5 STEPS TO A 5: 500 AP Chemistry Questions to Know by Test Day! (2012)
Chapter 3. States of Matter (Questions 91–150)
Questions 91–95 refer to the following descriptions of bonding in different types of solids.
(A) A lattice of closely packed cations with delocalized electrons throughout
(B) A lattice of cations and anions held together by electrostatic forces
(C) Strong, single covalent bonds connect every atom
(D) Strong, covalent bonds connect atoms within a sheet, while individual sheets are held together by weak intermolecular forces
(E) Strong, multiple covalent bonds including σ (sigma) and π (pi) bonds connect the atoms
91. Gold (Au)
92. Magnesium chloride (MgCl2)
93. Carbon dioxide (CO2)
94. Carbon (Cgraphite)
95. Carbon (Cdiamond)
Questions 96–98 refer to the following phase diagram of a pure substance.
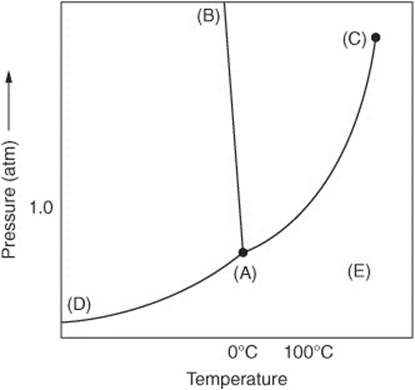
96. The point on the diagram that corresponds to the normal boiling point of the substance
97. The line on the graph that corresponds to the equilibrium between the solid and gas phases of the substance
98. All of the following are correct statements regarding the negative slope of the line indicated by the letter B except:
(A) As pressure increases, the temperature must decrease for the solid to form.
(B) As pressure increases, more heat must be removed from the compound in order to solidify.
(C) The freezing point of the compound is actually lower than the normal freezing point at pressures above 1 atm.
(D) The solid form of this compound may have a greater density than the liquid form of this compound.
(E) At low temperatures, a high pressure is required for a solid to form.
Questions 99 and 100 refer to the phase diagram for carbon dioxide.
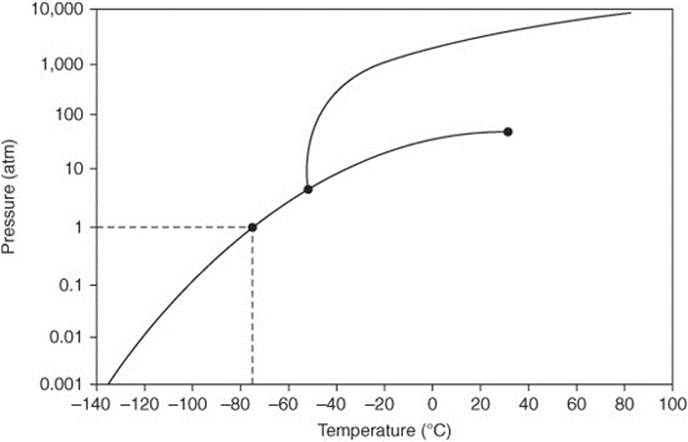
99. The temperature of a sample of pure solid is slowly raised from –100°C to 20°C at a constant pressure of 1 atm, what is the expected behavior of the substance?
(A) It melts to a liquid and then boils at –80°C.
(B) It melts to a liquid but does not boil until a temperature higher than 100°C is reached.
(C) It melts to a liquid and boils at about 30°C.
(D) It evaporates.
(E) It sublimes.
100. What is the expected behavior of the substance as the temperature is slowly raised from –100°C to –40°C at a constant pressure of 1 atm?
(A) It melts to a liquid.
(B) It sublimes to a vapor.
(C) It evaporates to a vapor.
(D) It first melts at approximately 80°C and then quickly evaporates.
(E) It first melts at approximately 80°C and then quickly sublimes.
101. Which of the following pure substances has the highest melting point?
(A) H2S
(B) C5H12
(C) I2
(D) SiO2
(E) S8
Questions 102–109 refer to the following diagram showing the temperature changes of 0.5 kg of water, starting as a solid. It is heated at a constant rate of 1 atm of pressure in an open container. Assume no mass is lost during the experiment.
