bond requires an input of 432 kJ mol−1 and forming and H–H bond results in 432 kJ of energy given off. There is no net energy change for breaking and forming the same bond.
261. (D) The ΔHrxn can be calculated by the formula:
![]()
Remember that the ΔH°f of elements in their standard states is zero. The stoichiometric coefficient of each species must be multiplied to the ΔH°f of that species to correctly calculate the total enthalpy change of the reaction.
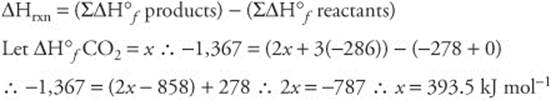
262. (B) We use the formula ![]()
Remember to multiply the ΔH°f of the compound by the stoichiometric coefficient in the balanced equation to get the total enthalpy change.
ΔHrxn = (9.7 – 2(34)) ∴ ΔHrxn = –58.3 kJ
(Questions 261 is similar.)
263. (D) The temperature decrease in the beaker indicates an endothermic reaction (the reaction absorbed heat from the environment), therefore ΔH > 0. The gas liberated indicates that entropy was increased, therefore ΔS > 0.
264. (D) The activation energies (Ea) for forward and reverse reactions are typically different. Activation energy is the energy difference between the reactants and the activated complex. If the forward reaction is exothermic, the Ea= (energy of activated complex) – (energy of reactants). For the reverse, endothermic reaction, the Ea includes the enthalpy change of the reaction. In other words, the Ea of the reverse reaction = (Eaexothermic rxn + ΔHrxn). If the Ea of the forward and reverse reactions are the same, the ΔH of the reaction must be zero.
265. (D) This is a Hess law problem.
The reaction we want is ![]() .
.
If we combine the other two reactions to produce this reaction, we can determine the ΔHrxn. Because enthalpy is a state function (it only depends on final and initial states), it doesn’t matter how we get there, only where we start and where we end up.
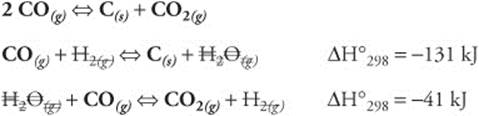
We had to reverse both reactions to cancel out intermediates and end up with the correct forward reaction, so we must reverse the signs of ΔH°298 for each reaction and add them ∴ ΔH°298 = –172 kJ.
266. (E) The Kp of a gaseous system at equilibrium will not change with pressure changes. When the pressure on a gaseous system in equilibrium increases, the equilibrium shifts to favor the side of the reaction with the least number of moles of gases. For a pressure increase to shift the equilibrium, there must be a different number of moles of gas in the products and the reactants. In reaction X, there’s 1 mole of gas in the reactants and 2 moles of gas in the products, therefore increasing the pressure will shift the reaction to favor the reactants. In reaction Y, there is an equal number of moles of gases in the products and reactants, so no shift will occur with a pressure change. In reaction Z, there are 2 moles of gas in the reactants and 1 in the products, so product formation will be favored under increased pressure. However, when the new equilibrium is established, the value of Kp will be the same. Even though partial pressures of the individual gases will change, their ratio (as defined by the equilibrium expression) will have the same value for Kp. The numerical values of Kp, Keq, and Ksp for a particular reaction only change with temperature.
267. (D) The Kp increases with increasing temperature for endothermic reactions and decreases with increasing temperature for exothermic reactions. If we imagine heat as a product of an exothermic reaction, adding more heat by increasing temperature pushes the equilibrium to the left, favoring the reactants. The major difference between adding more heat and adding more of a chemical product is that increasing the temperature is accomplished by continually adding more heat, so the reaction remains “pushed” to the left; whereas adding more of a chemical product transiently pushes the equilibrium to the left, but then the prior equilibrium reestablishes itself.
268. (D) Gases are the most entropic state of matter and solids are the least entropic state. Converting a gas to a solid decreases entropy, therefore Sfinal – Sinitial – 0.
269. (E) Thermodynamic data only quantify (or describe) energy and entropy changes in chemical reactions.
Chapter 7: Kinetics
For Questions 270 – 273:
(A) First-order for M, first-order reaction
(B) First order for M and N, second-order reaction
(C) First-order for M and second-order for N, third-order reaction
(D) Second-order for M and first-order for N, third-order reaction
(E) Second-order for N, second-order reaction
270. (E) If changing the concentration of M has no effect on reaction rate, we are looking for a rate law that does not include M.
271. (A) If the total concentration change of 2x doubles the reaction rate, we are looking for a first-order reaction ([2x]? = 2, ∴ ? = 1). Only A is a first-order reaction. In this case, the concentration change of N made no difference to the reaction rate, only the concentration change of M.
272. (D) If doubling the concentration of M quadruples the reaction rate, the reaction must be second-order with respect to M ([2x]? = 4, ∴ ? = 2). The effect of N was not considered, so we are only concerned with a rate law that contains [M]2.
273. (D) If doubling the concentration of reactants increases the reaction rate by a factor of 8, then the total reaction order must be 3 ([2x]? = 8, ∴ ? = 3). If no other information were given, both (C) and (D) are possible rate laws. However, if halving the concentration of N reduces reaction rate by fourfold, then the reactant order for N must be 2 ([½ x]? = ¼, ∴ ? = 2). Alternatively, we can invert the situation to read “doubling the concentration of N increases the reaction rate by fourfold,” which is a little easier to consider. We can eliminate (B) because we need a rate law that contains [N]2.
274. (D) This question is asking us about the effect of surface area on reaction rate. As a general rule, increasing the surface area of a solid will increase reaction rate. Breaking up a solid doesn’t affect its concentration [choice (C), although concentration isn’t a term typically applied to solids]. Choice (A) may seem alluring because it addresses (indirectly) the surface area issue (exposure), but the iron needs to react with sulfur, not oxygen, and the oxidation state of iron should be 0 to react with sulfur since it will losing electrons to reduce sulfur. If the iron were partially oxidized, it would be a less effecting reducing agent. (See Answer 275 for a detailed explanation of effect of surface area on reaction rate.)
275. (B) This question, like Questions 274, is asking us about the effect of surface area on reaction rate. However, two answer choices address surface area. If we had two cubes of wood, one with dimensions 2 × 2 × 2 and other with dimensions 4 × 4 × 4, their surface areas would be 24 and 96, respectively (the units are irrelevant). The surface area of the larger piece of wood is four times that of the smaller piece of wood which makes sense, the cube is basically two times as large and since area has a unit of is a length2, (2 × length)2 = 4 × the surface area. The volume of the 2 × 2 × 2 cube is 8, whereas the volume of the 4 × 4 × 4 cube is 64. As expected, the volume of the larger cube is eight times that of the smaller cube. The unit of volume is length3, so (2 × length)3 = 8.
Increasing surface area increases the rate of a reaction due to the greater exposure per unit of volume or mass.
When we revisit our cubes, the 2 × 2 × 2 cube has a surface area to volume ratio of ![]() , but the surface area to volume ratio of the 4 × 4 × 4 cube is
, but the surface area to volume ratio of the 4 × 4 × 4 cube is ![]() . Notice doubling the size decreases the surface area to volume ratio by one-half.
. Notice doubling the size decreases the surface area to volume ratio by one-half.
If the two solids are the same (wood, twigs, sawdust) then the composition (and concentration, which isn’t a term typically applied to solids) does not change when we break it into smaller pieces.
276. (D) Increasing the pressure only shifts the equilibrium (and thereby momentarily changes the rate of either the forward or reverse reaction) of a reaction that involves gases, and even then, the number of gaseous moles of reactant and product must differ or the pressure change has no effect. Increasing the temperature at which a reaction occurs will almost always increase the reaction rate, whether the reaction is exothermic or endothermic. (For explanations regarding the effect of surface area on reaction rate, see Answers 274 and 275.) (See Answer 11 for an except question strategy.)
277. (D) A very common misconception regarding the effect of temperature on reaction rate is that increased temperatures significantly increases the frequency of collisions. The small increase in collision frequency doesn’t actually have much effect on reaction rate.
In order for a chemical reaction to occur, the reactants must collide, but the collision itself will not form a product unless the reactants collide in the proper orientation to one another and with enough kinetic energy. When reactants collide in the right orientation and with enough energy to overcome the activation energy of the reaction, they produce a product (or a reaction intermediate, at least). This is called an effective collision.
Imagine Batman trying to fight off the bad guys under the misconception that more collisions (between him and the bad guy) will result in a higher reaction rate. He calculates that if he uses his fingertips instead of his fists, he can get in five times more collisions than with just his two fists alone. According to his logic, a fight that would have normally taken an hour should take only 12 minutes. He goes off into the night, finds the bad guys, and applies his new logic to their fight. He soon finds that poking is no substitute for punching. And just like collisions between reactants have to occur not only with enough energy but in an orientation that allows the reactants to collide in just the right position relative to each other, a punch delivered to the face or belly is going to be much more effective at stopping the bad guys than a punch in the arm or leg.
278. (C) Temperature increases the reaction rate of almost all chemical reactions, whether they are exothermic of endothermic (there are some cases for which this isn’t true, and these reactions have negative activation energies). Higher temperatures result in more effective collisions (collisions with enough energy and with the proper orientation or reactants relative to each other) by increasing the average kinetic energy of the particles in the reaction mixture, assuring that a greater number of collisions will occur with an energy that meets or exceeds the activation energy of the reaction, the energy barrier that must be overcome in order for the reaction to proceed. (See Answer 277 for a more in-depth explanation of effective collisions.)
279. (A) This question is very tricky. Choice (A) is almost correct—each radioactive species does have a particular half-life, but a particular element can have several different isotopes, each with its own half-life. (See Answer 11for an except question strategy.)
280. (C) In comparing Trials 1 and 2, the two trials in which the O2 concentration does not change, we see that the concentration of NO is increased by 1.6 (not quite 2). The rate increases by about 2.6-fold. If we were allowed to use a calculator (in some sections of the free response section), we could easily plug in ([1.6x]? = 2.6 fold rate increase). If we encounter a problem like this in the multiple choice section, we do an estimate: If the reactant order for NO were 1, doubling the concentration would double the rate, so a 1.6-fold increase in concentration should increase the rate by 1.6-fold. But the change we see is 2.6-fold. We can probably see that the reaction order isn’t 3, but maybe we’re unsure of 2. If the reactant order of NO is 2, and the concentration of NO was doubled, we’d expect the rate would increase by fourfold. If the concentration was increased by 1.6, 1.62 = 2.56, or 2.6.
We can apply the same methodology to the reactant order of O2. The two trials in which only the concentration of O2 changes are Trials 1 and 3. The O2 concentration increases from 0.035 to 0.045, an increase of about 1.3 times. The rate increases from 0.143 to 0.184. If we recognize that 0.143 is about two-thirds of 0.184, we can determine that the reaction rate increased by one-third. Therefore, with regard to O2, increasing the concentration by one-third increases the reaction rate by one-third. (Please see the important note from the author at the beginning of the book.)
281. (D) All we need to do here is substitute the data from one of the trials into the rate law. Use the trial that has the easiest numbers to crunch. Using the data from Trial 1:
Rate = k[NO] 2[O2]
k = rate/[NO]2[O2]
k = 0.143/[0.024]2[0.035]
k = 7,093 or 7.0 × 103
Remember, the numerical value of the rate constant, k, always increases with increasing temperature. So the value of k calculated for a particular reaction occurring at one temperature will not be the same in the same reaction performed at a different temperature. (Please see the important note from the author at the beginning of the book.)
