Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT II: Bioenergetics and Carbohydrate Metabolism
Chapter 14. Glycosaminoglycans, Proteoglycans, and Glycoproteins
I. OVERVIEW OF GLYCOSAMINOGLYCANS
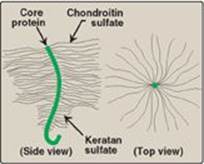
Glycosaminoglycans (GAGs) are large complexes of negatively charged heteropolysaccharide chains. They are generally associated with a small amount of protein (“core protein”), forming proteoglycans, which typically consist of up to 95% carbohydrate. [Note: This is in comparison to the glycoproteins, which consist primarily of protein with a variable (but typically small) amount of carbohydrate (see p. 165).] GAGs have the special ability to bind large amounts of water, thereby producing the gel-like matrix that forms the basis of the body’s ground substance, which, along with fibrous structural proteins such as collagen, elastin, and fibrillin-1, and adhesive proteins such as fibronectin, make up the extracellular matrix (ECM). The hydrated GAGs serve as a flexible support for the ECM, interacting with the structural and adhesive proteins, and as a molecular sieve, influencing movement of materials through the ECM. The viscous, lubricating properties of mucous secretions also result from the presence of GAGs, which led to the original naming of these compounds as mucopolysaccharides.
Figure 14.1 Repeating disaccharide unit.
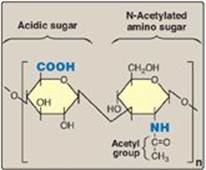
II. STRUCTURE OF GLYCOSAMINOGLYCANS
GAGs are long, unbranched, heteropolysaccharide chains composed of a repeating disaccharide unit [acidic sugar–amino sugar]n (Figure 14.1). [Note: A single exception is keratan sulfate, which contains galactose rather than an acidic sugar.] The amino sugar is either D-glucosamine or D-galactosamine, in which the amino group is usually acetylated, thus eliminating its positive charge. The amino sugar may also be sulfated on carbon 4 or 6 or on a nonacetylated nitrogen. The acidic sugar is either D-glucuronic acid or its C-5 epimer L-iduronic acid (Figure 14.2). These acidic sugars contain carboxyl groups that are negatively charged at physiologic pH and, together with the sulfate groups, give GAGs their strongly negative nature.
Figure 14.2 Some monosaccharide units found in glycosaminoglycans.
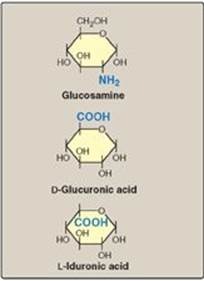
A. Relationship between glycosaminoglycan structure and function
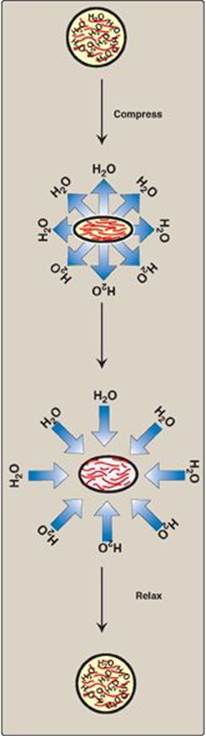
Because of their large number of negative charges, these heteropolysaccharide chains tend to be extended in solution. They repel each other and are surrounded by a shell of water molecules. When brought together, they “slide” past each other, much as two magnets with the same polarity seem to slide past each other. This produces the “slippery” consistency of mucous secretions and synovial fluid. When a solution of GAGs is compressed, the water is “squeezed out,” and the GAGs are forced to occupy a smaller volume. When the compression is released, the GAGs spring back to their original, hydrated volume because of the repulsion of their negative charges. This property contributes to the resilience of synovial fluid and the vitreous humor of the eye (Figure 14.3).
B. Classification of the glycosaminoglycans
The six major types of glycosaminoglycans are divided according to monomeric composition, type of glycosidic linkages, and degree and location of sulfate units. The structure of the GAGs and their distribution in the body is illustrated in Figure 14.4. All GAGs, except for hyaluronic acid, are sulfated and are found covalently attached to protein, forming proteoglycan monomers.
Figure 14.3 Resilience of glycosaminoglycans.
C. Proteoglycans
Proteoglycans are found in the ECM and on the outer surface of cells.
1. Structure of proteoglycan monomers: A proteoglycan monomer found in cartilage consists of a core protein to which up to 100 linear chains of GAGs are covalently attached. These chains, which may each be composed of up to 200 disaccharide units, extend out from the core protein and remain separated from each other because of charge repulsion. The resulting structure resembles a “bottle brush” (Figure 14.5). In cartilage proteoglycan, the species of GAGs include chondroitin sulfate and keratan sulfate. [Note: Proteoglycans are grouped into gene families that encode core proteins with common structural features. The aggrecan family (aggrecan, versecan, neurocan, and brevican), abundant in cartilage, is an example.]
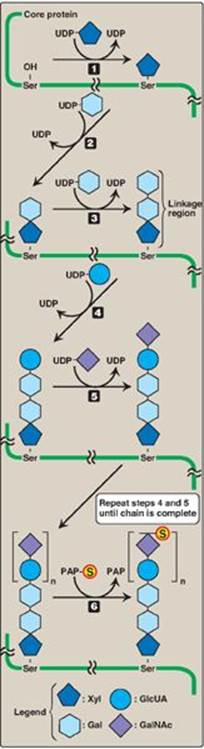
2. Linkage between the carbohydrate chain and the protein: This linkage is most commonly through a trihexoside (galactose-galactose-xylose) and a serine residue, respectively. An O-glycosidic bond is formed between the xylose and the hydroxyl group of the serine (Figure 14.6).
