Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT II: Bioenergetics and Carbohydrate Metabolism
Chapter 11. Glycogen Metabolism
I. OVERVIEW
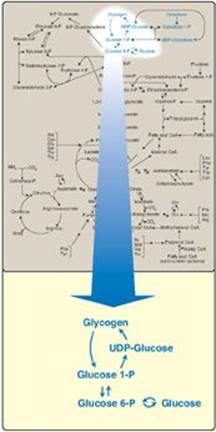
A constant source of blood glucose is an absolute requirement for human life. Glucose is the greatly preferred energy source for the brain, and the required energy source for cells with few or no mitochondria such as mature red blood cells. Glucose is also essential as an energy source for exercising muscle, where it is the substrate for anaerobic glycolysis. Blood glucose can be obtained from three primary sources: the diet, degradation of glycogen, and gluconeogenesis. Dietary intake of glucose and glucose precursors, such as starch (a polysaccharide), disaccharides, and monosaccharides, is sporadic and, depending on the diet, is not always a reliable source of blood glucose. In contrast, gluconeogenesis (see p. 117) can provide sustained synthesis of glucose, but it is somewhat slow in responding to a falling blood glucose level. Therefore, the body has developed mechanisms for storing a supply of glucose in a rapidly mobilizable form, namely, glycogen. In the absence of a dietary source of glucose, this sugar is rapidly released from liver and kidney glycogen. Similarly, muscle glycogen is extensively degraded in exercising muscle to provide that tissue with an important energy source. When glycogen stores are depleted, specific tissues synthesize glucose de novo, using amino acids from the body’s proteins as a primary source of carbons for the gluconeogenic pathway. Figure 11.1 shows the reactions of glycogen synthesis and degradation as part of the essential pathways of energy metabolism.
Figure 11.1 Glycogen synthesis and degradation shown as a part of the essential pathways of energy metabolism (see Figure 8.2, p. 92, for a more detailed view of the overall reactions of metabolism). P = phosphate; UDP = uridine diphosphate.
II. STRUCTURE AND FUNCTION OF GLYCOGEN
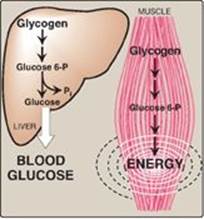
The main stores of glycogen are found in skeletal muscle and liver, although most other cells store small amounts of glycogen for their own use. The function of muscle glycogen is to serve as a fuel reserve for the synthesis of adenosine triphosphate (ATP) during muscle contraction. That of liver glycogen is to maintain the blood glucose concentration, particularly during the early stages of a fast (Figure 11.2; also see p. 329). [Note: Liver glycogen can maintain blood glucose for 10–18 hours.]
A. Amounts of liver and muscle glycogen
Approximately 400 g of glycogen make up 1%–2% of the fresh weight of resting muscle, and approximately 100 g of glycogen make up to 10% of the fresh weight of a well-fed adult liver. What limits the production of glycogen at these levels is not clear. However, in some glycogen storage diseases ([GSDs] see Figure 11.8), the amount of glycogen in the liver and/or muscle can be significantly higher. [Note: In the body, muscle mass is greater than liver mass. Consequently, most of the body’s glycogen is found in muscle.]
B. Structure of glycogen
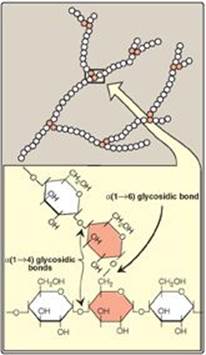
Glycogen is a branched-chain polysaccharide made exclusively from α-D-glucose. The primary glycosidic bond is an α(1→4) linkage. After an average of eight to ten glucosyl residues, there is a branch containing an α(1→6) linkage (Figure 11.3). A single glycogen molecule can have a molecular weight of up to 108 Da. These polymers of glucose exist in discrete cytoplasmic granules that also contain most of the enzymes necessary for glycogen synthesis and degradation.
C. Fluctuation of glycogen stores
Liver glycogen stores increase during the well-fed state (see p. 323) and are depleted during a fast (see p. 329). Muscle glycogen is not affected by short periods of fasting (a few days) and is only moderately decreased in prolonged fasting (weeks). Muscle glycogen is synthesized to replenish muscle stores after they have been depleted following strenuous exercise. [Note: Glycogen synthesis and degradation go on continuously. The differences between the rates of these two processes determine the levels of stored glycogen during specific physiologic states.]
Figure 11.2 Functions of muscle and liver glycogen. P = phosphate; Pi = inorganic phosphate.