282. (C) We can memorize the units of the rate constant or calculate them. Notice that the absolute value of the exponent for L and mol are always one less than the reaction order.

To calculate k, just substitute the units into the rate law.
Rate = k[NO]2[O2]
M s−11 = k (mol L−1)2 (mol L−1)
**Remember that the units of M are mol L−1**
mol L−1 s−1 = k mol3 L3
k = L2 mol−2 sec−1
(Please see the important note from the author at the beginning of the book.)
283. (C) The reaction order for NO is 2, so increasing its concentration would increase the reaction rate by 25 × ([5x]2 = 25-fold increase in rate). (Please see the important note from the author at the beginning of the book.)
284. (D) All we need to do is plug the numbers into the rate law, using the value of k determined in Questions 281.
Rate = k[NO]2[O2]
Rate = 7.3 × 103 [2 × 10−2]2 [4 × 10−2]
Rate = 1.1 × 10−1
(Please see the important note from the author at the beginning of the book.)
285. (E) The effect that the concentration of a particular reactant has on a reaction cannot be determined until the actual experiment of changing the concentration of that reaction and measuring the rate change that results is performed. In writing the equilibrium expression, the exponents that accompany reactions are taken from the balanced equation, but the equilibrium concentrations must be measured to actually determine the Keq for the reaction. (Please see the important note from the author at the beginning of the book.)
286. (D) We should certainly remember the statements in choices (A), (B), (C), and (E) as important facts about catalysts. Choice (D) is the opposite of what is true, and this is also important to remember: A catalyst does not differentiate between the forward and reverse reactions. The activated complex is the same whether a reaction occurs in the forward or reverse reaction, so decreasing the energy of the activated complex will decrease the activation energy in both directions by an equal amount. Because a catalyst increases the rates of both forward and reverse reactions equally, the presence of a catalyst does not change the Keq for a reaction. It doesn’t change the ΔHrxn, either (yet another important thing to remember about catalysts). (See Answer 11 for an except question strategy.)
287. (E) Statements I and II are standard ways of measuring reaction rates. The rate law (III) can be used to predict the rate of a chemical reaction. The reaction quotient (Q) can be used in conjunction with the equilibrium constant (Keq) to calculate how far a reaction is from equilibrium.
288. (A) Reaction rates are typically measured in one of two ways, the appearance of product over time, and/or the disappearance of one or more substrates over time. When using reaction data, it is vital to consider the stoichiometric coefficients when to compare relative rates of appearance or disappearance. For example, for each mole NO consumed by this reaction, one mole of H2 will be consumed as well and one mole of H2O will form, as well. However, only half of a mole of N2 will form. Related to time, N2 will be formed at half the rate as H2O formation, and half the rate at which NO and H2 are consumed.
289. (B) The slowest (rate determining) step in a multistep reaction governs the rate law of the reaction. CO is not involved in the rate determining step, so it is not included in the rate law. Because two NO2 molecules are necessary for collision (it is bimolecular), the reactant order for NO2 is two.
290. (D) It is more difficult to determine the rate law from a reaction mechanism in which an intermediate (NOBr, in this case) is involved in the slow (rate determining) step. Notice that the first, fast step is at equilibrium, so that the forward and reverse reactions are occurring at the same rate. We can write and equate a rate law for these reactions, then solve for [NOBr], which allows us to use the intermediate that we normally don’t include in the rate law, but need when it is part of the rate determining step.
kforward [NO][Br2] = kreverse [NOBr]
[NOBr] = kforward/kreverse [NO][Br2]
The rate law for the slow step, if NOBr was not an intermediate, would look like this:
Rate = k [NOBr][NO]
but now we substitute for [NOBr]
Rate = k2 (kf/kr)[NO][Br2][NO]
and simplify to get rate = krxn [NO]2[Br2].
The value of k rxn = k2kf/ kr.
291. (D) The numerical value of the rate constant, k, is irrelevant to answering this question. It is the unit of the rate constant that gives us the most information (at first). From the unit, we can deduce that this is a third-order reaction. Remember, the absolute value of the exponent for L and mol is 1 less than the reaction order. (For reaction order n, the units of the rate law will be L(n–1) mol−(n–1) sec−1.) (See Answer 282 for a table of the rate constant units and how to determine them.)
The rate constant represents an “adaptor” between the concentration of reactants and the reaction rate (at a particular temperature), it doesn’t tell us anything about the enthalpy changes that occur in the reaction, nor can it be used for comparing reaction rates with other reactions for which nothing but the rate constant is known. The units of the rate constant do provide a quantitative relationship between concentration changes and reaction rates. In this third-order reaction, doubling the concentrations of all the reactions will result in an eightfold increase in reaction rate ([2x]3 = 8 × rate), not an 8.1 × 1010-fold rate increase.
292. (B) Reactant orders can be positive, negative, or fractional. A negative reactant order indicates that the reaction rate increases as the reactant with the decreased concentration of that reactant.
293. (D) The activated complex formed during a chemical reaction without a catalyst must be different from the one formed by the same reaction with a catalyst. First, the potential energies are different. Second, the presence of a catalyst provides an alternative pathway for reaction, which means a different set of intermediates are formed. (See Answer 11 for an except question strategy.)
294. (E) The reaction is endothermic because the products have more potential energy than the reactants. The Ea is calculated by subtracting the energy of the reactants from the energy of the activated complex ∴ 510 – 75 = 435 kJ mol−1. The potential energy of the products is approximately 250 kJ mol−1 and the energy of the reactants is about 75 kJ mol−1 ∴ΔH = 250 – 75 = 175 kJ mol−1.
295. (D) Reaction rate is measured by product formation (or substrate consumption) over time and is a function of the number of effective collisions that occur in a given period of time.
296. (B) It takes between 10 and 15 minutes to decrease the amount of reactant by 50 percent. It takes another 10 to 15 minutes to decrease the amount of reactant by another 50 percent, therefore the reaction order is 1, and the half-life is between 10 and 15 minutes ∴ 12 minutes.
297. (E) The total reaction order is 3, so doubling both reactants will increase the reaction rate eightfold ([2x]3) = 8-fold increase in rate).
298. (B) In Trial 2, the concentration of X is cut by half, so we expect the rate to decrease by half since X is a first-order reactant (∴ R/2). The concentration of Y is doubled, however, and Y is a second-order reactant, so the change in reaction rate due to doubling [Y] = 4R. (R/2) × (4R) = 2R.
299. (D) In Trial 2, the concentration of X is cut by half so we expect the rate to decrease by one-fourth since X is a second-order reactant in this situation (∴ R/4). The concentration of Y is doubled, however, and since Y is a first-order reactant in this case, the change in reaction rate due to doubling is [Y] = 2R. (R/4) × (2R) = R/2.
300. (D) The units of the rate constant for a fourth-order reaction is L3 mol−3 sec−1. Remember that the absolute value of the exponents of L and mol is 1 less than the reaction order. (See the table under Answer 282 for a summary of the units of k.)
Chapter 8: Equilibrium
301. (D) Le Chatelier’s principle: If a chemical system at equilibrium experiences a change in concentration, volume, temperature, or partial pressure, the equilibrium shifts to counteract the change and a new equilibrium will be established. To increase the amount of MgO produced, a disturbance to the equilibrium must be applied that will be counteracted by the production of more MgO. Removing Mg(s) (or adding it) will not cause any change in the equilibrium because it is a solid. Solids don’t have a concentration and they are not represented in the equilibrium expression. Increasing the pressure on a gaseous system at equilibrium will cause the equilibrium to shift to favor the side of the reaction with the fewest moles of gas. Decreasing the pressure shifts the equilibrium to favor the side of the reaction with the most moles of gas. Changing the pressure of a gaseous system does not change the value of Kp, however. The ratio of partial pressures after the pressure change will equal the same value of Kp. Adding more O2(g) will increase MgO formation because the system will consume the O2 in an effort to counteract the increase in O2 pressure. (See table in Answer 307.)
302. (D) The reaction is endothermic because heat (energy) is on the reactant side of the equation. To shift the equilibrium to favor the reactants, the pressure could decrease (the reactants have greater number of moles of gas) or the temperature can be lowered (removing heat would pull the equilibrium to the left).
303. (E) This reaction is exothermic, so increasing the temperature favors the reactants by pushing the equilibrium to the left. The number of moles of gases on each side of the equation is the same, so pressure changes will not affect the equilibrium partial pressures.
304. (B) Increased pressure due to decreased volume shifts the equilibrium of a gaseous system to favor the side of the reaction with the fewest moles of gases (without changing the Kp). Equation (B) has an equal number of moles of gases in the products and reactants, so pressure changes will not change equilibrium partial pressures.
305. (D) Decreased water temperature and increased gas pressure above the water increase the solubility of a gas in water. Shaking the container will not result in a sustained increase in solubility (since the system is at equilibrium).
306. (B) If N2 is injected into the tank, it will react with H2 by consuming it, and therefore decreasing its partial pressure in the tank.
307. (C) The reaction is endothermic. The value for Kp only changes with temperature changes.

308. (C) The ratio is the equilibrium expression for the reverse, exothermic reaction. Only temperature changes can change the value of Keq. Pressure changes in a gaseous system will shift the equilibrium until the new equilibrium is reached. Although the actual partial pressures of each gas (at equilibrium) may be different, their ratio as calculated by the equilibrium expression will result in the same value of Kp.
309. (C) All we need to do is substitute the concentrations into the equilibrium expression.
![]()
310. (C) The reactant side of the equation has fewer moles of gas than the products, so increasing the pressure will shift the equilibrium to favor the reactants, not the products. The value for ΔH is positive, so the forward reaction is endothermic, which means that increasing the temperature will shift the equilibrium to favor the forward reaction, and decreasing the temperature favors the reverse reaction. Adding more X will increase the formation of Z as the system consumes it in an effort to counteract the increase in X pressure.
311. (B) This question is not just asking what will shift the equilibrium, it’s asking what will change the equilibrium constant for the reaction. The Keq of a reaction doesn’t change if we add more reactant or product, it only changes with temperature. The ratio in the question is written for the forward, endothermic reaction (the reverse reaction would be the inverse). (See table in Answer 308.)
312. (E) The equation tells us that for every one F2 that reacts, two F are produced. If we put the number of particles in the box into the equilibrium expression we get [F]2/[F2] = [42/9] = 1.8. The equilibrium constant will never be a negative number because there are no negative concentrations.
313. (E) The equilibrium expression for the reverse reaction inverts the products and reactants, so the Keq for the reverse reaction is simply the inverse, 1/Keq. The value of 1/2.5 × 1010 is easy to calculate because all we need to do is divide 1 by 2.5 (= 0.4) and reverse the sign on the exponent (10−10). However, 0.4 × 10−10 is more correctly written as 4.0 × 10−11.
314. (D) If 30 percent of XY3 dissociates in 1 kg of a 0.06 m solution, then out of the 0.06 mole of XY3 present, 0.018 mole dissociates and 0.042 mole remains in the form of XY3. Immediately, choice (A) can be eliminated. Each XY3 that dissociated produced 4 particles, one X3+ and three Y−, so when the 0.018 mole of XY3 dissociated, it created 0.018 × 4 – 0.072 mole of particles. The total particle count, then, is 0.042 mole undissociated particles plus 0.072 mole ions = 0.114 mole particles.