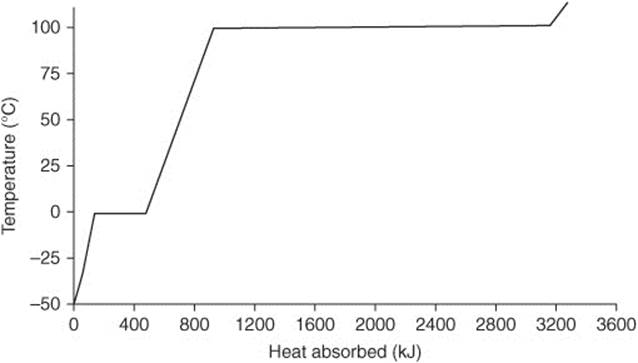
102. The sample of water requires the greatest input of energy during:
(A) The heating of ice from –50°C to 0°C
(B) The melting of ice at 0°C
(C) The heating of water from 0°C to 100°C
(D) The vaporization of water 100°C
(E) The heating of steam from 100°C to 120°C
103. Which of the following best describes what is happening at 0°C?
(A) The average kinetic energy of the particles is increasing as heat is being absorbed.
(B) The average distance between the molecules is decreasing.
(C) The number of hydrogen bonds between the molecules are increasing.
(D) The potential energy of the substance is decreasing.
(E) The substance is sublimating.
104. The heat of fusion is closest to:
(A) 75 kJ kg−1
(B) 150 kJ kg−1
(C) 300 kJ kg−1
(D) 600 kJ kg−1
(E) 750 kJ kg−1
105. The heat of vaporization is closest to:
(A) 750 kJ kg−1
(B) 1,500 kJ kg−1
(C) 1,800 kJ kg−1
(D) 2,300 kJ kg−1
(E) 3,000 kJ kg−1
106. The specific heat of ice is closest to:
(A) 2.0 kJ kg−1°C−1
(B) 4.2 kJ kg−1°C−1
(C) 6.0 kJ kg−1°C−1
(D) 8.4 kJ kg−1°C−1
(E) 10.0 kJ kg−1°C−1
107. How is the the disparity between the heat of fusion and the heat of vaporization best explained?
(A) It takes more hydrogen bonds for water to fuse than it does to vaporize.
(B) Water molecules are moving farther apart during fusion than during vaporization.
(C) Water molecules are moving closer together during fusion and farther apart during vaporization.
(D) Vaporization occurs at a higher kinetic energy than fusion.
(E) More hydrogen bonds are broken during vaporization.
108. The data in the heating curve graph can be used to calculate:
(A) The enthalpy of formation of water
(B) The enthalpy of hydrogen bond formation
(C) The specific heat of superheated steam
(D) The amount of time it takes for water to melt at 0°C
(E) The density of water at 50°C
109. All of the following are true regarding energy and entropy changes in the water during the experiment except:
(A) The energy of the water continuously increases.
(B) The kinetic energy of the water does not increase continuously.
(C) There are two points on the curve where only the potential energy and entropy of the water are increasing.
(D) The entropy of the water only increases during phase changes.
(E) The rearrangement of the water molecules during phase changes increases their potential energy.
Questions 110–112 refer to the choices in the following table.

110. The compound with the least or weakest intermolecular forces
111. The compound that can form hydrogen bonds with the strongest London dispersion forces
112. Nonpolar molecule of lowest volatility
113. The melting point of BeO is 2,507°C while the melting point of NaCl is 801°C. Explanations for this difference include which of the following?
I. Be2+ is more positively charged than Na+.
II. O2− is more negatively charged than Cl−.
III. The Cl− ion is larger than the O2− ion.
(A) I only
(B) II only
(C) III only
(D) I and II only
(E) I, II, and III
114. A pure liquid heated in an open container will boil when at the temperature at which the
(A) average kinetic energy of the liquid is equal to the average kinetic energy of the gas.
(B) average kinetic energy of the liquid equals the molar entropy of the gas.
(C) entropy of the liquid equals the entropy of the gas.
(D) entropy of the vapor above the liquid equals the entropy of the atmosphere.
(E) vapor pressure of the liquid equals the atmospheric pressure above the liquid.
115. At the top of a high mountain, water boils at 90°C (instead of 100°C, the boiling point of water at sea level). Which of the following best explains this phenomenon?
(A) Water at high altitudes contains a greater concentration of dissolved gases.
(B) Water molecules at high altitudes have higher kinetic energies due to the lower pressure on them.
(C) Equilibrium because water vapor pressure equals atmospheric pressure at a lower temperature.
(D) The vapor pressure of water increases with increasing altitude.
(E) Water found at high altitudes has fewer solutes and impurities that allows boiling to occur at lower temperatures.
116. Which of the following best describes the changes that occur in the forces of attraction between CO2 molecules as they change phase from a gas to a solid?
(A) C–O bonds are formed.
(B) Hydrogen bonds between CO2 molecules are formed.
(C) Ionic bonds between CO2 molecules are formed.
(D) London (dispersion) forces operate to form the solid.