3. Proteoglycan aggregates: The proteoglycan monomers associate with a molecule of hyaluronic acid to form proteoglycan aggregates. The association is not covalent but occurs primarily through ionic interactions between the core protein and the hyaluronic acid. The association is stabilized by additional small proteins called link proteins (Figure 14.7).
III. SYNTHESIS OF GLYCOSAMINOGLYCANS
The heteropolysaccharide chains are elongated by the sequential addition of alternating acidic and amino sugars donated by their uridine diphosphate (UDP)-derivatives. The reactions are catalyzed by a family of specific glycosyltransferases. The synthesis of GAGs is analogous to that of glycogen (see p. 126) except that GAGs are produced for export from the cell. Their synthesis occurs, therefore, primarily in the Golgi, rather than in the cytosol.
Figure 14.4 Structure and distribution of glycosaminoglycans (GAGs). Sulfate groups (![]() ) are shown in all possible positions. GlcUA = glucuronic acid; IdUA = iduronic acid; GalNAc = N-acetylgalactosamine; GlcNAc = N-acetylglucosamine; GlcN = glucosamine; Gal = galactose.
) are shown in all possible positions. GlcUA = glucuronic acid; IdUA = iduronic acid; GalNAc = N-acetylgalactosamine; GlcNAc = N-acetylglucosamine; GlcN = glucosamine; Gal = galactose.
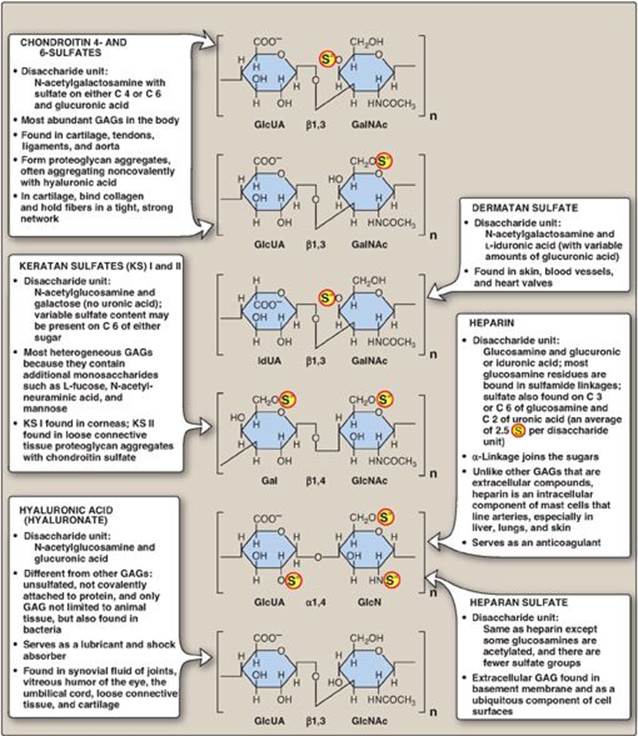
Figure 14.5 “Bottle-brush” model of a cartilage proteoglycan monomer.
A. Synthesis of amino sugars
Amino sugars are essential components of GAGs, glycoproteins, and glycolipids and are also found in some antibiotics. The synthetic pathway of amino sugars is very active in connective tissues, where as much as 20% of glucose flows through this pathway.
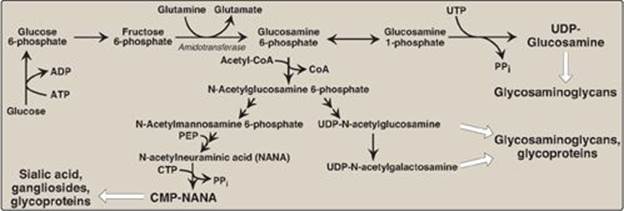
1. N-Acetylglucosamine and N-acetylgalactosamine: The monosaccharide fructose 6-phosphate is the precursor of N-acetyl-glucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), and the sialic acids, including N-acetylneuraminic acid (NANA). In each of these sugars, a hydroxyl group of the precursor is replaced by an amino group donated by glutamine (Figure 14.8). [Note: The amino groups are then almost always acetylated.] The UDP-derivatives of GlcNAc and GalNAc are synthesized by reactions analogous to those described for UDP-glucose synthesis (see p. 126). These nucleotide sugars are the activated forms of the monosaccharides that can be used to elongate the carbohydrate chains.
2. N-Acetylneuraminic acid: NANA, a nine-carbon, acidic monosaccharide, is a member of the family of sialic acids, each of which is acylated at a different site. These compounds are usually found as terminal carbohydrate residues of oligosaccharide side chains of glycoproteins; glycolipids; or, less frequently, of GAGs. The carbons and nitrogens in NANA come from N-acetylmannosamine and phosphoenolpyruvate (an intermediate in the glycolytic pathway; see p. 102). [Note: Before NANA can be added to a growing oligosaccharide, it must be converted into its active form by reacting with cytidine triphosphate (CTP). The enzyme CMP-NANA synthetasecatalyzes the reaction. This is the only nucleotide sugar in human metabolism in which the carrier nucleotide is a monophosphate.]
Figure 14.6 Linkage region of glycosaminoglycans.
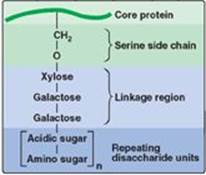
Figure 14.7 Proteoglycan aggregate.