315. (C) Ksp is the solubility product constant, the equilibrium constant for a solid in equilibrium with its ions. It is measured in a saturated solution at equilibrium. The molar solubility is the number of moles that can be dissolved to produce 1 L of a saturated solution of that substance. What simplifies Ksp calculations is that there is always a solid reactant in these problems but solids are not included in equilibrium expressions, so they will never have a denominator.
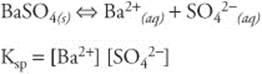
Let x =[Ba2+] = [SO42–] (They are produced in a 1:1 ratio.)
Ksp = x2 = 1.1 × 10−10
x = ~1 × 10−5
(See Answer 316 for a more complicated variation of this problem.)
316.![]()
Let x = [Ca2+]
F− and Ca2+ are produced in a 2:1 ratio, so [F−] is 2x. Because Ksp is an equilibrium constant, the [F−] must be raised to the exponent that is its stoichiometric coefficient, 2.
Ksp = [Ca2+] [2 F−1]2
Ksp = 4x3
4.0 × 10−11 = 4x3 ∴ x3 = 1.0 × 10−11 ∴ x = (1.0 × 10−11)1/3 M
Remember that the [Ca2+] = x, but the [F−] = 2x.
(See Answer 315 for a simpler variation of this problem.)
317. (C) This is the reverse process of Questions 315 and 316, but we’ll use the same principles. There is also an extra step involved because instead of being given a value for the molar solubility of one of the dissociation products (given which we can deduce the concentrations of the other), we are given the pH. The pH = 10, so we know the solution is basic.
pH + pOH = 14, if pOH = 4 then [OH−] is 1 × 10−4 M
M(OH)2 ⇔ M2+ + 2 OH−
Let x = [M2+]
[OH−] = 2x = 1 × 10−4 ∴ x = 0.5 × 10−4, or 5 × 10−5 M
Ksp = [M+2] [2 OH−]2 ∴ Ksp = [x] [2x]2 = 4x3
Since x = 5 × 10−5, 4x3 = 5 × 10−13.
318. (A) The solubility of Fe(OH)2 decreases with increased pH because the higher concentrations of OH− ions pushes the reaction toward the formation of solid Fe(OH)2. Decreasing the pH increases its solubility because the extra H+ ions in solution will combine with the hydroxides to produce H2O, pushing the reaction to the left. The value of Ksp does not depend on the pH directly, but since one of the aqueous products of the reaction is OH−, the Kspalready takes the pH into account.
319. (A) The Ksp for BaCrO4 is much smaller than that of CaCrO4, so we know that it is much less soluble. The difference of six orders of magnitude (10−10 versus 10−4) tells us that it is possible to precipitate almost all of the Ba2+ions without precipitating any CaCrO4. The trick to solving this problem is to understand how to produce a saturated CaCrO4 solution. This will leave Ca2+ ions in solution, but will overwhelm the Ba2+ ions.
Ksp = [Ca2+][CrO42–]
7 × 10−4 = [0.2][CrO42–] ∴ [CrO42–] = ~3.5 × 10−3 M.
320. (E) The only determinant of the vapor pressure of a particular substance at equilibrium with its liquid is the temperature. The amount of surface area will affect the rates of evaporation (and condensation) and the volume of the container will affect how many moles of vapor are present, but not the vapor pressure at equilibrium.
321. (C) The vapor pressure of a liquid is determined at equilibrium at a particular temperature, so we can consider this as we would an equilibrium question. Just as the Keq of an endothermic reaction increases with increasing temperature (and the Keq of exothermic reactions decreases with increasing temperature), higher temperatures favor vaporization over condensation until a new equilibrium is reached, and it will include a larger fraction of vapor than the equilibrium condition at a lower temperature. Choice (A) is not true and the system in choice (B) is not at equilibrium. For a system at equilibrium (and at constant temperature), the rates of forward and reverse processes are the same, so (D) is not true. Finally, the ΔH of a forward and reverse process (or reaction) are equal in magnitude, but opposite in sign.
322. (C) At atmospheric pressures, N2, O2, and Ar condense at –195°C, –183°C, and –186°C respectively. CO2 does not condense into a liquid at atmospheric pressures, but becomes a solid at about –80°C (see the phase diagrams for H2O and CO2 above Questions 96 and 99, respectively).
323. (A) An adiabatic system is one in which heat does not enter or leave the system. Entropy is a measure of the degree of disorder or dispersion of energy in a system, and it typically increases with the addition of heat. The liquid state of a pure substance has greater entropy than its solid state because the particles in a liquid are more disordered than the particles in a solid.
During the melting process, the change in entropy is equal to the heat of fusion divided by the melting point in K. The term melting implies a displacement from equilibrium by continued heat transfer over time to drive the melting process. However, in this question, the system is adiabatic, that is, no heat is entering or leaving the system, it is at a constant temperature.
The phase equilibrium of a pure substance is independent of the ratio of the solid phase to the liquid phase. Equilibrium exists even if only the tiniest bit of solid is present in the solid–liquid mixture. For that portion of the solid that melted, there is an entropy increase. The remaining mass of liquid and solid is at equilibrium and has no entropy change.
324. (B) The strength of oxyacids increases with (1) increasing electronegativity of the central atom (the atom the O—H attaches to), (2) other atoms of high electronegativity in the compound, and (3) an increasing number of oxygen atoms. The pH of a solution depends on the concentration on H+ in solution and therefore the concentration of the acid in solution, but the strength of an acid is intrinsic to the acid and does not depend on the concentration.
325. (C) The Ka for hypoiodous acid is 2 × 10−11. The stability of the conjugate base also tells us something about the strength of the acid OI−, which picks up protons in solution rather easily, meaning that more HOI is present at equilibrium. If less HOI dissociates, the acid is weaker. (See Answer 324 for a list of description of factors that affect the strength of oxyacids.)
326. (C) The names of oxyacids are derived from the polyatomic anions that the protons are attached to.
In the case of ClO− series:

The ClO− ion is named hypochlorite because it has the least number of oxygen atoms in the series. The compounds are named relative to each other, not by their absolute number of oxygen atoms. Once we’ve identified the polyatomic oxyanion, we name the acid accordingly:
Oxyanions ending in –ite become –ous acid.
Oxyanions ending in –ate become –ic acid.
327. (D) The equation shows the reaction of the conjugate base of HOCl. The formation of an OH− ion means we can use Kb, the base ionization constant, to figure out the unknowns in the reaction.
![]()
328. (D) The AP Chemistry exam is unlikely to give us a question like this in the multiple choice section, however there is a mandatory equilibrium problem in the free response section, so we need to know how to set up an ICE-box. See choice (D). (1) The initial concentration of reactants is taken from the question. Water is a pure liquid and is therefore not considered in the equilibrium expression. (2) The initial concentration of both products is zero. (3) The change in concentrations will always be (–) for the reactants and (+) for the products, but their stoichiometric coefficients from the balanced equation MUST be accounted for.
For example, if 2 A → 3 B, then the change in [A] = –2x and the change in [B] = +3x.
329. (C) The rate of the forward reaction continues to decrease until about 1 second. The instantaneous reaction rate is determined by calculating the slope of the tangent to the point of interest on the concentration versus time curve. At point X, the rate of the forward reaction is still decreasing and the rate of the reverse reaction is still increasing. At point X on this graph, the slope of curve A is the inverse of the slope of curve B.
330. (D) Le Chatlier’s principle says that when a chemical system at equilibrium experiences a change in concentration, the equilibrium shifts to counteract the change imposed on it. Once the system regains its equilibrium state, the ratio of products to reactants stays the same (only temperature changes affect the Keq of the reaction).
Chapter 9: Acid–Base Chemistry
331. (B) Definition of a Bronsted-Lowry acid.
332. (D) Definition of a Lewis base.
333. (E) Definition of an Arrhenius base.
334. (D) Coordinate covalent bonds are covalent bonds in which one atom donates both electrons to the bond, instead of each atom donating one electron. There is no difference in the character of the bond, only how it forms. Lewis bases always form coordinate covalent bonds with acids since Lewis bases are electron pair donors.
335. (B) Choice (B) is a mixture of a strong base and a weak base.
336. (C) Choice (C) is a mixture of a strong acid and a neutral salt. The Cl− ion is a very poor conjugate base and will not pick up any protons from the solution, so the pH is 0. (A good fact to memorize: The pH of a 1 M concentration of a strong acid = 0.)
337. (A) A weak base and a weak acid will neutralize each other.
338. (E) An alkaline buffer consists of a weak base and the salt of its conjugate acid. A buffer resists changes in pH by acting like a “proton sponge,” giving them to the solution when the [H+] decreases, and absorbing them from the solution when the [H+] increases.
339. (D) An acidic buffer consists of a weak acid and the salt of its conjugate base. A buffer resists changes in pH by acting like a “proton sponge,” giving them to the solution when the [H+] decreases, and absorbing them from the solution when the [H+] increases.
340. (B) We can use the expression for Ka.
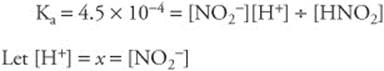
Let [HNO2] = 0.02 – x but because the value of Ka is so low and we will not be calculating the value of the [H+] to more than two or three significant figures, the value of (0.02 – x) is indistinguishable from 0.02, so we can drop the x in the denominator and greatly simplify our math.
![]()
341. (E) In the reaction, F− is the proton acceptor, the base, and H2O is the proton donor, or acid. When dealing with Bronsted–Lowry acids and bases, the acid and base are always the reactants, and their conjugates are always the products. The base, once it accepts an H+, becomes the conjugate acid and the acid, once it donates an H+, becomes the conjugate base.
342. (A) When dealing with Bronsted–Lowry acids and bases, the acid and base are always the reactants and their conjugates are always the products. Since H2O is a reactant, it can only be an acid or base, not a conjugate. Be careful with questions that ask about the reverse reaction. For example, water is the conjugate acid of the OH− ion in the reverse of this reaction. But in the forward reaction, H2O is giving the H− to NH3, so it is an acid.
343. (E) An increased number of hydrogens does not make an oxyacid a stronger acid. If an acid has more than one dissociable proton, it is a polyprotic acid. The Ka of the first H+ is the highest, and the Ka decreases for each successive H+. (See Answer 324 for a list of factors that affect the strength of oxyacids.) (See Answer 11 for an except question strategy.)
344. (E) When mixing two solutions, only concentrations intermediate to the solutions being mixed can be achieved. In this case, we can’t make a solution that is less concentrated than 0.25 M and we can’t get more concentrated than 0.35 M, so all concentrations achievable by mixing these two solutions, no matter what the ratios, will be between these two values.
345. (C) The Ka for the acid HA is 5.0 × 10−9. A Ka of such small magnitude means the acid is very weak. Compared to 0.5 M concentration, the amount that dissociates is almost negligible in calculations in which the answer will contain two to three significant figures. This makes these calculations much easier because you can ignore the value of x in the denominator, as follows. Start by setting up the chemical equation and the equilibrium expression for the value of Ka:
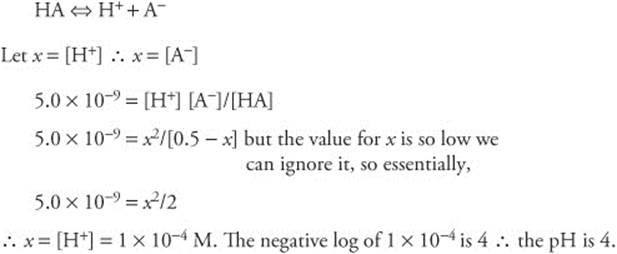
346. (B) An equilibrium constant of 2.5 × 103 is large, and so the products are greatly favored in this reaction. Y− is the proton acceptor in the forward reaction and X− plays that role in the reverse reaction. But since the forward reaction is favored so much more than the reverse, we can infer that Y− is a stronger base than X−. We can also infer that HX is a stronger acid than HY, but that is not an answer choice. As for conjugate acids and bases, X− is the conjugate base of HX, and HY is the conjugate acid of Y−. A conjugate acid will differ from its base by one more H+. A conjugate base will differ from its acid by one less H+.