III. SYNTHESIS OF GLYCOGEN (GLYCOGENESIS)
Glycogen is synthesized from molecules of α-D-glucose. The process occurs in the cytosol and requires energy supplied by ATP (for the phosphorylation of glucose) and uridine triphosphate (UTP).
A. Synthesis of uridine diphosphate glucose
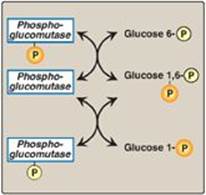
α-D-Glucose attached to uridine diphosphate (UDP) is the source of all the glucosyl residues that are added to the growing glycogen molecule. UDP-glucose (Figure 11.4) is synthesized from glucose 1-phosphate and UTP by UDP-glucose pyrophosphorylase (Figure 11.5). Pyrophosphate (PPi), the second product of the reaction, is hydrolyzed to two inorganic phosphates (Pi) by pyrophosphatase. The hydrolysis is exergonic, ensuring that the UDP-glucose pyrophosphorylase reaction proceeds in the direction of UDP-glucose production. [Note: Glucose 1-phosphate is generated from glucose 6-phosphate by phosphoglucomutase. Glucose 1,6-bisphosphate is an obligatory intermediate in this reversible reaction (Figure 11.6).]
B. Synthesis of a primer to initiate glycogen synthesis
Glycogen synthase makes the α(1→4) linkages in glycogen. This enzyme cannot initiate chain synthesis using free glucose as an acceptor of a molecule of glucose from UDP-glucose. Instead, it can only elongate already existing chains of glucose and, therefore, requires a primer. A fragment of glycogen can serve as a primer in cells whose glycogen stores are not totally depleted. In the absence of a glycogen fragment, a protein called glycogenin can serve as an acceptor of glucose residues from UDP-glucose (see Figure 11.5). The side-chain hydroxyl group of a specific tyrosine in the protein serves as the site at which the initial glucosyl unit is attached. Because the reaction is catalyzed by glycogenin itself via autoglucosylation, glycogenin is an enzyme. Glycogenin then catalyzes the transfer of the next few molecules of glucose from UDP-glucose, producing a short, α(1→4)-linked glucosyl chain. This short chain serves as a primer that is able to be elongated by glycogen synthase as described below [Note: Glycogenin stays associated with and forms the core of a glycogen granule.]
Figure 11.3 Branched structure of glycogen, showing α(1→4) and α(1→6) glycosidic bonds.
C. Elongation of glycogen chains by glycogen synthase
Elongation of a glycogen chain involves the transfer of glucose from UDP-glucose to the nonreducing end of the growing chain, forming a new glycosidic bond between the anomeric hydroxyl group of carbon 1 of the activated glucose and carbon 4 of the accepting glucosyl residue (see Figure 11.5). [Note: The nonreducing end of a carbohydrate chain is one in which the anomeric carbon of the terminal sugar is linked by a glycosidic bond to another compound, making the terminal sugar nonreducing (see p. 84).] The enzyme responsible for making the α(1→4) linkages in glycogen is glycogen synthase. [Note: The UDP released when the new α(1→4) glycosidic bond is made can be phosphorylated to UTP by nucleoside diphosphate kinase (UDP + ATP ![]() UTP + ADP; see p. 296).]
UTP + ADP; see p. 296).]
Figure 11.4 The structure of UDP-glucose, a nucleotide sugar.
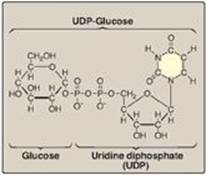
Figure 11.5 Glycogen synthesis. UTP = uridine triphosphate; UDP = uridine diphosphate; PPi = pyrophosphate; Pi = inorganic phosphate.
D. Formation of branches in glycogen
If no other synthetic enzyme acted on the chain, the resulting structure would be a linear (unbranched) chain of glucosyl residues attached by α(1→4) linkages. Such a compound is found in plant tissues and is called amylose. In contrast, glycogen has branches located, on average, eight glucosyl residues apart, resulting in a highly branched, tree-like structure (see Figure 11.3) that is far more soluble than the unbranched amylose. Branching also increases the number of nonreducing ends to which new glucosyl residues can be added (and also, as described later, from which these residues can be removed), thereby greatly accelerating the rate at which glycogen synthesis can occur and dramatically increasing the size of the glycogen molecule.
Figure 11.6 Interconversion of glucose 6-phosphate and glucose 1-phosphate by phosphoglucomutase. P = phosphate.