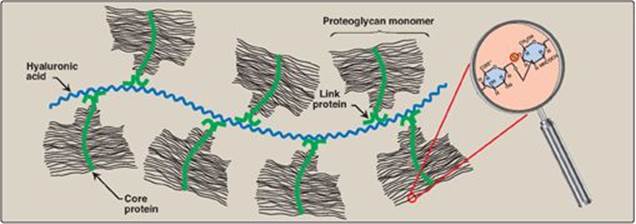
Figure 14.8 Synthesis of the amino sugars. UTP = uridine triphosphate; UDP = uridine diphosphate; CoA = coenzyme A; PEP = phosphoenoylpyruvate; CTP = cytidine triphosphate; CMP = cytidine monophosphate; PPi = pyrophosphate.
B. Synthesis of acidic sugars
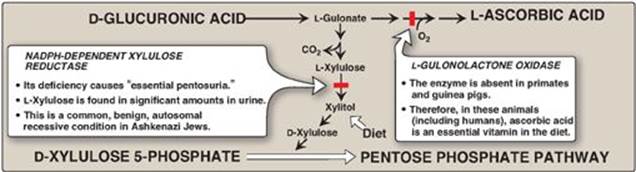
D-Glucuronic acid, whose structure is that of glucose with an oxidized carbon 6 (–CH2OH → –COOH) and its C-5 epimer, L-iduronic acid, are essential components of GAGs. Glucuronic acid is also required in detoxification reactions of a number of insoluble compounds, such as bilirubin (see p. 282), steroids, and many drugs, including the statins (see p. 224). In plants and mammals (other than guinea pigs and primates, including humans), glucuronic acid serves as a precursor of ascorbic acid (vitamin C). The uronic acid pathway also provides a mechanism by which dietary D-xylulose can enter the central metabolic pathways.
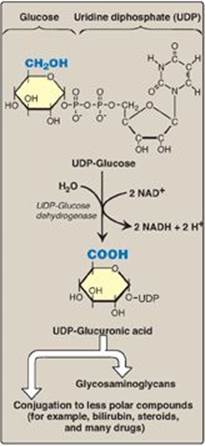
1. Glucuronic acid: Glucuronic acid can be obtained in small amounts from the diet. It can also be obtained from the lysosomal degradation of GAGs, or from glucose 6-phosphate via the uronic acid pathway. The end product of glucuronic acid metabolism in humans is D-xylulose 5-phosphate, which can enter the pentose phosphate pathway and produce the glycolytic intermediates glyceraldehyde 3-phosphate and fructose 6-phosphate (Figure 14.9; see also Figure 13.2, p. 146). The active form of glucuronic acid that donates the sugar in GAG synthesis and other glucuronidation reactions is UDP-glucuronic acid, which is produced by oxidation of UDP-glucose (Figure 14.10).
2. L-Iduronic acid synthesis: Synthesis of L-iduronic acid residues occurs after D-glucuronic acid has been incorporated into the carbohydrate chain. Uronosyl 5-epimerase causes epimerization of the D- to the L-sugar.
C. Synthesis of the core protein
The core protein is synthesized by ribosomes on the rough endoplasmic reticulum (RER) and enters the RER lumen. The protein is then glycosylated by membrane-bound glycosyltransferases located in the Golgi.
Figure 14.9 Metabolism of glucuronic acid. NADPH = reduced nicotinamide adenine dinucleotide phosphate.
D. Synthesis of the carbohydrate chain
Carbohydrate chain formation begins by synthesis of a short linkage region on the core protein on which carbohydrate chain synthesis will be initiated. The most common linkage region is formed by the transfer of a xylose from UDP-xylose to the hydroxyl group of a serine (or threonine) catalyzed by xylosyltransferase. Two galactose molecules are then added, completing the trihexoside. This is followed by sequential addition of alternating acidic and amino sugars (Figure 14.11) and epimerization of some D-glucuronyl to L-iduronyl residues.
E. Addition of sulfate groups
Sulfation of a GAG occurs after the particular monosaccharide to be sulfated has been incorporated into the growing carbohydrate chain. The source of the sulfate is 3’-phosphoadenosyl-5’-phosphosulfate ([PAPS], a molecule of adenosine monophosphate with a sulfate group attached to the 5’-phosphate). The sulfation reaction is catalyzed by sulfotransferases. [Note: An example of the synthesis of a sulfated GAG, chondroitin sulfate, is shown in Figure 14.11.] PAPS is also the sulfur donor in glycosphingolipid synthesis (see p. 210).
A defect in the sulfation of the growing glycosaminoglycan chains results in one of several autosomal recessive disorders, the chondrodystrophies, that affect the proper development and maintenance of the skeletal system.
Figure 14.10 Oxidation of UDP-glucose to UDPglucuronic acid. NAD(H) = nicotinamide adenine dinucleotide.
IV. DEGRADATION OF GLYCOSAMINOGLYCANS
GAGs are degraded in lysosomes, which contain hydrolytic enzymes that are most active at a pH of approximately 5. Therefore, as a group, these enzymes are called acid hydrolases. [Note: The low pH optimum is a protective mechanism that prevents the enzymes from destroying the cell should leakage occur into the cytosol where the pH is neutral.] The half-lives of GAGs vary from minutes to months and are influenced by the type of GAG and its location in the body.
A. Phagocytosis of extracellular glycosaminoglycans
Because GAGs are extracellular or cell-surface compounds, they must first be engulfed by an invagination of the cell membrane (phagocytosis), forming a vesicle inside of which the GAGs are to be degraded. This vesicle then fuses with a lysosome, forming a single digestive vesicle in which the GAGs are efficiently degraded (see p. 150 for a discussion of phagocytosis).