347. (D) The equivalence point where the number of moles of base (or acid) added equals the number of moles of acid initially present (or base). It is midway along the steep part of the curve, the inflection point. The pH at the equivalence point will be 7 for a strong acid/ strong base titration, but will be greater than 7 when titrating a weak acid with a strong base. It will be below 7 when titrating a weak base with a strong acid. This is because the equivalence point is not when the actual number of OH− ions is equal to the actual number of H+ ions; it is, in this case, when the number of OH− ions added equals the number of H+ ions initially present, that is, neutralization.
348. (B) The region of the curve between 0 and about 25 mL NaOH added is the buffer region. At the lower end of the buffer region, the amount of weak acid exceeds that of the conjugate base and toward the end, the conjugate base is present in much greater concentrations. Midway, the weak acid/conjugate base concentrations are about equal.
349. (A) A buffer resists changes in pH by acting like a “proton sponge,” giving them to the solution when the [H+] decreases, and absorbing them from the solution when the [H+] increases. A buffer consists of an acid or base and the salt of its conjugate base or acid. The region of the curve between 0 and about 25 mL NaOH added is the buffer region. The weak acid and its conjugate base are both present and very little neutralization has occurred.
350. (D) The question is asking us to identify the compound that would produce a basic solution. LiF is a soluble salt (any ionic with an alkali metal is soluble) that produces F− upon dissociation. F− is a good conjugate base and readily picks up protons in solution, increasing the pH.
351. (E) Conjugate acid/base pairs always differ by one proton (H+). If we are looking for a conjugate acid, it means that NH3 is acting as a base, accepting a proton. The conjugate acid would be NH4+, one more H+.
352. (B) Two handy rules regarding pH and pOH are:
![]()
This problem can be solved in two ways. (1) If the pH = 6, the pOH = 8 ∴ the [OH−] = 1 × 10−8. (2) If the pH = 6, the [H+] = 1 × 10−6 the [OH−] < 1 × 10−8. This makes sense because we expect the [OH−] < 1 × 10−7 in an acidic solution (pH 6).
353. (B) To answer this question correctly we must remember that the pH scale is a log10 scale, so a pH of 6 is 10 times more acidic than a pH of 7 and 100 times more acidic than a pH of 8. If we want to acidify the pH by 1 point on the pH scale, we must either reduce the [OH−] by 10 and increase the [H+] by 10 (an increase in one will always result in a decrease of the other, see the two rules in Answer 352). So we must dilute the basic solution tenfold to decrease the pH from 13 to 12.
354. (E) The values for K1, K2, and K3 decrease successively, so we expect the anion of the third deprotonation to be present in the lowest concentration. We can also consider that only a small fraction of H3C6H5O7 will lose a proton, leaving H2C6H5O7− behind. Let’s say 1 percent to make it simple, although it’s actually much lower than that. Out of the small number of H2C6H5O7− ions, only a small fraction of those will lose the second proton, leaving HC6H5O72– behind. Again, let’s say 1 percent for simplicity. That means that 1 percent of 1 percent (0.01 percent) of the original H3C6H5O7 loses two protons. Finally, if 1 percent of HC6H5O72– loses the third proton, only 1 percent of 0.01 percent of the original H3C6H5O7 would have lost all three protons (0.0001 percent).
355. (C) A buffer resists changes in pH by acting like a “proton sponge,” giving them to the solution when the [H+] decreases and absorbing them from the solution when the [H+] increases. A buffer consists of an acid or base, and the salt of its conjugate base or acid. Choice I represents a weak base and its conjugate acid, and choice III represents a weak acid and its conjugate base. Choice II, however, represents a strong acid and the salt of its conjugate base. The conjugate bases of strong acids are very weak and are not able to pick up protons in solution, so they are not good buffers.
356. (E) 0.2L × 0.2 mol/L = 0.04 mole Sr(OH)2 but × two OH− per Sr(OH)2, so 0.08 mole OH–
0.8 × .8 × 2 = 1.28
1.36 M OH = pH of 14.
357. (D) The final volume is 200 mL. We can use M1V1 = M2V2 to solve this problem: (100)(0.002) = (M2)(200) = 0.001 M, or just realize that the concentration has been halved by adding an equal volume of water to the solution. HCl is a strong acid and so the concentration of HCl is equal to the [H+]. pH = –log [H+] ∴ –log [1 × 10−3] = 3.
358. (D) The final volume will be 20 mL. We can use M1V1 = M2V2. (5)(0.02) = (M2) (20) = 0.005 M but that is the molarity of the BaOH2 solution, not the [OH−] concentration. There are two OH− per BaOH2 so the concentration of OH− is two times that of the [BaOH2] ∴ 0.01 M. The pOH = –log [OH−] = –log [1 × 10−2] = 2 ∴ pH = 12.
359. (C) Phenolphthalein changes from clear in acid to pink in base. We add the phenolphthalein to the acid and titrate with the base. When the acid we are titrating turns pink, we know the endpoint has been reached.
360. (C) The numbers on the graduations differ on the two burettes, so we need to be careful to read both burettes and not just take the difference between the heights of them. Since it’s the same solution, we can read from the meniscus or we can read from the edge. It doesn’t matter in a question like this because we’ll get the same answer either way. We will read from the edge because it’s closest to a whole number, 10, on the first burette, and 41.5 on the second burette.
The change in volume = Vfinal – Vinitial = 41.5 – 10 = 31.5 mL.
361. (C) We use MaVa = MbVb, but we need to remember that Sr(OH)2 has two moles of OH− ions per mole Sr(OH)2. [(Ma)(35) = (0.5 × 2)(25)] × Ma = ~0.7 M.
Chapter 10: Electrochemistry
362. (B) The answer to this question is very clear if we know a simple fact: Zn takes on only one oxidation state, +2. (A trick for remembering the oxidation states of Ag, Zn, and Al: they are connected diagonally on the periodic table, and as we progress from Ag to Zn to Al, the oxidation states are +1, +2, and +3.)
363. (E) The sum of all the oxidation states of all the atoms must sum to the overall charge of the compound. In this case, the oxidation state of perchloric acid is zero:
H = +1 and O = –2 (× 4) = –8 ∴ Cl = +7
364. (E) The sum of the oxidation numbers on the atoms in the chromite ion, ![]() must equal the charge on the compound, –2. O = –2 (× 4) – –8 ∴ 2 Cr = +6 ∴ 1 Cr = +3. The answer choices provide some complicated compounds for assigning oxidation numbers. Try the easiest ones first. Cr2O3 looks promising because it only has three oxygen atoms and no charge. The –2 charge on
must equal the charge on the compound, –2. O = –2 (× 4) – –8 ∴ 2 Cr = +6 ∴ 1 Cr = +3. The answer choices provide some complicated compounds for assigning oxidation numbers. Try the easiest ones first. Cr2O3 looks promising because it only has three oxygen atoms and no charge. The –2 charge on ![]() negated the effect of the extra oxygen, so the Cr atom in Cr2O3 should be the same (and it is). Chromium takes on oxidation states ranging from –2 to +6. Negative oxidation states are not common for metals. Cr2(O2CCH3)4 is chromium (II) acetate, and features a quadruple bond(which occurs extremely rarely). Cr(CO)6 is chromium hexacarbonyl, a compound in which Cr takes on a zero oxidation state in this compound. The Cr in dichromate,
negated the effect of the extra oxygen, so the Cr atom in Cr2O3 should be the same (and it is). Chromium takes on oxidation states ranging from –2 to +6. Negative oxidation states are not common for metals. Cr2(O2CCH3)4 is chromium (II) acetate, and features a quadruple bond(which occurs extremely rarely). Cr(CO)6 is chromium hexacarbonyl, a compound in which Cr takes on a zero oxidation state in this compound. The Cr in dichromate, ![]() , has an oxidation state of +6, and potassium peroxochromate, K3[Cr(O2)4], features a +5 oxidation state for Cr.
, has an oxidation state of +6, and potassium peroxochromate, K3[Cr(O2)4], features a +5 oxidation state for Cr.
365. (E) The electrical conductivity of a solution is determined mainly by the concentration of its ions and its temperature. The concentration of ions in solutions equimolar with respect to compounds varies according to solubility and the van’t Hoff factor (i). Strong acids and bases, and soluble salts are good electrolytes because they dissociate completely. H3PO4 is a weak acid and therefore does not dissociate into 3 H+ and a ![]() as we might expect; however, if dissolved in a basic solution, it can. The Ka values for the protons are 7.5 × 10−3, 6.2 × 10−8, and 4.8 × 10−13. For a given acid in solution, if the pH < pKa “the proton is on” and if the pH < pKa, “the proton is off.”
as we might expect; however, if dissolved in a basic solution, it can. The Ka values for the protons are 7.5 × 10−3, 6.2 × 10−8, and 4.8 × 10−13. For a given acid in solution, if the pH < pKa “the proton is on” and if the pH < pKa, “the proton is off.”
366. (A) Some oxidation and reduction reactions are spontaneous (exergonic). Our table of Standard Reduction Potentials is an incomplete list of them. Reductions that have a positive E° value are exergonic, which means the reverse reaction, or oxidation, is endergonic. The bottom of the table would have endergonic reductions and exergonic oxidations. Notice reducing F2 is very exergonic and reducing Li+ is very endergonic. The reduction of (1 M) Cl2produces 1.36 V, while the oxidation of (1 M) Br− requires 1.07 V. The difference between them, 0.29 V, is still favorable (+ΔE = spontaneous) so this redox reaction is spontaneous and can generate electricity. We can think of this in terms of how much Cl2 “wants” to be reduced versus how much Br− “doesn’t want” to be oxidized. The difference in the ΔE° values quantifies how greatly their “desires” differ and which species will get what it wants. Comparing the E° values for the two elements tells us that the ability of Cl2 to take electrons from Br− is greater than the ability of Br− to prevent Cl2 from taking them.
367. (C) Statement I is not true because Cu starts out in the solid, reduced form and ends up as a 2+ ion, so it is certainly not the oxidizing agent. (Agents get the opposite of what they do. If the agent oxidizes, it gets reduced.) Statement II is also not true. The oxidation state of hydrogen does not change in the reaction. It is +1 as an ion and +1 in water.
368. (C) We almost certainly have heard the mnemonic device that applies to all electro-chemical cells (galvanic and electrolytic), An Ox, Red Cat, which translates to Anode = Oxidation, Reduction at the Cathode. The two reactions are written as reductions, and so one of them will need to be written in reverse. Since they are opposite signs, we should flip the nonspontaneous reaction, the one with the –ΔE°. Silver is reduced at the cathode producing 0.80 V, and Zn is oxidized at the anode, producing 0.76 V. Total cell potential = 1.56 V. The Zn+ ion does not participate in this reaction.
369. (D) See explanation for Answer 368.
370. (D) See Answers 243 and for examples of how to assign oxidation numbers.