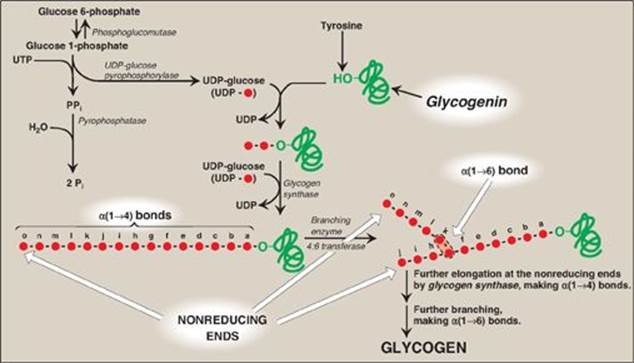
1. Synthesis of branches: Branches are made by the action of the branching enzyme, amylo-α(1→4)→α(1→6)-transglucosidase. This enzyme removes a set of six to eight glucosyl residues from the nonreducing end of the glycogen chain, breaking an α(1→4) bond to another residue on the chain, and attaches it to a non-terminal glucosyl residue by an α(1→6) linkage, thus functioning as a 4:6 transferase. The resulting new, nonreducing end (see “j” in Figure 11.5), as well as the old nonreducing end from which the six to eight residues were removed (see “o” in Figure 11.5), can now be further elongated by glycogen synthase.
2. Synthesis of additional branches: After elongation of these two ends has been accomplished, their terminal six to eight glucosyl residues can be removed and used to make additional branches.
IV. DEGRADATION OF GLYCOGEN (GLYCOGENOLYSIS)
The degradative pathway that mobilizes stored glycogen in liver and skeletal muscle is not a reversal of the synthetic reactions. Instead, a separate set of cytosolic enzymes is required. When glycogen is degraded, the primary product is glucose 1-phosphate, obtained by breaking α(1→4) glycosidic bonds. In addition, free glucose is released from each α(1→6)–linked glucosyl residue (branch point).
A. Shortening of chains
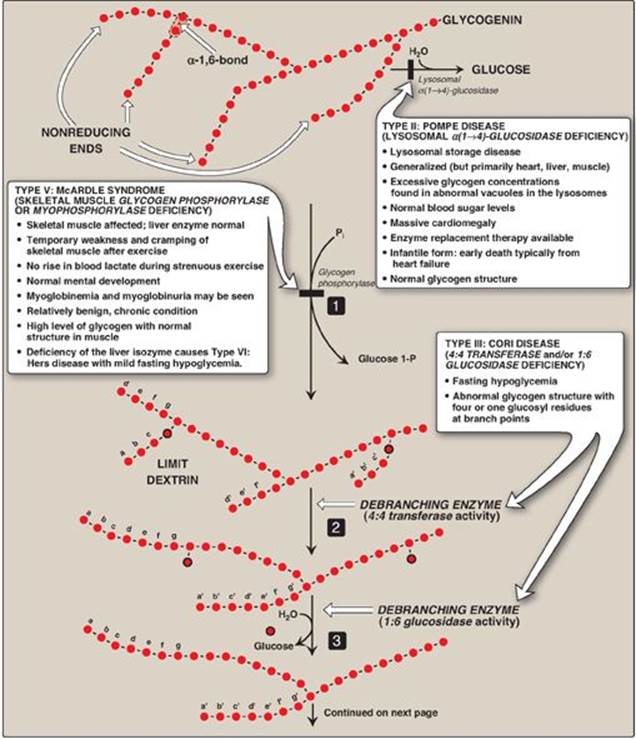
Glycogen phosphorylase sequentially cleaves the α(1→4) glycosidic bonds between the glucosyl residues at the nonreducing ends of the glycogen chains by simple phosphorolysis (producing glucose 1-phosphate) until four glucosyl units remain on each chain before a branch point (Figure 11.7). [Note: Phosphorylase contains a molecule of covalently bound pyridoxal phosphate that is required as a coenzyme.] The resulting structure is called a limit dextrin, and phosphorylase cannot degrade it any further (Figure 11.8).
B. Removal of branches
Branches are removed by the two enzymic activities of a single bifunctional protein, the debranching enzyme (see Figure 11.8). First, oligo-α(1→4)→α(1→4)-glucantransferase activity removes the outer three of the four glucosyl residues attached at a branch. It next transfers them to the nonreducing end of another chain, lengthening it accordingly. Thus, an α(1→4) bond is broken and an α(1→4) bond is made, and the enzyme functions as a 4:4 transferase. Next, the remaining glucose residue attached in an α(1→6) linkage is removed hydrolytically by amylo-α(1→6)-glucosidase activity, releasing free glucose. The glucosyl chain is now available again for degradation by glycogen phosphorylase until four glucosyl units in the next branch are reached.