B. Lysosomal degradation of glycosaminoglycans
The lysosomal degradation of GAGs requires a large number of acid hydrolases for complete digestion. First, the polysaccharide chains are cleaved by endoglycosidases, producing oligosaccharides. Further degradation of the oligosaccharides occurs sequentially from the nonreducing end of each chain (see p. 127), the last group (sulfate or sugar) added during synthesis being the first group removed (by sulfatases or exoglycosidases). Examples of some of these enzymes and the bonds they hydrolyze are shown in Figure 14.12. [Note: Endo- and exoglycosidases are also involved in the lysosomal degradation of glycoproteins (see p. 170) and glycolipids (see p. 210). Deficiencies in these enzymes result in the accumulation of partially degraded carbohydrates, resulting in tissue damage.]
Multiple sulfatase deficiency is a rare lysosomal storage disease in which all sulfatases are nonfunctional due to a defect in the formation of formylglycine, an amino acid derivative required at the active site for enzymic activity to occur.
Figure 14.11 Synthesis of chondroitin sulfate.
V. MUCOPOLYSACCHARIDOSES
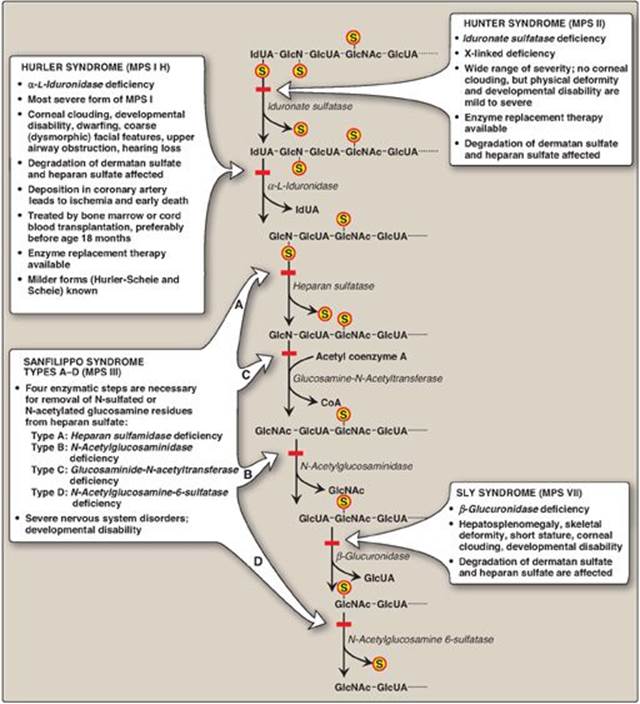
The mucopolysaccharidoses are hereditary diseases (1:25,000 live births) caused by a deficiency of any one of the lysosomal hydrolases normally involved in the degradation of heparan sulfate and/or dermatan sulfate (see Figure 14.12). They are progressive disorders characterized by lysosomal accumulation of GAGs in various tissues, causing a range of symptoms, such as skeletal and extracellular matrix deformities, and intellectual disability. All are autosomal recessive disorders except Hunter syndrome, which is X linked. Children who are homozygous for any one of these diseases are apparently normal at birth and then gradually deteriorate. In severe cases, death occurs in childhood. There currently is no cure. Incomplete lysosomal degradation of GAGs results in the presence of oligosaccharides in the urine. These fragments can be used to diagnose the specific mucopolysaccharidosis by identifying the structure present on the nonreducing end of the oligosaccharide, because that residue would have been the substrate for the missing enzyme. Diagnosis is confirmed by measuring the patient’s cellular level of the lysosomal hydrolases. Bone marrow and cord blood transplants, in which transplanted macrophages produce the enzymes that degrade GAGs, have been used to treat Hurler and Hunter syndromes, with limited success. Enzyme replacement therapy is available for both syndromes but does not prevent neurologic damage.
Figure 14.12 Degradation of the glycosaminoglycan heparan sulfate by lysosomal enzymes, indicating sites of enzyme deficiencies in some representative mucopolysaccharidoses (MPSs). [Note: Deficiencies in the degradation of keratan sulfate result in Morquio syndrome, A and B. Deficiencies in the degradation of dermatan sulfate result in Maroteaux-Lamy syndrome.] GlcUA = glucuronic acid; IdUA = iduronic acid; GalNAc = N-acetylgalactosamine; GlcNAc = N-acetylglucosamine; GlcN = glucosamine; S = sulfate.
VI. OVERVIEW OF GLYCOPROTEINS
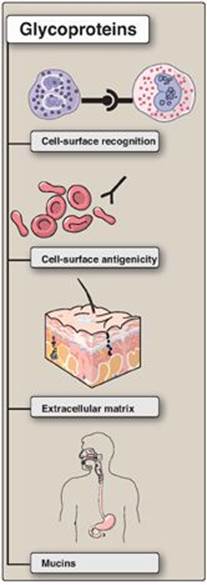
Glycoproteins are proteins to which oligosaccharides are covalently attached. They differ from the proteoglycans in that the length of the carbohydrate chain in glycoproteins is relatively short (usually two to ten sugar residues in length, although they can be longer), whereas it can be very long in the GAGs of proteoglycans (see p. 157). In addition, whereas GAGs have repeating disaccharide units, the carbohydrates of glycoproteins do not have serial repeats. The glycoprotein carbohydrate chains are often branched instead of linear and may or may not be negatively charged. Glycoproteins contain highly variable amounts of carbohydrate but typically less than that in GAGS. For example, immunoglobulin IgG contains less than 4% of its mass as carbohydrate, whereas human gastric glycoprotein (mucin) contains more than 80% carbohydrate. Membrane-bound glycoproteins participate in a broad range of cellular phenomena, including cell-surface recognition (by other cells, hormones, and viruses), cell-surface antigenicity (such as the blood group antigens), and as components of the ECM and of the mucins of the gastrointestinal and urogenital tracts, where they act as protective biologic lubricants. In addition, almost all of the globular proteins present in human plasma are glycoproteins, although albumin is an exception. (See Figure 14.13 for a summary of some of the functions of glycoproteins.) [Note: Glycosylation is the most common posttranslational modification of proteins.]