371. (C) Aluminum gets reduced (its oxidation state changes from +3 to 0) and oxygen gets oxidized (its oxidation state changes from –2 to 0). An Ox, Red Cat, which is Anode – Oxidation, Reduction at the Cathode.
372. (D) An ampere quantifies the rate at which charge (measured in Coulombs, C) flows in C/sec. The charge on one proton or electron is +/– 1.6 × 10−19 C (the +1 and –1 we use in other chemistry chapters refer to an elementary charge, the numerical value of which is +/– 1.6 × 10−19 C). A faraday is the magnitude of the electrical charge on one mole of electrons (or protons). A current of 15 amperes means 15 C, or 15 mole electrons, are available per second (pass into the cell), for 20 minutes (1,200 seconds). The numbers 20 and 60 in the numerator convert minutes to seconds. The number 27 converts grams of Al into moles.
The number 15 in the numerator converts the amperage and time into Coulombs: amps (C/s) × time (sec, from the product of 20 × 60) = Coulombs. 1 mole e− = 96,500 C, so if 18,000 C are 0.187 mole e− enter the cell. Each mole of Al requires 3 moles of e− to get reduced, so 0.187 ÷ 3 = 0.062 mole of Al will get reduced. At 27 g mol−1, that’s 1.7 g Al. An interesting fact about aluminum is that it’s the third most abundant element on earth, but its electrolytic production is very energy intensive. Approximately 4 percent of the electricity consumption in the United States is used for the production of aluminum.
373. (D) The reduction of X2+ to X is the reverse of Reaction 1, so the DE° of the reaction is –2.27 V, but the oxidation of Ag also occurs. Reaction 2 gives us the reduction of Ag+, which produces 0.80 V, so its oxidation is –0.8 V. Subtracting the oxidation of Ag (–0.80 V) from Reaction 1 (–2.27 V) leaves us with the half-reaction for the reduction of X and a standard reduction potential of –1.47 V.
374. (D) An ampere quantifies the rate at which charge (measured in Coulombs, C) flows in one second, so the unit of current is C/sec. The charge on one proton or electron is +/– 1.6 × 10−19 C (the +1 and –1 we use in other chemistry chapters refers to an elementary charge, the numerical value of which is +/– 1.6 × 10−19 C). A faraday is the magnitude of the electrical charge on one mole of electrons (or protons). 1.00 ampere = 1 C/sec. The oxidation state on Fe in FeCl3 is +3, so it will require 3 moles of e– to reduce 1 mole Fe3+.
3 moles of e− = 96,500 C × 3 = 289,500 C required. Delivered at a rate of 1.00 C/sec, that would require 289,500 seconds (more than 80 hours).
375. (B) A simple way to eliminate some answer choices is to count electrons. We need to remember we are dealing with the Zn2+ ion, which has two fewer electrons than Zn ∴30 – 2 = 28 electrons. Choices (B), (D), and (E) have 28 electrons so we’ve already eliminated two choices. The electrons of highest energy (n) are lost first, so the Zn atom loses its 4s electrons leaving a full d subshell. That leaves only choice (B).
376. (D) The number of positive charges in the nucleus of Zn2+ is still 30, but now those 30 protons are only pulling on 28 electrons.
377. (A) Use the mnemonic An Ox, Red Cat, which comes to Anode = Oxidation, Reduction at the Cathode. The question is, which species—Zn or H2—gets oxidized. We may remember that in the hydrogen electrode, 2 H+ are reduced to H2, but if we don’t, that’s fine. We know the cell is spontaneous because it is a galvanic cell and therefore the E° of the cell must have a positive number. Since the reduction of Zn2+ is negative, the oxidation is positive, and our cell will work spontaneously. The hydrogen electrode is the cathode, so choices III and IV do not apply.
378. (D) The salt bridge has two functions in the galvanic cell. (1) It completes the circuit by providing a bridge of mobile ions between the two half cells and (2) it provides ions to the half cells extending the life of the battery.
At the anode, the oxidation of solid Zn produces Zn2+ ions in solution. The salt bridge neutralizes the excess positive charges by providing NO3− ions. At the cathode, H+ ions are being reduced to H2 gas, so the salt bridge provides K+ ions to replace the lost cations, neutralizing the Cl− ions.
379. (E) It’s not possible to measure the E° of a half reaction (or half cell) so the reduction of 2 H+ to H2 was given the arbitrary value of zero and all the other standard reduction potentials were measured relative to it.
380. (C) We know the cell is spontaneous because it is a galvanic cell, not an electrolytic cell. Spontaneous cells have a negative Δ G and the sum of the oxidation and reduction potentials must be positive.
381. (E) The Nernst equation relates half-cell concentrations to the E of the cell by E = E° = (0.0257 V/n) ln Q, where Q is the reaction quotient. As the cell operates, the flow of electrons from the anode to the cathode results in the formation of product and the consumption of reactant. This increases product concentration and decreases reactant concentration. As the cell approaches equilibrium, E = 0 (and Q = Keq). Zn2+ is the product of the oxidation that occurs at the anode.
382. (A) A sacrificial anode is also called a galvanic anode, and is a metal with a more negative electrochemical potential. This allows the sacrificial anode, the Zn, to be oxidized preferentially to the “cathode,” in this case, the iron.
383. (B) Electrical energy = electrical potential × the base unit of charge = Voltage × Coulombs. A volt = 1 J C−1 ∴ electrical energy can be expressed in joules. The sum of the two half-cells only determines the maximum voltage of the cell, not how much energy the cell can generate.
384. (C) In an electrochemical wet cell, concentration does matter. The E° values on the table of Standard Reduction Potentials are for standard conditions that include 1.0 M concentrations. The Nernst equation is used to calculate the electromotive force (voltage) of a cell under nonstandard conditions. (See Answer 11 for an except question strategy.)
385. (B) From the balanced redox equation we can see that Li(s) is oxidized, and then we remember: An Ox, Red Cat: Anode = Oxidation, Reduction at theCathode.
386. (E) Choices (A) through (D) make lithium-ion batteries superior to many other types of dry cells, but they are very reactive. They can explode if not handled with care. (See Answer 11 for an except question strategy.)
387. (A) All electrochemical cells abide by An Ox, Red Cat:
Anode = Oxidation, Reduction at the Cathode. However, galvanic (voltaic) cells are spontaneous and convert chemical energy into electrical energy whereas electrolytic cells require energy to cause nonspontaneous redox reactions (electrolysis).
Chapter 11: Nuclear Chemistry
388. (E) When balancing nuclear reactions, remember: (1) Conservation of mass number (the number of protons and neutrons are equal in the products and reactants) and (2) Conservation of atomic number (the number of nuclear charges in the products and reactants are the same).
Total mass number in reactants = 236
Total atomic number in reactants = 92
Mass number in products must = 236 ∴ 236 – 141 – (3 × 1) = 92
Atomic number in products must = 92 ∴ 92 – 55 = 37, rubidium
389. (B) See Answer 388.
Total mass number in reactants = 31
Total atomic number in reactants = 15
Mass number in products must = 31 ∴ 31 – 1 = 30
Atomic number in products must = 15 ∴ 15 – 0 = 15, phosphorus
390. (B) See Answer 388.
Total mass number in products = 18
Total atomic number in products = 9
Mass number in reactants must = 18 ∴ 18 – 4 = 14
Atomic number in reactants must = 9 ∴ 9 – 2 = 7, nitrogen
391. (D) Choice (D) = gamma radiation (γ), a high-frequency form of electromagnetic radiation (EMR). Visible light is a very low frequency of EMR relative to gamma, but the basic properties of EMR are its wave- and particle-like properties, and its lack of mass.
392. (A) Choice (A) is alpha decay. An alpha particle is a helium nucleus, with a mass number of 4. It makes it the most massive particle and therefore least penetrative form of radioactive decay. (For a given value of kinetic energy, more massive particles have lower speed.)
393. (D) Choice (D) = gamma radiation (γ), the highest frequency of electromagnetic radiation (EMR) known. The frequency of EMR is proportional to its energy, so gamma radiation is the highest energy EMR known. The frequency of gamma radiation is on the order of magnitude of 1019 Hz, and the wavelengths are less than the diameter of an atom. Gamma radiation is ionizing because it has enough energy to dislodge electrons from atoms and molecules and produce free radicals in the body.
394. (A) Choice (A) is an alpha particle, a helium nucleus with a 2= charge. Other charged decay particles have a +1 or –1 charge.
395. (B) Choice (B) is beta decay, a form of decay in which a neutron turns into a proton and electron. The electron is ejected from the nucleus as a beta-particle (0−1e or 1−1β), and the atomic number increases by 1, leaving the mass number intact (so the number of neutrons decreases by 1). Beta-decay decreases the neutron to proton ratio of an unstable nucleus.
396. (C) Nuclear binding energy is an indication of the stability of a nucleus. The binding energy is the energy required to break up a nucleus into protons and neutrons. The nucleus with the highest binding energy per nucleon is the most stable.
397. (A) Choice (A) is false because the curve represents binding energy per nucleon, not per nuclide. More importantly, the stability of a nucleus is the sum of two forces, attractive and repulsive. The net attractive force is what ultimately determines the stability of the nucleus, not the total attractive force (without considering the repulsive forces). (See Answer 11 for an except question strategy.)
398. (C) The definition of mass defect. The binding energy is the energy required to break up a nucleus into protons and neutrons.
399. (D) The lost mass is accounted for by Einstein’s famous mass–energy equivalence equation, E = mc2. Since the value of c2 = 9 × 1016, a tiny piece of mass lost from the nucleus is transformed into a lot of energy.
400. (E) Statement I correctly accounts for the chemical reactivity of the alkali metals. Chemical reactivity (or stability) is a function of electron configuration, whereas radioactivity is a function of nuclear stability (neutron-to-proton ratio). Statement II is incorrect; radioactive nuclei increase their stability by decaying, often transmuting into another species of atom. Statement III is correct about all the non-noble gas elements; they all form molecules that are more stable than the lone atoms of the element. The “motivation” of atoms is to fill their valence shell by giving, taking, or sharing electrons with other atoms.
401. (A) This is the strong nuclear force, and it only works when the protons very close to each other are on the order of about 10−15 m. The nucleus only carries positive charges (protons) although it can create and emit electrons (β-particles, 00e−) through β-decay. The nucleus is dense, so the protons can’t be spread out enough to escape the like-charge repulsion. The neutrons don’t form a cage around the protons. There would need to be a force that holds the neutrons together in cage formation, and that force would need to exceed the repulsive force of the like-charge repulsion to be an effective cage. Besides the fact that it isn’t true, it doesn’t explain anything about the forces; it only implies a new force exerted by a different particle.
402. (C) The average mass (1 × 10−22 g) ÷ the volume of the nucleus (4/3 π (5 × 10−13cm)3) = 2 × 1014g cm−3 (π in this equation = 3.14, the ratio of the diameter of a circle to its circumference. It does not refer to the bond). We didn’t have to do all that math, however. To estimate an order of magnitude we use 10−22 ÷ (10−13)3 = 10−22 ÷ 10−39 = 1017. That is three orders of magnitude off, which is 1,000 times too big (a big error!), but the AP Chemistry exam won’t give us a calculation on the multiple choice section of the exam that requires a calculator to compute in a reasonable amount of time. A question with gigantic or infinitesimal numbers will offer significantly different quantities as answer choices so you can round generously.