Figure 11.7 Cleavage of an α(1→4)-glycosidic bond. PLP= pyridoxal phosphate; Pi = inorganic phosphate; P = phosphate.
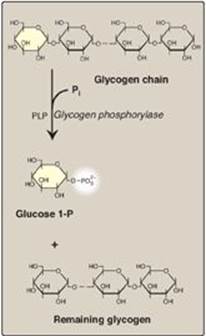
Figure 11.8 Glycogen degradation, showing some of the glycogen storage diseases (GSDs). [Note: A GSD can also be caused by defects in branching enzyme, an enzyme of synthesis, resulting in Type IV: Andersen disease and causing death in early childhood from liver cirrhosis.] Pi = inorganic phosphate; P = phosphate. Glycogen degradation, showing some of the glycogen storage diseases (GSDs).
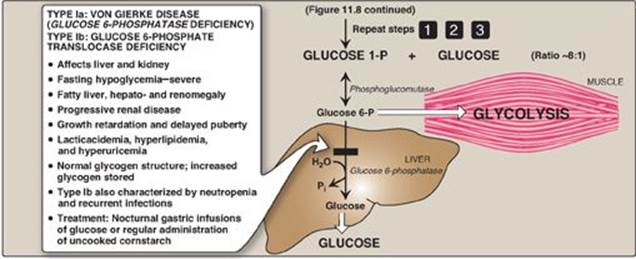
C. Conversion of glucose 1-phosphate to glucose 6-phosphate
Glucose 1-phosphate, produced by glycogen phosphorylase, is converted in the cytosol to glucose 6-phosphate by phosphoglucomutase (see Figure 11.6). In the liver, glucose 6-phosphate is transported into the endoplasmic reticulum (ER) by glucose 6-phosphate translocase. There it is converted to glucose by glucose 6-phosphatase (the same enzyme used in the last step of gluconeogenesis; see p. 121). The glucose then is transported from the ER to the cytosol. Hepatocytes release glycogen-derived glucose into the blood to help maintain blood glucose levels until the gluconeogenic pathway is actively producing glucose. [Note: In the muscle, glucose 6-phosphate cannot be dephosphorylated and sent into the blood because of a lack of glucose 6-phosphatase. Instead, it enters glycolysis, providing energy needed for muscle contraction.]
D. Lysosomal degradation of glycogen
A small amount (1%–3%) of glycogen is continuously degraded by the lysosomal enzyme, α(1→4)-glucosidase (acid maltase). The purpose of this pathway is unknown. However, a deficiency of this enzyme causes accumulation of glycogen in vacuoles in the lysosomes, resulting in the serious glycogen storage disease (GSD) Type II: Pompe disease (see Figure 11.8). [Note: Type II: Pompe disease is the only GSD that is a lysosomal storage disease.]
Lysosomal storage diseases are genetic disorders characterized by the accumulation of abnormal amounts of carbohydrates or lipids primarily due to their decreased lysosomal degradation.
V. REGULATION OF GLYCOGENESIS AND GLYCOGENOLYSIS
Because of the importance of maintaining blood glucose levels, the synthesis and degradation of its glycogen storage form are tightly regulated. In the liver, glycogenesis accelerates during periods when the body has been well fed, whereas glycogenolysis accelerates during periods of fasting. In skeletal muscle, glycogenolysis occurs during active exercise, and glycogenesis begins as soon as the muscle is again at rest. Regulation of glycogen synthesis and degradation is accomplished on two levels. First, glycogen synthase and glycogen phosphorylase are hormonally regulated (by phosphorylation/dephosphorylation) to meet the needs of the body as a whole. [Note: Phosphorylation of glycogen phosphorylase is catalyzed by glycogen phosphorylase kinase (see p. 132).] Second, these same enzymes are allosterically regulated (by effector molecules) to meet the needs of a particular tissue.
Figure 11.9 Stimulation and inhibition of glycogen degradation. AMP = adenosine monophosphate; cAMP = cyclic AMP; GTP = guanosine triphosphate; P = phosphate; PPi = pyrophosphate; R = regulatory subunit; C = catalytic subunit.