Figure 14.13 Functions of glycoproteins.
VII. OLIGOSACCHARIDE STRUCTURE
The oligosaccharide components of glycoproteins are generally branched heteropolymers composed primarily of D-hexoses, with the addition in some cases of neuraminic acid (a nonose) and of L-fucose, a 6-deoxyhexose.
A. Structure of the linkage between carbohydrate and protein
The oligosaccharide may be attached to the protein through an N- or an O-glycosidic link (see p. 86). In the former case, the sugar chain is attached to the amide group of an asparagine side chain and, in the latter case, to the hydroxyl group of either a serine or threonine side chain. [Note: In the case of collagen, there is an O-glycosidic linkage between galactose or glucose and the hydroxyl group of hydroxylysine (see p. 47).]
B. N- and O-linked oligosaccharides
A glycoprotein may contain only one type of glycosidic linkage (N- or O-linked) or may have both types within the same molecule.
1. O-Linked oligosaccharides: The O-linked oligosaccharides may have one or more of a wide variety of sugars arranged in either a linear or a branched pattern. Many O-linked oligosaccharides are found in extracellular glycoproteins or as membrane glycoprotein components. For example, O-linked oligosaccharides on the surface of red blood cells help provide the ABO blood group determinants.
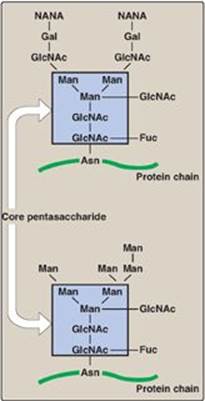
2. N-linked oligosaccharides: The N-linked oligosaccharides fall into two broad classes: complex oligosaccharides and high-mannose oligosaccharides. Both contain the same pentasaccharide core shown in Figure 14.14, but the complex oligosaccharides contain a diverse group of additional sugars, for example, GlcNAc, GalNAc, L-fucose, and NANA, whereas the high-mannose oligosaccharides contain primarily mannose.
Figure 14.14 Complex (top) and high-mannose (bottom) N-linked oligosaccharides. [Note: Members of each class contain the same pentasaccharide core.] NANA = N-acetylneuraminic acid; Gal = galactose; GlcNAc = N-acetylglucosamine; Man = mannose; Fuc = fucose; Asn = asparagine.
VIII. SYNTHESIS OF GLYCOPROTEINS
Proteins destined to function in the cytoplasm are synthesized on free cytosolic ribosomes. However, proteins, including glycoproteins, that are destined for cellular membranes, lysosomes, or to be exported from the cell, are synthesized on ribosomes attached to the RER. These proteins contain specific signal sequences that act as molecular “address labels,” targeting the proteins to their proper destinations. An N-terminal hydrophobic sequence initially directs these proteins to the RER, allowing the growing polypeptide to be extruded into the lumen. The proteins are then transported via secretory vesicles to the Golgi complex, which acts as a sorting center (Figure 14.15). In the Golgi, those glycoproteins that are to be secreted from the cell (or are targeted for lysosomes) are packaged into vesicles that fuse with the cell (or lysosomal) membrane and release their contents. Those that are destined to become components of the cell membrane are integrated into the Golgi membrane, which buds off, forming vesicles that add their membrane-bound glycoproteins to the cell membrane. [Note: The membrane glycoproteins are, thus, oriented with the carbohydrate portion on the outside of the cell (see Figure 14.15).]
A. Carbohydrate components of glycoproteins
The precursors of the carbohydrate components of glycoproteins are nucleotide sugars, which include UDP-glucose, UDP-galactose, UDP-GlcNAc, and UDP-GalNAc. In addition, guanosine diphosphate (GDP)-mannose, GDP-L-fucose (which is synthesized from GDP-mannose), and CMP-NANA may donate sugars to the growing chain. [Note: When the acidic NANA is present, the oligosaccharide has a negative charge at physiologic pH.] The oligosaccharides are covalently attached to the R groups of specific amino acids in the protein, where the three-dimensional structure of the protein determines whether or not a specific amino acid is glycosylated.
B. Synthesis of O-linked glycosides
The synthesis of the O-linked glycosides is very similar to that of the GAGs (see p. 158). First, the protein to which the oligosaccharides are to be attached is synthesized on the RER and extruded into its lumen. Glycosylation begins with the transfer of GalNAc (from UDP-GalNAc) onto the R-group of a specific serine or threonine.
1. Role of glycosyltransferases: The glycosyltransferases responsible for the stepwise synthesis of the oligosaccharides are bound to the membranes of the Golgi apparatus. They act in a specific order, without using a template as is required for DNA, RNA, and protein synthesis (see Unit VI) but, rather by recognizing the actual structure of the growing oligosaccharide as the appropriate substrate.
C. Synthesis of the N-linked glycosides
The synthesis of N-linked glycosides occurs in the lumen of the RER and requires the participation of the phosphorylated form of dolichol (dolichol pyrophosphate), a lipid of the ER membrane (Figure 14.16). The initial product is processed in the ER and Golgi.