403. (C) One half-life = →, so 30 g → 15 g → 7.5 g → 3.75 g → 1.875 g → 0.94 g. The decay to less than 1 gram took five half-lives (five arrows). 325 days ÷ 5 half-lives = 65 days per half-life.
404. (B) In four half-lives, 93.75 percent of a sample decays (see table below). 24 days ÷ 4 half-lives = 6 days per half-life.

405. (C) After three half-lives, 12.5 percent of a sample remains undecayed (see table in Answer 404). If three half-lives take 30 days, each half-life = 10 days.
406. (E) There are six, two-year half-lives in 12 years. The table in Answer 404 shows that after five half-lives, about 3 percent of the radioisotope remains undecayed (about 1.9 g out of the original 60). One more half-life would reduce that by half, leaving less than, but close to, 1 gram.
407. (B) When an atom’s mass number stays the same but the atomic number increases, the atom has undergone a β− (beta) decay. When the mass number of an atom stays the same but the atomic number decreases, the atom has undergone a β− (positron) decay. About 0.01 percent of K atoms are 40K. β–decay decreases the proton-to-neutron ratio by converting a neutron into a proton and an electron (β-particle, 00e−), the proton stays in the nucleus (so the atomic number increases by one) and the β–particle, the electron, is ejected. Because the total number of protons and neutrons stayed the same, the mass number doesn’t change.
408. (E) Radioactive decay displays first-order kinetics. Choice (E) is a plot of a first-order reaction (with respect to substrate disappearance). Choice (B) is a plot of a second-order reaction (with respect to substrate disappearance).
Chapter 12: Descriptive
409. (D) Silicon combines with oxygen to form a variety of silicates. The most common silicate is silicon dioxide, which has a number of different crystalline and amorphous forms. Silicon dioxide is a covalent network solid, and the formula SiO2 is the empirical formula of the solid.
410. (C) NaCl is the salt most responsible for the salinity of sea water and the main ingredient in table salt (which may also contain minute amounts of iodine salts, added to prevent iodine deficiency). Any ionic compound with alkali metal is soluble, and compounds formed with alkali and alkali earth metals (groups 1 and 2) as the only metals are generally not colored.
411. (B) CuSO4 is a deliquescent compound. It is white when anhydrous (without water) and turns blue upon hydration. (See Answer 454 for a summary of properties related to deliquescence.)
412. (D) Most chlorides salts are soluble, with the notable exception of AgCl, HgCl2, and PbCl2. The process of elimination may have allowed us to identify this compound since NH4NO3 and NaCl are both white, KMNO4 is purple, and CuSO4 is white or blue depending on its state of hydration.
413. (A) Potassium permanganate, KMnO4 contains manganese. The presence of transition metals in compounds often results in bright colors, even though the pure metals are mostly silver (with the notable exceptions of copper and gold). KMnO4 is a strong oxidizing agent.
414. (B) CuSO4 is a deliquescent compound, which means it has a high affinity for water molecules and can absorb them from the air. CuSO4 changes color when it hydrates (it turns blue), making CuSO4 an excellent desiccant(because we know when it’s full of water and needs to be dried). A desiccant is a substance used to create or sustain a dry environment by absorbing water from it.
415. (E) Ammonium nitrate, NH4NO3, is very soluble. (All ionic compounds containing the ![]() ion or the NO3− ion are soluble, and this one has both.) It is used for fertilizers not only because it is high in nitrogen, but because the two forms of nitrogen (ammonium and nitrate) are the most readily absorbed and used by plants. The most abundant gas in the atmosphere is N2 (78 percent). The least abundant is CO2 (less than 1 percent, but it has considerable effects). Plants can only use the carbon dioxide in the atmosphere as a substrate in organic syntheses, they cannot use N2 as a source of nitrogen. Only a few kinds of bacteria can metabolize N2 into a form that is usable by plants.
ion or the NO3− ion are soluble, and this one has both.) It is used for fertilizers not only because it is high in nitrogen, but because the two forms of nitrogen (ammonium and nitrate) are the most readily absorbed and used by plants. The most abundant gas in the atmosphere is N2 (78 percent). The least abundant is CO2 (less than 1 percent, but it has considerable effects). Plants can only use the carbon dioxide in the atmosphere as a substrate in organic syntheses, they cannot use N2 as a source of nitrogen. Only a few kinds of bacteria can metabolize N2 into a form that is usable by plants.
416. (A) Propane is a gaseous alkane that is often compressed into a liquid for transport. It is a by-product of natural gas processing and the fractional distillation of petroleum. Hydrocarbons are useful fuels because their combustion has a negative free energy change of great magnitude and yields a great number of moles of gas that expand rapidly at the high temperatures produced by the reaction. (They are highly reduced, so there’s a lot to oxidize.)
417. (E) Trichlorofluoromethane is a chlorofluorocarbon. It is colorless, practically odorless, and boils at room temperature. Clorofluorocarbons, or CFCs, were used as refrigerants until it became clear that CFCs cause the breakdown of ozone in the presence of ultraviolet light, the frequencies of electromagnetic radiation (1015 to 1016 Hz, sec−1) from which the ozone layer protects Earth’s surface.
418. (C) Hydrogen peroxide is a strong oxidizing agent, which makes it useful for killing bacteria (by oxidative stress). But it also may harm the cells of the body (by oxidative stress), which may prevent healing. Hydrogen peroxide is a natural by-product of all aerobic organisms, but cells contain enzymes that decompose H2O2 to O2 and H2O at the (low) concentrations produced in cells (as compared to the 3-percent solution in the pharmacy).
419. (D) Hydrogen sulfide has the odor of rotten eggs. It is produced by the decomposition of organic matter under anaerobic conditions and is also found in volcanic gases.
420. (B) Remember that the hydrogen halides other than HF (HCl, HBr, and HI) are strong acids. HF is considered weak, but it is still highly corrosive, hence its use in etching glass. Because of its reaction with glass (and metal), HF must be stored in plastic containers.
421. (C) (See Answer 94 for an explanation of how graphite conducts electricity.)
422. (E) All amino acids contain the carboxyl and amino functional groups. The carboxyl group = COOH (physiological pH, 7.2 – 7.4, is above the pKa of the carboxyl group, so it is in its deprotonated form, COO−, in body fluids) and the amino group = NH2 (physiological pH is below the pKa of the amino group so it is in its protonated form, NH3+, in body fluids). The four elements C, H, O, and N make up 96 percent of living matter (easily remembered as CHON).
423. (D) This question is really asking us to recognize SiO2 as a covalent network solid (or at least not as a gas) as opposed to asking us to recognize the rest of the answer choices as gases (although it’s a good idea to be able to). (See Answer 11 for an except question strategy.)
For Questions 424–428:
(A) Isoamyl acetate (banana oil)
(B) Ethyl methyl ether
(C) Benzoic acid
(D) Benzone
(E) Glucose
424. (D) The C=O and the ending “one” make this the ketone.
425. (E) Glucose has five hydroxyl groups per molecule making it very much water soluble.
426. (A) Isoamyl acetate is an ester and is responsible for the fruity odor found in bananas and many other sweet-smelling items.
427. (C) The carboxylic acid group is represented by –COOH.
428. (B) An ether has two alkyl chains attached to a singly bonded oxygen.
Chapter 13: Laboratory procedure
429. (E) It is not only acceptable but it is standard practice to rinse a burette with the solution that will be added to it before it is filled with the solution. This ensures that any water or impurities introduced into the burette during cleaning will be removed. Any water or impurities left in the burette will dilute or contaminate the solution, decreasing precision and accuracy. For our safety and the well-being of the balance, never place hot objects on a balance. For some substances, this will introduce error into the weighing, as well (air currents are produced around hot objects, and some substances will pick up mass from or lose mass to the environment depending on its temperature).
Adding hot water to a volumetric flask is also considered unsafe, mainly because the mouth of the flask has a small diameter. Using 5 mL of phenolphthalein to titrate 20 mL of acid means that 5 out of 25 mL total volume of our solution is the phenolphthalein indicator. That’s 20 percent! Finally, remember the strained but useful rhyme “Slowly you ought–ta add acids to wat-tah.” The dissociation of an acid (or base) in water is typically exothermic, but for the strong acid and bases, it’s strongly exothermic. The heat produced by the dissociation can rapidly increase the temperature of the water. Slowly adding acid to water allows the water to absorb excess heat from the dissociation as you pour, so the heat produced can be regulated by the person pouring. It also prevents a harmful splash back. Even if some of the liquid does splash back, it’s likely going to be the water that was displaced by the acid that gets splashed instead of the undiluted acid. (See Answer 430 for a detailed explanation of why we add base to water slowly.)
430. (D) Remember the rhyme, “Slowly you ought–ta add bases to wat-tah.” The dissociation of strong base in water is highly exothermic. Adding the base slowly to water allows the water to absorb excess heat from the dissociation and allows the pourer to adjust the pour accordingly. If the base is added too quickly, or an insufficient amount of water is added to a base, the container can get too hot and can even boil. It can also splatter out of the container due to the vigorous reaction. The composition of the splatter will be a hot (possibly boiling), concentrated solution of a strong base. (See Answer 429 for a detailed explanation of why we add acids to water slowly.)
431. (D) The best way to deal with an acid spill is to rinse the area to remove excess acid, then neutralize it with a weak base. Never use strong acids or bases to neutralize a base or acid spill, especially on skin. Acids and bases neutralize each other in a highly exothermic reaction that can thermally burn skin (in addition to chemically burning it).
432. (B) The most direct and efficient method to determine the molarity of this solution is to measure its volume. Two pieces of information are needed to calculate molarity (M = mol/L), mol and L. The student already knows how many moles of acetic acid are present, so all that needs to be done is to measure the total volume and plug it into the expression for molarity.
433. (B) The most direct and efficient method to determine the molality is to measure the mass of the solution. The student needs two pieces of information to calculate molality (m = mol/kg solvent), mol and kg solvent. The student knows the mass of the phosphoric acid, so the number of moles can be calculated. Also, the mass of the phosphoric acid has to be subtracted from the total mass to determine the mass of the solvent, which is needed for the expression.
434. (E) The conductivity of a solution is a measure of its ability to conduct electricity. It can be quantified by measuring the resistance to the flow of electricity between two electrodes in the solution.
435. (B) Chromatography is a general term for a laboratory procedure used to separate the components of a mixture. Paper chromatography uses a two-phase system, a solvent and paper, to separate the components of a solution according to their differential solubilities in (or affinities for) the solvent and the paper.
436. (A) The concentration of a colored solution can be determined by colorimetry, or visible-light spectrophotometry. The more concentrated the solution, the greater the absorbance (or less transmittance) at a particular wavelength. Using standard solutions of known concentrations, a graph of absorbance (or transmittance) versus concentration is constructed so that any absorbance (or transmittance) value can be linked to a concentration by interpolation (obtaining “implied” data from areas on a graph within discrete, measured data points).
437. (D) Gravimetric analysis is a method for analyzing the amount of a substance by weighing the products of its reaction with something else that is known. For example, we could determine the concentration of sulfate ions in a solution taking a sample of known volume, adding Ba2+ ions until a precipitate stops forming, and then weighing (after filtering and washing) the precipitate. Because we can measure the mass of the precipitate, we know the molar masses of BaSO4 and the stoichiometry of the reaction (Ba2+ and SO42– react in a 1:1 ratio), and we can figure out how much sulfate was in the original solution. Differential precipitation exploits the different solubilities of ions in the presence of other ions or under different conditions to selectively remove them from a solution. (See Answer 438 for a description of a similar method of analysis, titration.)