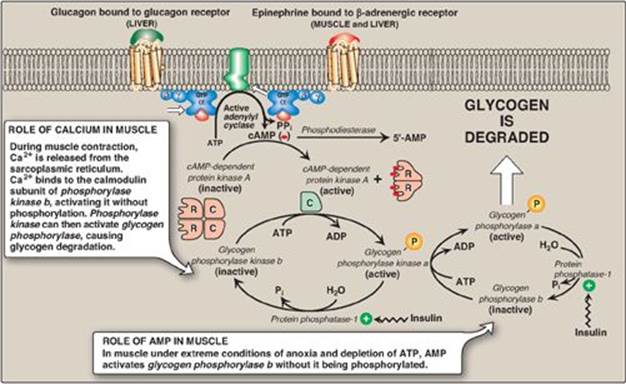
A. Activation of glycogen degradation
The binding of hormones, such as glucagon or epinephrine, to plasma membrane G protein–coupled receptors (GPCRs) signals the need for glycogen to be degraded, either to elevate blood glucose levels or to provide energy for exercising muscle.
1. Activation of protein kinase A: Binding of glucagon or epinephrine to their specific hepatocyte GPCR, or of epinephrine to a specific myocyte GPCR, results in the G protein–mediated activation of adenylyl cyclase. This enzyme catalyzes the synthesis of cyclic adenosine monophosphate (cAMP), which activates cAMP-dependent protein kinase A (PKA), as described on page 95. PKA is a tetramer, having two regulatory subunits (R) and two catalytic subunits (C). cAMP binds to the regulatory subunits, releasing individual catalytic subunits that are active (Figure 11.9). PKA then phosphorylates several enzymes of glycogen metabolism, affecting their activity. [Note: When cAMP is removed, the inactive tetramer, R2C2, is again formed.]
2. Activation of phosphorylase kinase: Phosphorylase kinase exists in two forms: an inactive “b” form and an active “a” form. Active PKA phosphorylates the inactive “b” form of phosphorylase kinase, producing the active “a” form (see Figure 11.9).
3. Activation of glycogen phosphorylase: Glycogen phosphorylase also exists in two forms: the dephosphorylated, inactive “b” form and the phosphorylated, active “a” form. Active phosphorylase kinase is the only enzyme that phosphorylates glycogen phosphorylase b to its active “a” form, which then begins glycogenolysis (see Figure 11.9).
4. Summary of the regulation of glycogen degradation: The cascade of reactions listed above results in glycogenolysis. The large number of sequential steps serves to amplify the effect of the hormonal signal (that is, a few hormone molecules binding to their receptors results in a number of PKA molecules being activated that can each activate many phosphorylase kinase molecules). This causes the production of many active glycogen phosphorylase a molecules that can degrade glycogen.
5. Maintenance of the phosphorylated state: The phosphate groups added to phosphorylase kinase and phosphorylase in response to cAMP are maintained because the enzyme that hydrolytically removes the phosphate, protein phosphatase-1 (PP1), is inactivated by inhibitor proteins that are also phosphorylated and activated in response to cAMP (see Figure 11.9). [Note: PP1 is activated by a signal cascade initiated by insulin (see p. 311). Insulin also activates the phosphodiesterase that degrades cAMP, and, thus, insulin opposes the effects of glucagon and epinephrine.]
Figure 11.10 Hormonal regulation of glycogen synthesis. [Note: In contrast to glycogen phosphorylase, glycogen synthase is inactivated by phosphorylation.] cAMP = cyclic adenosine monophosphate; P = phosphate; PPi = pyrophosphate; R = regulatory subunit; C = catalytic subunit.
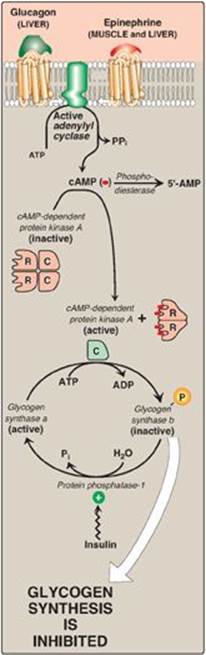
B. Inhibition of glycogen synthesis
The regulated enzyme in glycogenesis is glycogen synthase. It also exists in two forms, the active “a” form and the inactive “b” form. However, for glycogen synthase, in contrast to phosphorylase kinase and phosphorylase, the active form is dephosphorylated, whereas the inactive form is phosphorylated (Figure 11.10). Glycogen synthase a is converted to the inactive “b” form by phosphorylation at several sites on the enzyme, with the level of inactivation proportional to its degree of phosphorylation. Phosphorylation is catalyzed by several different protein kinases that are regulated by cAMP or other signaling mechanisms (see C. below). Glycogen synthase b can be reconverted to the “a” form by PP1. Figure 11.11 summarizes the covalent regulation of glycogen metabolism.