(E) CO2 molecules form a crystal around a nucleation point.
117. All of the following changes occur as H2O freezes except:
(A) Ionic bonds form between the water molecules.
(B) The water takes on a crystalline structure.
(C) The density of the water decreases.
(D) The mass of the water does not change.
(E) The number of hydrogen bonds between the water molecules increases.
118. Which of the following statements accounts for the increase in boiling points of the elements going down group 18 (the noble gases)?
(A) The London (dispersion) forces increase.
(B) Atoms with a large radius are closer together.
(C) Atoms of higher mass move more slowly on average than atoms of lower mass.
(D) Dipole–dipole interactions increase.
(E) The kinetic energy of the atoms decreases with increasing mass.
119. Which of the following is expected to have the highest boiling point based on the strength of intermolecular forces?
(A) Xe
(B) Br2
(C) Cl2
(D) N2
(E) O2
120. Which of the following must be true of a pure, covalent solid heated slowly at its melting point until about half the compound has turned into liquid?
(A) The sum of the intermolecular forces holding the solid together decrease to zero as the solid continues to melt.
(B) Covalent bonds are broken as the solid melts.
(C) The temperature increases and the average kinetic energy of the molecules in the liquid phase increases.
(D) The volume increases as the substance becomes a liquid.
(E) The average kinetic energy of the substance remains the same.
Questions 121–127 refer to the following gases at 0°C and 1 atm.
(A) He (molar mass 4)
(B) Xe (131)
(C) O2 (32)
(D) CO2 (44)
(E) CO (28)
121. Has the greatest density
122. The particles (atoms or molecules) of this gas have an average speed closest to that of N2 molecules at STP
123. Has the greatest rate of effusion
124. Has the lowest rate of effusion
125. Requires the lowest temperature and highest pressure to liquefy
126. The gas that has the greatest London dispersion forces
127. The gas that condenses at the highest temperature at 10 atm
128. Under which of the following conditions do gases behave most ideally?
(A) Low pressure and temperature
(B) High pressure and temperature
(C) High pressure, low temperature
(D) Low pressure, high temperature
(E) Any temperature if the pressure is less than 0.821 atm
129. At 298 K and 1 atm, a 0.5-mol sample of O2(g) and a separate 0.75-mol sample of CO2(g) have the same:
(A) Mass
(B) Density
(C) Average molecular speed
(D) Average molecular kinetic energy
(E) Number of atoms
130. At STP, a 0.2-mol sample of CO2(g) and a separate 0.4-mol sample of N2O(g) have the same:
(A) Mass
(B) Volume
(C) Average molecular speed
(D) Number of atoms
(E) Chemical properties
131. The temperature at which 32.0 g of O2 gas will occupy 22.4 L at 4.0 atm is closest to:
(A) 90 K
(B) 273 K
(C) 550 K
(D) 950 K
(E) 1,900 K
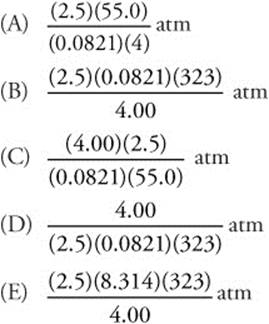
132. The pressure exerted by 2.5 mol of an ideal gas placed in a 4.00-L container at 55°C is given by which of the following expressions?
133. Gases N2(g) and H2(g) are added to a previously evacuated container and react at a constant temperature according to the following chemical equation:
![]()
If the initial pressure of N2(g) was 1.2 atm, and that of H2(g) was 3.8 atm, what is the partial pressure of NH3(g) when the partial pressure of N2(g) has decreased to 0.9 atm?
(A) 0.30 atm
(B) 0.60 atm
(C) 0.9 atm
(D) 1.8 atm
(E) 3.8 atm
134. Which of the following gases behaves least ideally?
(A) Ne
(B) CH4
(C) CO2
(D) H2
(E) SO2
135. Which of the following gases will behave most ideally?

136. Equal masses of Ne and Ar are placed in a rigid, sealed container. If the total pressure in the container is 1.2 atm, what is the partial pressure of Ar?
(A) 0.20 atm
(B) 0.40 atm
(C) 0.60 atm
(D) 0.80 atm
(E) 2.40 atm
137. A flask contains 0.5 mol of SO2(g), 1 mol of CO2(g), and 1 mol of O2(g). If the total pressure in the flask is 750 mmHg, what is the partial pressure of SO2(g)?