438. (C) Titration is a laboratory method that is used to determine an unknown concentration of a known reactant by studying its reaction with a known concentration of a different known reactant. An indicator that the reaction has occurred, or has finished occurring, is required. The two volumes, the concentration of the known solution and the stoichiometry of the reaction, are then used to calculate the unknown concentration. For example, in Answer 437, we could have used a Ba2+ solution of known concentration to calculate the number of moles of ![]() if we had a sensitive way to determine when the very last
if we had a sensitive way to determine when the very last ![]() ions precipitated (an indicator that lets us know we matched up each every
ions precipitated (an indicator that lets us know we matched up each every ![]() ion present with a Ba2+ ion). Then we could use M1V1 = M2V2 because we know the molarity of the Ba2+ solution (Solution 1), we measured the volume needed to “capture” each
ion present with a Ba2+ ion). Then we could use M1V1 = M2V2 because we know the molarity of the Ba2+ solution (Solution 1), we measured the volume needed to “capture” each ![]() (we know they react in a 1:1 ratio), and we know the volume of the
(we know they react in a 1:1 ratio), and we know the volume of the ![]() solution (Solution 2) we titrated. (See Answer 437 for a description of a similar method of analysis, gravimetric analysis.)
solution (Solution 2) we titrated. (See Answer 437 for a description of a similar method of analysis, gravimetric analysis.)
439. (E) A liquid in an open container boils when the vapor pressure above the liquid reaches the pressure atmosphere. Pressure cookers are used to cook faster and at higher temperatures than 100°C. The temperature of water in an open container at sea level cannot exceed 100°C, no matter how much heat is added. Increasing the pressure in a sealed container is the equivalent of increasing the atmospheric pressure around an open container. In order to evaporate (and boil), the water molecules must have enough kinetic energy (KE) to reach “escape velocity.” The greater the pressure pushing down on the surface of the liquid, the greater velocity (and therefore KE) required to escape. Therefore, a higher temperature (average KE) is required to boil it. Answer choice (A) is not correct because the student would have to add a lot of salt to make the water boil significantly higher (the Kb of water is only 0.52 K/m).
440. (E) The volume of most substances changes with temperature, but the mass and the amount of particles don’t change with temperature (as long as they are closed systems). Measurements that deal with volume directly (like molarity and density) can be unreliable if the temperature is changed significantly (what constitutes a significant temperature change depends on the substance).
441. (B) A pipet will most accurately transfer a particular volume of solution. A flask is not used for the accurate measurement of volumes. A graduated cylinder is appropriate for measuring volumes, but is not the best choice for the transfer of a specific volume (too much pouring).
442. (C) The question contains the limiting measurement for significant digits, 50.00 mL.
443. (D) A volumetric flask of the proper volume (each flask measures only one volume) is the best piece of equipment for preparing solutions or measuring specific volumes that require a high degree of accuracy. The bottom of the flask is wide and fits most of the sample, but the neck of the volumetric flask is very narrow so the area at the surface of the solution is small. The error incurred by adding an extra mm (in height) of water to a cylinder with an area of 1 cm2 is insignificant (surface area × length = volume, 0.1 mL in this case) when compared to adding an extra mm of water to a cylinder with an area of 25 cm2 (2.5 mL) for two vessels of the same volume.
444. (B) ![]() and
and ![]() are both water soluble ions no matter what other ions are present so the only way to retrieve them from an aqueous solution is to evaporate the water and collect the dry solid.
are both water soluble ions no matter what other ions are present so the only way to retrieve them from an aqueous solution is to evaporate the water and collect the dry solid.
Chapter 14: Data Interpretation
445. (C) Astatine is the heaviest known halogen and is produced by the radioactive decay of other elements. Its short half-life (7–8 hours, depending on the isotope) prevents it from being purified in significant quantities. The astatine purified in the question decayed into bismuth. The bismuth then decayed into lead. We didn’t have to know the decay series of astatine to answer this question correctly. Because it has an atomic number of 85, we know it is radioactive and will likely transmutate into other elements.
446. (E) Each compound is only used once, so we can eliminate answer choices even if we’re not sure how the particular compound in the question reacts with ammonia.
1 = Silver nitrate
2 = Barium chloride
3 = Copper (II) nitrate
4 = Mercury (I) nitrate
5 = Fe3+ ions
447. (A) See Answer 446.
448. (C) At low concentrations, NH3 is a weak base and produces hydroxide ions, OH−, that combine with Ni2+ to form Ni(OH)2, which is normally insoluble. The excess NH3 allows it to form a stable, soluble, complex ion with Ni2+ instead of precipitating with the OH−.
449. (C) Solid Zn reduces H+ ions in solution to produce H2 (Zn is above H in the activity series, and can therefore be oxidized by it). When the ![]() ion is present in acidic solutions, CO2 gas is produced according to this (worth memorizing) reaction:
ion is present in acidic solutions, CO2 gas is produced according to this (worth memorizing) reaction:
![]()
NH3, however, will not produce a gas when combined with HCl. Instead, the weak base will partially neutralize the strong acid, producing a soluble salt (NH4Cl) that will remain in solution with the excess H+ ions.
450. (D) Na2CO3 is a very soluble salt (due to the Na+) and the CO32– it produces in solution is a weak base. It will pick up protons to form ![]() and even H2CO3. When the CO32– ion is present in acidic solutions, CO2 gas is produced (see the equation in Answer 449).
and even H2CO3. When the CO32– ion is present in acidic solutions, CO2 gas is produced (see the equation in Answer 449).
451. (D) The Law of Multiple Proportions states that if two elements can combine to form more than one compound, then there is a small, whole-number ratio comparing the masses in which one element combines with the fixed mass of the other. The simplest way to illustrate this is with H2O and H2O2.

If we hold the mass of hydrogen constant, we can see that the ratio of the mass of oxygen in H2O2 compared to water is 16:8 or 2:1. There is no “law of stoichiometry.” If there were, it would be a derived from the laws of definite proportions, multiple proportions, and conservation of mass.
452. (A) Finding the empirical formula only requires knowing the mole ratio in which the elements in a compound combine.
453. (C) Each of the compounds contained 28 g, or ½ mol, Fe. The tricky part is that the masses of oxygen are the masses of molecular oxygen, not atomic oxygen. To find the number of moles of oxygen atoms, we need to find the number of moles of molecular oxygen and double it (there are two oxygen atoms in each molecule). In the first compound, ½ mole Fe combined with ½ mole oxygen atoms (¼ mole O2 molecules) in a 1:1 mole ratio (FeO). In the second compound, ½ mole Fe combined with ¼ mole oxygen atoms (1/8 mole O2 molecules) in a 2:1 mole ratio (Fe2O). In the third compound, ½ mole Fe combined with ~2/3 mole oxygen atoms (~1/3 mole O2 molecules) in a 3:4 mole ratio (Fe3O4).
454. (D) Efflorescence is the loss of water from a salt crystal upon exposure to air. Deliquescence is the property of having a strong affinity to absorb water from the air. It is a property of a good dessicant, a substance used to create and/or maintain a state of dryness. Hydrophilic is synonymous with the term water-soluble. (See Answer 11 for an except question strategy.)
455. (B) Blue, or dry, CoCl2 has a molar mass of 130 g mol−1, so 13 g = 0.1 mole. The sample absorbed 4 g of water from the atmosphere during handling, which is equal to ~0.2 mole of water (twice the number of moles of CoCl2). The formula for the purple hydrate is thus CoCl2 · 2 H2O. The sample absorbed 7 additional g of water, for a total of 11 g, or 0.6 mole of water, making the formula for the red hydrate CoCl2 · 6 H2O.
456. (B) With the exception of gold and copper, the color of all of the pure transition metals (as reduced metals) is silver. The compounds of transition metals are colored, and these colors of are due to electron transitions that occur when the metal bonds with other elements. The oxidation state of the transition metal determines the color of the complex. The two most common types of electron transitions are electron transfers and d-d transitions.
Electron transfer: In a complex ion, ligands (ions or molecules that bond with the central metal) surround the metal and form a coordination complex. Often, the ligand transfers one or more of its electrons to the central metal atom (they are commonly thought of as Lewis bases). In complexes in which the central atom is a transition metal, the electrons involved in the transfer are easily excited by wavelengths in the visible light spectrum, causing some wavelengths of light to be absorbed and others to be reflected. The reflected wavelengths are those we see as colors.
d–d transitions: In transition metal complexes, the d orbitals vary in energy level. These differences in energy correspond to the wavelengths of light that can be absorbed (and reflected).
457. (B) The molar mass of a compound can be found by its vapor density. The formula is derived from the ideal gas law: PV = nRT but substitute (mass, m, in g/MM (molar mass)) for n (the number of moles) and solve for molar mass (MM).
![]()
458. (C) If the temperature was not actually brought up to 100°C but the calculation was still done using the value 373 K, the molar mass calculated would have been larger (T is in the numerator in the equation derived in Answer 457). (See Answer 11 for an except question strategy.)
459. (B) The molar mass (MM) of a compound can be found by measuring its effect on the freezing point or boiling point of a liquid. In this case, we know that 10 g of solute added to 50 grams of water raised the boiling point by 2°C.
The formula we use to determine freezing point depression or boiling point elevation is ΔT = Kbmi, where Kb is the ebullioscopic constant, a constant that allows us to relate the molality of a solution to the boiling or freezing point of the solvent (subscript b is for boiling point). The Kb and Kf are specific to a particular solvent. The van’t Hoff factor, i, is the ratio of the number of moles of particles a compound produces in solution relative to the number of moles of particles of compound added.
The formula ΔT = Kbm i can be used to determine the numerical value of m (molality = mol solute/kg solvent). The mass of the solute divided by its molar mass (MM) gives us the number of moles of solute; while the number of moles of solute divided by the mass of the solvent gives us the molality. Therefore, we can rewrite the m expression as m = (mass of solute/(MM)(kg solvent)). We know the mass of the solute and we calculated m with the equation ΔT = Kbmi, so now we rearrange our molality expression to solve for MM: MM = mass of solute/(kg solvent)(m).
Alternatively, we could substitute the expression for m, (mass of solute in g, grams/(MM)(kg solvent)), directly into the equation ΔT = Kbmi and solve for MM:
![]()
460. (C) The point in the curve where the temperature (average kinetic energy) is stable means the potential energy of the substance is changing (decreasing in this case). The absence of a temperature change in a substance that is gaining or losing energy is an indication that the substance is undergoing a phase change.
461. (C) The temperature of the solution increases about 1°C and then decreases again to 70°C over the course of about three minutes. The average slope of the line during that time period is close to zero, and indicates the approximate freezing point of the solution.
462. (B) Use ΔT = Kfmi, i = 1 (no dissociation).
![]()
463. (B) ΔT = Kfmi, i = 1 (no dissociation)
ΔT = 12.5°C, Kf= 5 ∴m = ΔT/Kf = 12.5/5 = 2.5m
molality = mol solute/kg solvent ∴ moles solute = (m) × (kg solvent)
moles solute = (2.5)(0.10) = 0.25 mol solute
moles solute = mass solute/molar mass, solving for molar mass:
molar mass = mass solute/mol solute = 20 g/0.25 mol = 80 g mol−1
464. (C) Diffraction is the spreading out of waves when they’re passed through small openings. Diffraction is a property of waves, and to occur, the wavelengths of the waves must be comparable in length to the size of the opening through which they are being passed. The pattern that emerges is similar (and probably indistinguishable from) an interference pattern, with alternating dark and light bands. X-rays have wavelengths comparable to the spaces between the ions in an ionic crystal lattice and will produce a diffraction pattern which is used to analyze the lattice structure of the crystal.