Figure 11.11 Summary of the hormone-mediated covalent regulation of glycogen metabolism. cAMP = cyclic AMP; PKA = protein kinase A.
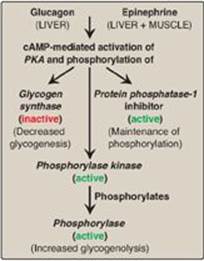
C. Allosteric regulation of glycogen synthesis and degradation
In addition to hormonal signals, glycogen synthase and glycogen phosphorylase respond to the levels of metabolites and energy needs of the cell. Glycogenesis is stimulated when substrate availability and energy levels are high, whereas glycogenolysis is increased when glucose and energy levels are low. This allosteric regulation allows a rapid response to the needs of a cell and can override the effects of hormone-mediated covalent regulation.
1. Regulation of glycogen synthesis and degradation in the well- fed state:
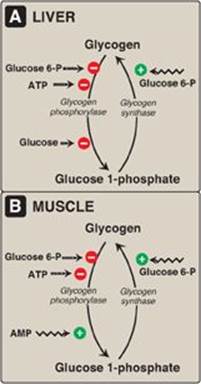
In the well-fed state, glycogen synthase b in both liver and muscle is allosterically activated by glucose 6-phosphate, which is present in elevated concentrations (Figure 11.12). In contrast, glycogen phosphorylase a is allosterically inhibited by glucose 6-phosphate, as well as by ATP, a high-energy signal in the cell. [Note: In liver, but not muscle, nonphosphorylated glucose is also an allosteric inhibitor of glycogen phosphorylase a, making it a better substrate for PP1.]
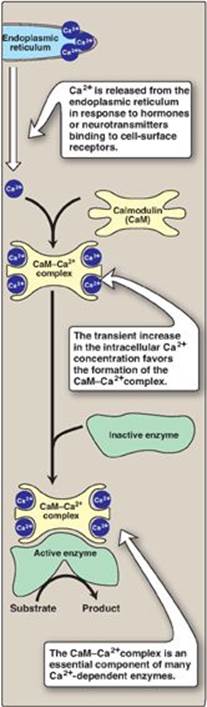
2. Activation of glycogen degradation by calcium: Ca2+ is released into the cytoplasm in muscle in response to neural stimulation and in liver in response to epinephrine binding to α1-adrenergic receptors. The Ca2+ binds to calmodulin (CaM), the most widely distributed member of a family of small, calcium-binding proteins. The binding of four molecules of Ca2+ to CaM triggers a conformational change such that the activated Ca2+–CaM complex binds to and activates protein molecules, often enzymes, that are inactive in the absence of this complex (see Figure 11.13). Thus, CaM functions as an essential subunit of many complex proteins. One such protein is the tetrameric phosphorylase kinase, whose b form is activated by the binding of Ca2+ to its δ subunit (CaM) without the need for the kinase to be phosphorylated by PKA. [Note: Epinephrine at β-adrenergic receptors signals through a rise in cAMP, not Ca2+ (see p. 131).]
a. Calcium activation of muscle phosphorylase kinase: During muscle contraction, there is a rapid and urgent need for ATP. This energy is supplied by the degradation of muscle glycogen to glucose, which can then enter glycolysis. Nerve impulses cause membrane depolarization, which promotes Ca2+ release from the sarcoplasmic reticulum into the sarcoplasm of myocytes. The Ca2+ binds the CaM subunit, and the complex activates muscle phosphorylase kinase b (see Figure 11.9).
Figure 11.12 Allosteric regulation of glycogen synthesis and degradation. A. Liver. B. Muscle. P = phosphate.
b. Calcium activation of liver phosphorylase kinase: During physiologic stress, epinephrine is released from the adrenal medulla and signals the need for blood glucose. This glucose initially comes from hepatic glycogenolysis. Binding of epinephrine to hepatocyte α-adrenergic GPCRs activates a phospholipid-dependent cascade (see p. 205) that results in movement of Ca2+ from the ER into the cytoplasm. A Ca2+–CaM complex forms and activates hepatic phosphorylase kinase b. [Note: The released Ca2+ also helps to activate protein kinase C that can phosphorylate (therefore, inactivate) glycogen synthase a.]
3. Activation of glycogen degradation in muscle: Muscle glycogen phosphorylase is active in the presence of the high adenosine monophosphate (AMP) concentrations that occur under extreme conditions of anoxia and ATP depletion. AMP binds to glycogen phosphorylase b, causing its activation without phosphorylation (see Figure 11.9). [Note: Recall that AMP also activates phosphofructokinase-1 of glycolysis (see p. 99), allowing glucose from glycogenolysis to be oxidized.]