1. Synthesis of dolichol-linked oligosaccharide: First, as with the O-linked glycosides, the protein is synthesized on the RER and enters its lumen. However, the protein does not become glycosylated with individual sugars. Instead, a lipid-linked oligosaccharide is first constructed. This consists of dolichol (an ER membrane lipid 80–100 carbons long) attached through a pyrophosphate linkage to an oligosaccharide containing N-GlcNAc, mannose, and glucose. The sugars to be added sequentially to the dolichol by the membrane-bound glycosyltransferases are first N-GlcNAc, followed by mannose and glucose (see Figure 14.16). The entire 14-sugar oligosaccharide is then transferred from the dolichol to the amide nitrogen (N) of an asparagine in the protein to be glycosylated by a protein-oligosaccharide transferase present in the ER. [Note: Tunicamycin inhibits N-linked glycosylation.]
Congenital disorders of glycosylation (CDGs) are syndromes caused primarily by defects in the N-linked glycosylation of proteins, either oligosaccharide assembly (Type I) or processing (Type II).
Figure 14.15 Transport of glycoproteins through the Golgi apparatus and their subsequent release or incorporation into a lysosome or the cell membrane.
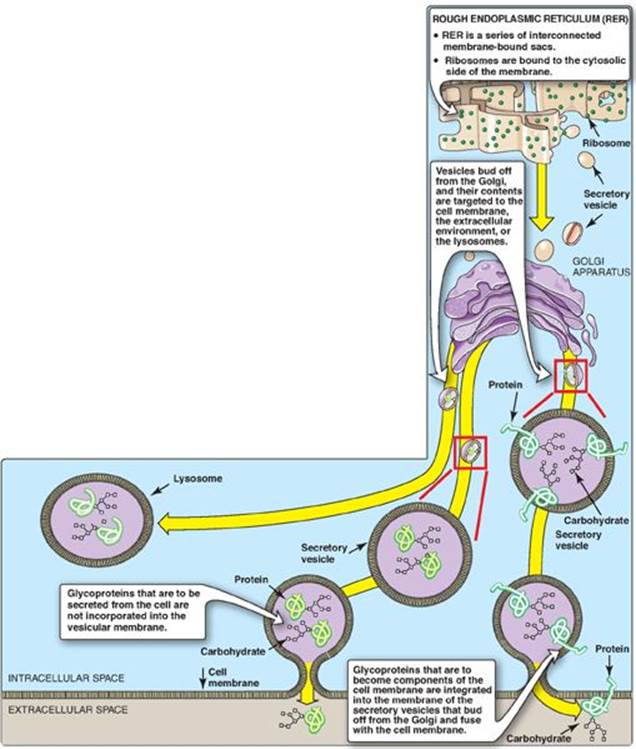
Figure 14.16 Synthesis of N-linked glycoproteins. ![]()
![]() = terminal group (fucose or N-acetylneuraminic acid); mRNA = messenger RNA; Asn = asparagine.
= terminal group (fucose or N-acetylneuraminic acid); mRNA = messenger RNA; Asn = asparagine.
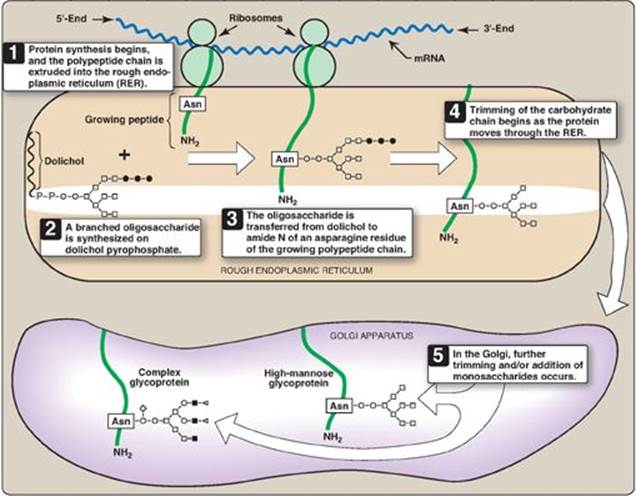
2. Final processing of N-linked oligosaccharides: After incorporation into the protein, the N-linked oligosaccharide is processed by the removal of specific mannosyl and glucosyl residues as the glycoprotein moves through the RER. Finally, the oligosaccharide chains are completed in the Golgi by addition of a variety of sugars (for example, N-GlcNAc, N-GalNAc, and additional mannoses, and then fucose or NANA as terminal groups) to produce a complex glycoprotein. Alternatively, they are not processed further, leaving branched, mannose-containing chains in a high-mannose glycoprotein (see Figure 14.16). The ultimate fate of N-linked glycoproteins is the same as that of the O-glycoproteins linked (for example, they can be released by the cell or become part of a cell membrane). In addition, N-linked glycoproteins can be targeted to the lysosomes. [Note: Nonenzymatic glycosylation of proteins is known as glycation (see p. 345).]
Figure 14.17 Mechanism for transport of N-linked glycoproteins to the lysosomes. Asn = asparagine; Man = mannose; P = phosphate; Pi = inorganic phosphate.