465. (E) The calculation for determining the percent mass of a hydrate = (mass of dry sample/mass of hydrated sample) × 100. If the sample was calculated to have only 26 percent water, it’s because the mass of the dry sample was greater than its actual mass, making the dry compound a larger percent of the hydrate than it actually is. Deliquescent compounds readily absorb water from the atmosphere, so the most likely cause of the increased mass of the dry compound is that it wasn’t really dry; it still had water molecules attached to it when it was weighed.
466. (A) Mg is above hydrogen in the activity series, so we know that it can be oxidized by the H+ ions in the HCl solution. The Mg will reduce 2 H+ to H2 gas. Zn is also above hydrogen in the activity series, but the Zn in Zn(NO3)2 has already been oxidized (it’s in the form of Zn2+), and so it has no electrons with which to reduce H+.
Na+ in its elemental form could certainly reduce H+, but not in its oxidized form. The CO3−2 will produce CO2 gas in an acidic solution, not H2 gas, according to the equation
![]()
467. (C) We are titrating a weak base with a strong acid. HCl is a strong acid, but since the pH at the equivalence point is < 7, we know we are titrating a weak base (the pH of the equivalence point of a strong acid-base titration = 7). Methyl red is the best indicator listed for this titration because it changes color with the pH range of the equivalence point of the titration (the equivalence point pH is it about 5 and methyl red undergoes a color change between pH 4.4 and 6.2).
468. (A) A buffer solution resists changes in pH by acting like a “proton sponge,” giving them to the solution when the [H+] decreases and absorbing them from the solution when the [H+] increases. The buffer region in this titration consists of the weak base and its conjugate acid. The buffer region is always at the beginning of the titration, when the weak base and its conjugate acid are present in fairly high concentrations, before too much neutralization has occurred. In this titration, the buffer region lies between pH 11 and 8. Phenolphthalein would remain pink in the buffer region and transition to clear at the end of it.
469. (B) The CO32– ion would have produced CO2 gas in an acidic solution (Sample 1) according to the equation
![]()
CaSO4 and BaSO4 both form an insoluble white precipitate, while aqueous NH3 precipitates green Ni(OH)2 and forms a colored solution when it reacts with water and ammonia according to the equation
![]()
470. (D) The purpose of collecting a gas over water is to keep the gas at a constant pressure as more gas particles are collected. Gases are highly compressible, so their volume changes with changes in pressure. Measuring the amount of gas in a closed, rigid container requires that both pressure and volume be measured.
An eudiometer, a device used to collect gas over water, works by displacing water with gas, but the pressure of the gas always equilibrates to the atmospheric pressure because the gas pushing down on the surface of the water in the eudiometer is the same (at equilibrium) as the pressure of the atmosphere pushing the water up in the eudiometer (by pushing down on the surface of the water in the container in which the eudiometer is placed).
471. (D) HCl and NH3 are very water soluble gases. A large number of the gases will dissolve in the water. They must pass through to be collected, greatly reducing the yield. CO2 is slightly soluble, so it’s not the best gas to collect over water, but a relatively small fraction will be lost to its dissolving in (and reacting with) water compared to HCl and NH3.
472. (B) The gas collected in the eudiometer is actually a mixture of two gases—the gas intentionally collected and water vapor that evaporated in the eudiometer. The partial pressure of the water vapor is determined solely by the temperature. At 22°C, the temperature at which the experiment was performed, the vapor pressure of water is 19.8 torr. The total pressure of the gases is 770 torr (the atmospheric pressure). Dalton’s law of partial pressures states that the total pressure of a mixture of gases is the sum of the partial pressures of each of the gases in the mixture ∴ 770 – 19.8 = 750.2 torr. (See Answer 470 for an explanation of why the total pressure of the gases in the eudiometer is equal to the atmospheric pressure.)
473. (B) masscup+water – masscup = masswater
masscup+water+ice – masscup+water = massice
474. (E) The mass of the ice measured would have been greater than the actual mass of the ice, so the heat of fusion calculated would be too low. The units of Hfusion = kJ/gram or kJ/mol. Either way, a larger mass would have increased the value of the denominator and decreased the value of Hfusion.
475. (C) To calculate the heat of fusion of ice using this method, the specific heat of the ice must be known since the temperature of the ice must be raised from –20 to 0°C before it melts. In addition, the heat used to raise the temperature of the ice and to melt it comes from the loss of heat of the liquid water, so the specific heat of liquid water must also be known. Hfus = (total heat lost by liquid water) – (heat gained by ice to reach 0°C).
476. (B) 0.4 M = 0.4 mole per liter, or 0.4 mol/L solution × 0.250 L = 0.1 mole KOH (since the L cancels). Remember to convert to L; do not use mL. The molar mass of KOH = 56 g mol−1 ∴ 0.1 mole = 5.6 g.
477. (D) This question refers to an electrolytic cell. Two hours reduced 230 grams (10 mol) of Na+ into Na. Since 1 mole of electrons is needed to reduce 1 mole of Na+ ions, 10 moles of electrons must have passed into the cell in two hours. Fe3+ requires 3 moles of electrons per mole, so we intuitively know we’ll get a little more than one-third the number of moles of Fe metal. 10 moles electron × (1 mol Fe3+/3 mol electrons) = 3.3 moles Fe3+ ions can be reduced. The molar mass of Fe is 56 g mol−1 ∴ 3.3 moles Fe weighs about 185 g.
478. (D) We don’t really need to calculate the final concentrations of ions in the resulting solution because they are all present in the same volume, so the total number of moles of each species is enough to compare their relative concentrations.
0.1 L of2 mol/L Pb(NO3)2 = 0.2 mole Pb(NO3)2, which yields 0.2 mole Pb2+ ions and
2 × 0.2 = 0.4 mole NO3− ions.
0.1 L of3 mol/L NaCl = 0.3 mole NaCl, which yields 0.3 mole Na+ and 0.3 mole Cl− ions.
PbCl2 will precipitate out of solution in a 1:2 ratio of Pb2+ to Cl−. 0.2 mol Pb2+ will precipitate 0.4 mole Cl− ions, but there are only 0.3 mole, so just about all the Cl- ions will be taken out of solution by the 0.15 mole Pb2+ ions (0.3 mole Cl− will combine with 0.15 mole Pb2+ leaving 0.2 – 0.15 = 0.05 mole Pb2+ behind).
479. (D) Differential precipitation can separate ions mixed in a solution.
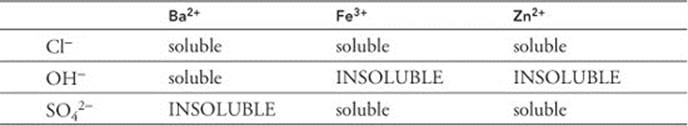
480. (A) An electron gets excited (raised to an orbital of higher energy) when its atom or molecule absorbs energy. The excited electrons are not stable at the higher energy levels, and when they drop back down to ground state, they emit energy. That energy is often lost as a photon (but there are other ways for energy to be lost). The energy of the photon is proportional to the energy change of the electron.
481. (D) The photoelectric effect demonstrated the particle-like properties of light (or more generally, electromagnetic radiation).
482. (D) E = hv ∴ E = (6.63 × 10−34)(4.4 × 1014) = 2.9 × 10−19 J.
483. (B) The energy of a photon is given by the equation E = hv, where h = 6.63 × 10−34 J. s and v = the frequency. Make sure to use the frequency and not the wavelength.
484. (C) Each line in line spectra corresponds to an energy change of an electron. Any one electron can produce several lines according to the energy transitions it experiences. (See Answer 11 for an except question strategy.)
485. (C) Waves can be imagined as vibrations produced by an oscillator. When the waves from an oscillator are polarized, all the vibrations occur in one plane.
486. (D) The spin of an electron is either +½ or –½, so only two spots would be expected to appear on the screen. The electrons spinning with +½ spin would be deflected in one direction by the field, and the electrons with the –½ spin would be deflected in the opposite direction.
487. (E) Emission lines represent the energy transitions of the electrons in an atom when energized. A magnetic field applied to the gas will not change the magnitude of the energy transitions of electrons occupying the same orbitals, but applying the field will cause the electrons to behave as magnets within the field. Electrons of opposite spins are like opposing poles of a magnet. The field will affect them in opposite directions, splitting the emission lines they produce.
488. (B) See Answer 486.
489. (C) The photoelectric effect is evidence that light (or more generally, electromagnetic radiation) has particle-like properties. When photons of a high enough frequency strike a metal plate, electrons are dislodged from the plate. Waves do not have this property. All photons are massless, they travel at the same speed in the same medium, and their energy is determined only by their frequency.
490. (E) When J.J. Thomson performed his cathode ray experiment to determine the charge-to-mass ratio of electrons, he knew the cathode ray was composed of electrons. Their deflection in magnetic and electric fields indicated they were charged. A variation of Young’s double-slit experiment using electrons instead of photons showed that electrons have the wave-like property of interference.
491. (B) Cancelling out units is the easiest way to solve this problem. We need to combine C and C/g to get g, so we can flip C/g to g/C and the C unit cancel:
–1.602 × 10−19 C × (1 gram/−1.76 × 108 C) = (1.602 × 10−19 C) × (–1.76 × 108)−1.
492. (D) Neutrons are the only particles in the list that have no charge. Any charged particle will be deflected when passed through an electric or magnetic field.
493. (B) Liquids with strong intermolecular forces have a high surface tension due to the strong attraction of surface molecules to each other. Strong forces of attraction between the molecules in a liquid cause the surface molecules to stick to each tightly, creating the appearance of a film. The greater the tension of the film, the steeper the meniscus it creates.
494. (E) Viscosity is defined as a fluid’s resistance to flow. Liquids with strong intermolecular forces will have a high viscosity because the particles in the liquid resist moving past one another.
495. (E) See Answer 494.
496. (E) Miscible typically refers to two or more liquids that are soluble in each other. Miscible sounds like mixable, and they can be thought of as synonyms. Water and glycerol can both form hydrogen bonds (The –ol ending in glycerol tells us it’s an alcohol and ∴ has O—H bonds. Glycerol is actually a three-carbon compound with a hydroxyl group on each carbon, for a total of three.). The high surface-tensions of water and glycerol and the high viscosity of glycerol indicate that their particles experience strong intermolecular forces of attraction.
497. (C) A Newton (N) is a unit of force. Surface tension is measured by the force required to break the surface of a liquid. The surface tension of castor oil is expected to be lower than water and glycerol since the only intermolecular forces of attraction in oils are weak London dispersion forces. We would expect the surface tension to be similar to that of olive oil but larger than benzene (castor oil and olive oil are triglycerides, or triacylglycerols), which have very high molar masses (in several hundred g mol−1).
498. (B) The breaking of van der Waals forces (intermolecular forces of attraction, IMFs) is endothermic. Higher temperatures disrupt IMFs, decreasing surface tension and viscosity.
499. (B) A very small fraction of the molecules of a weak acid dissociate in solution, so we expect slightly more than 1 M of particles (but much less than two).
500. (E). A good electrolyte produces a high concentration of ions in solution. Compound E produces three moles of ions per mol compound when dissolved. Soluble salts and strong acids and bases are excellent electrolytes because they completely dissociate in solution, producing at least two moles of ions per mole of compound.