Figure 11.13 Calmodulin mediates many effects of intracellular Ca2+.
VI. GLYCOGEN STORAGE DISEASES
These are a group of genetic diseases that are caused by defects in enzymes required for glycogen degradation or, more rarely, glycogen synthesis. They result either in formation of glycogen that has an abnormal structure or in the accumulation of excessive amounts of normal glycogen in specific tissues as a result of impaired degradation. A particular enzyme may be defective in a single tissue, such as liver (resulting in hypoglycemia) or muscle (causing muscle weakness), or the defect may be more generalized, affecting a variety of tissues. The severity of the GSDs ranges from fatal in early childhood to mild disorders that are not life threatening. Some of the more prevalent GSDs are illustrated in Figure 11.8. [Note: Only one GSD is lysosomal because glycogen metabolism occurs primarily in the cytosol.]
VII. CHAPTER SUMMARY
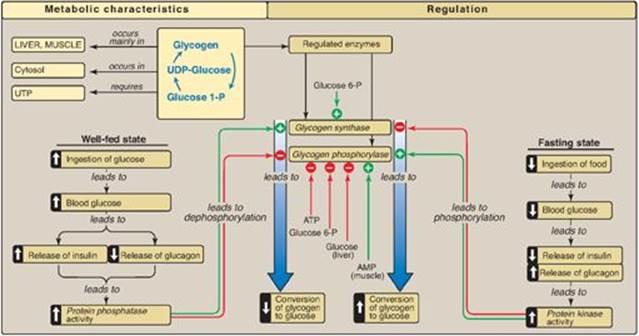
The main stores of glycogen in the body are found in skeletal muscle, where they serve as a fuel reserve for the synthesis of ATP during muscle contraction, and in the liver, where they are used to maintain the blood glucose concentration, particularly during the early stages of a fast. Glycogen is a highly branched polymer of α-D-glucose. The primary glycosidic bond is an α(1→4) linkage. After about eight to ten glucosyl residues, there is a branch containing an α(1→6) linkage. Uridine diphosphate (UDP)-glucose, the building block of glycogen, is synthesized from glucose 1-phosphate and UTP by UDP-glucose pyrophosphorylase (Figure 11.14). Glucose from UDP-glucose is transferred to the nonreducing ends of glycogen chains by primer-requiring glycogen synthase, which makes α(1→4) linkages. The primer is made by glycogenin. Branches are formed by amylo-α(1→4)→α(1→6)-transglucosidase (common name, glucosyl 4:6 transferase), which transfers a set of six to eight glucosyl residues from the nonreducing end of the glycogen chain (breaking an α(1→4) linkage), and attaches it with an α(1→6) linkage to another residue in the chain. Pyridoxal phosphate–requiring glycogen phosphorylase cleaves the α(1→4) bonds between glucosyl residues at the nonreducing ends of the glycogen chains, producing glucose 1-phosphate. This sequential degradation continues until four glucosyl units remain before a branch point. The resulting structure is called a limit dextrin that is degraded by the bifunctional debranching enzyme. Oligo-α(1→4)→α(1→4)-glucantransferase (common name, glucosyl 4:4 transferase) removes the outer three of the four glucosyl residues at a branch and transfers them to the nonreducing end of another chain, where they can be converted to glucose 1-phosphate by glycogen phosphorylase. The remaining single glucose residue attached in an α(1→6) linkage is removed hydrolytically by the amylo-(1→6) glucosidaseactivity of debranching enzyme, releasing free glucose. Glucose 1-phosphate is converted to glucose 6-phosphate by phosphoglucomutase. In the muscle, glucose 6-phosphate enters glycolysis. In the liver, the phosphate is removed by glucose 6-phosphatase, releasing free glucose that can be used to maintain blood glucose levels at the beginning of a fast. A deficiency of the phosphatase causes glycogen storage disease Type 1a (Von Gierke disease). This disease results in an inability of the liver to provide free glucose to the body during a fast. It affects both glycogen degradation and gluconeogenesis. Glycogen synthesis and degradation are reciprocally regulated to meet whole-body needs by the same hormonal signals (namely, an elevated insulin level results in overall increased glycogenesis and decreased glycogenolysis, whereas an elevated glucagon, or epinephrine, level causes increased glycogenolysis and decreased glycogenesis). Key enzymes are phosphorylated by a family of protein kinases, some of which are cyclic adenosine monophosphate dependent (a compound increased by glucagon and epinephrine). Phosphate groups are removed by protein phosphatase-1 (active when its inhibitor is inactive in response to elevated insulin levels). Glycogen synthase, phosphorylase kinase, and phosphorylase are also allosterically regulated to meet tissues needs. In the well-fed state, glycogen synthase is activated by glucose 6-phosphate, but glycogen phosphorylase is inhibited by glucose 6-phosphate as well as by ATP. In the liver, glucose also serves an an allosteric inhibitor of glycogen phosphorylase. The Ca2+ released from the endoplasmic reticulum in muscle during exercise and in liver in response to epinephrine activates phosphorylase kinase by binding to the enzyme’s calmodulin subunit. This allows the enzyme to activate glycogen phosphorylase, thereby causing glycogen degradation. AMP activates glycogen phosphorylase in muscle.