(A) 750 mmHg
(B) 375 mmHg
(C) 350 mmHg
(D) 300 mmHg
(E) 150 mmHg
138. A 2-L container will hold approximately 3 grams of which of the following gases at 0°C and 1 atm?
(A) CO2
(B) H2O
(C) Cl2
(D) O2
(E) NH3
139. A 2-L flask contains 0.50 mole of SO2(g), 0.75 mole of O2(g), 0.75 mole of CH4(g), and 1.00 mole CO2(g). The total pressure in the flask is 800 mmHg. What is the partial pressure of O2(g) in the flask?
(A) 125 mmHg
(B) 188 mmHg
(C) 200 mmHg
(D) 250 mmHg
(E) 375 mmHg
140. HCl and NH3 gases are released into opposite ends of a 1-meter (100-cm), vertical glass tube at 25° C. Their reaction quickly produces a white fog of ammonium chloride. If the two gases are released at exactly the same time, which of the following most closely approximates where the ammonium chloride fog would form?
(A) 20 cm from the side where NH3 was released
(B) 40 cm from the side where NH3 was released
(C) In the middle (50 cm from either side)
(D) 65 cm from the side where NH3 was released
(E) 80 cm from the side where NH3 was released
141. A 2-L container will hold about 7 g of which of the following gases at 0°C and 1 atm?
(A) SO2
(B) CO2
(C) N2
(D) Cl2
(E) C4H8
142. Which of the following gases, when collected over water, would produce the greatest yield (the highest percent collected)?
(A) CH4
(B) HCN
(C) SO2
(D) HCl
(E) NH3
143. Which of the following best explains why a hot-air balloon rises?
(A) The rate of diffusion of the hot air inside the balloon is greater than the rate of diffusion of the colder air surrounding the balloon.
(B) The pressure on the walls of the balloon is greater than the atmospheric pressure.
(C) The difference in temperature and pressure between the air inside and outside the balloon creates an upward acting current.
(D) The average density of the balloon is less than that of the surrounding air.
(E) The higher pressure of the surrounding air pushes on the sides of the balloon, squeezing it up to higher altitudes.
144. A rigid metal container contains Ne gas. Which of the following is true of the gas in the tank when additional Ne is added at a constant temperature?
(A) The pressure of the gas decreases.
(B) The volume of the gas increases.
(C) The total number of gas molecules remains the same.
(D) The average speed of the gas molecules remains the same.
(E) The average distance between the gas molecules increases.
145. Equal numbers of moles of Ar(g), Kr(g), and Xe(g) are placed in a rigid glass vessel at room temperature. If the container has a pin hole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the remaining gases after some effusion has occurred?
(A) PAr < PKr < PXe
(B) PXe < PKr < PAr
(C) PKr < PAr < PXe
(D) PAr < PXe < PKr
(E) PAr = PKr = PXe
146. Which of the following gases has the greatest average molecular speed at 298 K?
(A) He
(B) H2
(C) N2
(D) O2
(E) Ne
Questions 147–149 refer to the following situation.
In a laboratory experiment, a student reacts Na2CO3 (106 g mol−1) with HCl. Water displacement is used to measure the amount of CO2 produced (the gas over water is collect in a eudiometer).
147. If the student reacts 10.6 g Na2CO3 in 250 ml of 2.50 M HCl, how many moles of CO2 gas would one expect to collect?
(A) 0.10 mol CO2
(B) 0.25 mol CO2
(C) 0.325 mol CO2
(D) 0.63 mol CO2
(E) 1.625 mol CO2
148. The volume of gas the student collects is significantly less than expected because the CO2 gas
(A) can react with water.
(B) is denser than water vapor.
(C) has a molar mass larger than N2 and O2 and therefore cannot displace the air above the water in the eudiometer.
(D) has a molar mass larger than N2 and O2, and therefore has a lower average speed at the same temperature.
(E) is not the gas that is actually produced by the reaction.
149. The total atmospheric pressure of the laboratory (760 mmHg), as well as the temperature of the water (22°C) and the volume of gas (502 mL) in the eudiometer, are known. Which additional data, if any, is needed to calculate the number of moles of CO2 gas collected during the experiment?
(A) The temperature of the gas collected
(B) The mass of the gas in the eudiometer
(C) The volume of H2O(l) in the eudiometer
(D) The vapor pressure of water at the temperature of the water in the eudiometer
(E) No other information is needed
150. Three gases, 1.6 g He (4 g mol−1), 4 g Ar (40 g mol−1), and 26 g Xe (131 g mol−1), are added to a previously evacuated rigid container. If the total pressure in the tank is 2.1 atm, the partial pressure of Xe(g) is closest to:
(A) 0.2 atm
(B) 0.3 atm
(C) 0.4 atm
(D) 0.6 atm
(E) 0.8 atm