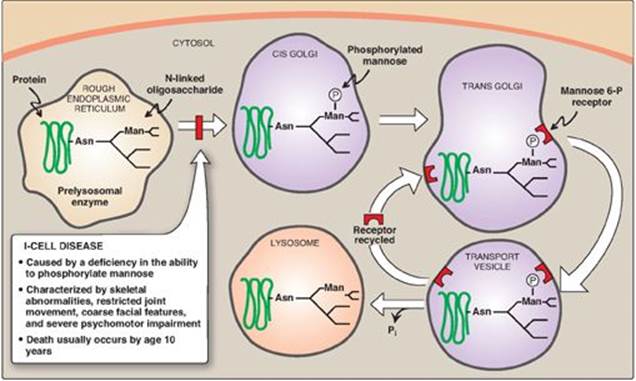
3. Enzymes destined for lysosomes: N-linked glycoproteins being processed through the Golgi can be phosphorylated on carbon 6 of one or more specific mannosyl residues. Mannose 6-phosphate receptors, located in the Golgi apparatus, bind the mannose 6-phosphate residues of these targeted enzymes, which are then packaged into vesicles and sent to the lysosomes. I-cell disease is a rare lysosomal storage disease in which the acid hydrolasesnormally found in lysosomes are absent, resulting in an accumulation of substrates normally degraded by these enzymes. [Note: I-cell disease is so-named because of the large inclusion bodies seen in cells of patients with this disease.] In addition, high amounts of lysosomal enzymes are found in the patient’s plasma and urine, indicating that the targeting process to lysosomes (rather than the synthetic pathway of these enzymes) is deficient. Individuals with I-cell disease are lacking the phosphotransferase needed to phosphorylate the mannose residues of potential lysosomal enzymes, causing the enzymes to be secreted (by default), rather than being targeted to lysosomal vesicles (Figure 14.17). I-cell disease is characterized by skeletal abnormalities, restricted joint movement, coarse (dysmorphic) facial features, and severe psychomotor impairment. [Note: Because I-cell disease has features in common with the mucopolysaccharidoses and sphingolipidoses (see p. 211), it is termed a mucolipidosis.] Currently, there is no cure, and death from cardiopulmonary complications usually occurs by age 10 years.
IX. LYSOSOMAL DEGRADATION OF GLYCOPROTEINS
Degradation of glycoproteins is similar to that of the GAGs (see p. 162). The lysosomal acid hydrolases are each generally specific for the removal of one component of the glycoprotein. They are primarily exoenzymes that remove their respective groups in the reverse order of their incorporation (“last on, first off”). If any one degradative enzyme is missing, degradation by the other exoenzymes cannot continue. A group of very rare, autosomal recessive diseases called the glycoprotein storage diseases (oligosaccharidoses), caused by a deficiency of any one of the degradative enzymes, results in accumulation of partially degraded structures in the lysosomes. For example, α-mannosidosis type 1 is a progressive, fatal deficiency of the enzyme, α-mannosidase. Presentation is similar to Hurler syndrome, but immune deficiency is also seen. Mannose-rich oligosaccharide fragments appear in the urine. Diagnosis is by enzyme assay.
X. CHAPTER SUMMARY
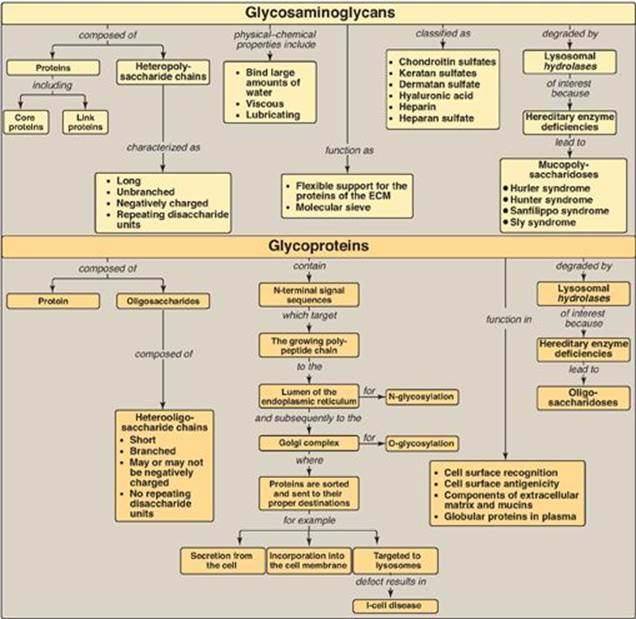
Glycosaminoglycans (GAGs) are long, negatively charged, unbranched, heteropolysaccharide chains generally composed of a repeating disaccharide unit [acidic sugar–amino sugar]n (Figure 14.18). The amino sugar is either D-glucosamine or D-galactosamine in which the amino group is usually acetylated, thus eliminating its positive charge. The amino sugar may also be sulfated on carbon 4 or 6 or on a nonacetylated nitrogen. The acidic sugar is either D-glucuronic acid or its C-5 epimer L-iduronic acid. GAGs bind large amounts of water, thereby producing the gel-like matrix that forms the basis of the body’s ground substance. The viscous, lubricating properties of mucous secretions are also caused by the presence of GAGs, which led to the original naming of these compounds as mucopolysaccharides. There are six major types of GAGs, including chondroitin 4- and 6-sulfates, keratan sulfate, dermatan sulfate, heparin, heparan sulfate, and hyaluronic acid. All of the GAGs, except hyaluronic acid, are found covalently attached to protein, forming proteoglycan monomers, which consist of a core protein to which the linear GAG chains are covalently attached. The proteoglycan monomers associate with a molecule of hyaluronic acid to form proteoglycan aggregates. GAGs are synthesized in the Golgi. The polysaccharide chains are elongated by the sequential addition of alternating acidic and amino sugars, donated by their UDP-derivatives. D-glucuronate may be epimerized to L-iduronate. The last step in synthesis is sulfation of some of the amino sugars. The source of the sulfate is 3′-phosphoadenosyl-5′-phosphosulfate. The completed proteoglycans are secreted into the extracellular matrix or remain associated with the outer surface of cells. GAGs are degraded by lysosomal acid hydrolases. They are first broken down to oligosaccharides, which are degraded sequentially from the nonreducing end of each chain. A deficiency of any one of the hydrolasesresults in a mucopolysaccharidosis. These are hereditary disorders in which GAGs accumulate in tissues, causing symptoms such as skeletal and extracellular matrix deformities and intellectual disability. Examples of these genetic diseases include Hunter and Hurler syndromes. Glycoproteins are proteins to which oligosaccharides are covalently attached. They differ from the proteoglycans in that the length of the glycoprotein’s carbohydrate chain is relatively short (usually two to ten sugar residues long, although they can