Figure 11.14 Key concept map for glycogen metabolism in the liver. [Note: Glycogen phosphorylase is phosphorylated by phosphorylase kinase, the “b” form of which can be activated by calcium.] UDP = uridine diphosphate; UTP = uridine triphosphate; P = phosphate.
Study Questions
Choose the ONE best answer.
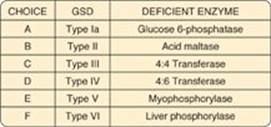
For Questions 11.1–11.4, match the deficient enzyme to the clinical finding in selected glycogen storage diseases (GSDs).
11.1 Exercise intolerance, with no rise in blood lactate during exercise
Correct answer = E. Myophosphorylase deficiency prevents glycogen degradation in muscle, depriving muscle of glycogen-derived glucose, resulting in decreased glycolysis and its anaerobic product, lactate.
Correct Answer = D. 4:6 Transferase (branching enzyme) deficiency, a defect in glycogen synthesis, results in formation of glycogen with fewer branches and decreased solubility.
Correct answer = B. Acid maltase [a(1→4)-glucosidase] deficiency prevents degradation of any glycogen brought into lysosomes. A variety of tissues are affected, with the most severe pathology resulting from heart damage.
Correct answer = A. Glucose 6-phosphatase deficiency prevents the liver from releasing free glucose into the blood, causing severe fasting hypoglycemia, lacticacidemia, hyperuricemia, and hyperlipidemia.
11.2 Fatal, progressive cirrhosis and glycogen with longer-than-normal outer chains
11.3 Generalized accumulation of glycogen, severe hypotonia, and death from heart failure
11.4 Severe fasting hypoglycemia, lacticacidemia, hyperuricemia, and hyperlipidemia
11.5 Epinephrine and glucagon have which one of the following effects on hepatic glycogen metabolism?
A. Both glycogen phosphorylase and glycogen synthase are activated by phosphorylation but at significantly different rates.
B. Glycogen phosphorylase is inactivated by the resuting rise in calcium, whereas glycogen synthase is activated.
C. Glycogen phosphorylase is phosphorylated and active, whereas glycogen synthase is phosphorylated and inactive.
D. The net synthesis of glycogen is increased.
Correct answer = C. Epinephrine and glucagon both cause increased glycogen degradation and decreased synthesis in the liver through covalent modification (phosphorylation) of key enzymes of glycogen metabolism. Glycogen phosphorylase is phosphorylated and active (“a” form), whereas glycogen synthase is phosphorylated and inactive (“b” form). Glucagon does not cause a rise in calcium.
11.6 In contracting skeletal muscle, a sudden elevation of the sarcoplasmic calcium concentration will result in:
A. activation of cyclic adenosine monophosphate (cAMP)-dependent protein kinase A.
B. conversion of cAMP to AMP by phosphodiesterase.
C. direct activation of glycogen synthase b.
D. direct activation of phosphorylase kinase b.
E. inactivation of phosphorylase kinase a by the action of protein phosphatase-1.
Correct answer = D. Ca2+ released from the sarcoplasmic reticulum during exercise binds to the calmodulin subunit of phosphorylase kinase, thereby allosterically activating the “b” form of this enzyme. The other choices are not caused by an elevation of cytosolic calcium.
11.7 Explain why the hypoglycemia seen with Type Ia glycogen storage disease (glucose 6-phosphatase deficiency) is severe, whereas that seen with Type VI (liver phosphorylase deficiency) is mild.
With Type Ia, the liver is unable to generate free glucose either from glycogenolysis or gluconeogenesis because both processes produce glucose 6-phosphate. With Type VI, the liver is still able to produce free glucose from gluconeogenesis, but glycogenolysis is inhibited.