be longer), may be branched, and does not contain serial disaccharide units. Membrane-bound glycoproteins participate in a broad range of cellular phenomena, including cell-surface recognition (by other cells, hormones, and viruses), cell-surface antigenicity (such as the blood group antigens), and as components of the extracellular matrix and of the mucins of the gastrointestinal and urogenital tracts, where they act as protective biologic lubricants. In addition, almost all of the globular proteins present in human plasma are glycoproteins. Glycoproteins are synthesized in the rough endoplasmic reticulum (RER) and the Golgi. The precursors of the carbohydrate components of glycoproteins are nucleotide sugars. O-linked glycoproteins are synthesized in the Golgi by the sequential transfer of sugars from their nucleotide carriers to the hydroxyl group of a serine or threonine residue in the protein. N-linked glycoproteins contain varying amounts of mannose. They are synthesized by the transfer of a preformed oligosaccharide from its ER membrane lipid carrier, dolichol pyrophosphate, to the amide N of an asparagine residue in the protein. A deficiency in the phosphorylation of mannose residues in N-linked glycoprotein enzymes destined for the lysosomes results in I-cell disease. Glycoproteins are degraded in lysosomes by acid hydrolases. A deficiency of any one of these enzymes results in a lysosomal glycoprotein storage disease (oligosaccharidosis), resulting in accumulation of partially degraded structures in the lysosome.
Figure 14.18 Key concept map for glycosaminoglycans and glycoproteins. ECM = extracellular matrix.
Study Questions
Choose the ONE best answer.
14.1 Mucopolysaccharidoses are hereditary lysosomal storage diseases. They are caused by:
A. defects in the degradation of glycosaminoglycans.
B. defects in the targeting of enzymes to lysosomes.
C. an increased rate of synthesis of the carbohydrate component of proteoglycans.
D. an insufficient rate of synthesis of proteolytic enzymes.
E. the synthesis of abnormally small amounts of core proteins.
F. the synthesis of heteropolysaccharides with an altered structure.
Correct answer = A. The mucopolysaccharidoses are caused by deficiencies in any one of the lysosomal acid hydrolases responsible for the degradation of glycosaminoglycans (not proteins). The enzyme is correctly targeted to the lysosome, so blood levels of the enzyme do not increase, but it is nonfunctional. In these diseases, synthesis of the protein and carbohydrate components of proteoglycans is unaffected, both in terms of structure and amount.
14.2 The presence of the following compound in the urine of a patient suggests a deficiency in which one of the enzymes listed below?
![]()
A. Galactosidase
B. Glucuronidase
C. Iduronidase
D. Mannosidase
E. Sulfatase
Correct answer = E. Degradation of glycoproteins follows the rule “last on, first off.” Because sulfation is the last step in the synthesis of this sequence, a sulfatase is required for the next step in the degradation of the compound shown.
14.3 An 8-month-old boy with coarse facial features, skeletal abnormalities, and delays in both growth and development is diagnosed with I-cell disease based on his presentation and on histologic and biochemical testing. I-cell disease is characterized by:
A. decreased production of cell-surface O-linked glycoproteins.
B. elevated levels of acid hydrolases in the blood.
C. an inability to N-glycosylate proteins.
D. increased synthesis of proteoglycans.
E. oligosaccharides in the urine.
Correct answer = B. I-cell disease is a lysosomal storage disease caused by deficiency of a protein essential for synthesis of the mannose 6-phosphate signal that targets acid hydrolases to the lysosome. This results in secretion of these enzymes from the cell and accumulation of materials within the lysosome due to impaired degradation. None of the other choices relate in any way to I-cell disease or lysosomal function. Oligosaccharides in the urine are characteristic of the muco- and polysaccharidoses but not I-cell disease (a mucolipidosis).
14.4 An infant with corneal clouding has dermatan sulfate and heparin sulfate in his urine. Decreased activity of which of the enzymes listed below would confirm the suspected diagnosis of Hurler syndrome?
A. α-L-Iduronidase
B. β-Glucuronidase
C. Glycosyltransferase
D. Iduronate sulfatase
Correct answer = A. Hurler syndrome, a defect in the lysosomal degradation of glycosaminoglycans (GAGs) with corneal clouding, is due to a deficiency in α-L-iduronidase. β-glucuronidase is deficient in Sly syndrome, and iduronate sulfatase is deficient in Hunter syndrome. Glycosyltransferases are enzymes of GAG synthesis.
14.5 Distinguish between glycoproteins and proteoglycans.
Glycoproteins are proteins to which short, branched, oligosaccharide chains are attached. Proteoglycans consist of a core protein to which long, unbranched, glycosaminoglycan (GAG) chains are attached. GAGs are large complexes of negatively charged heteropolysaccharides composed of repeating [acidic sugar–amino sugar]n disaccharide units.