Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT V: Integration of Metabolism
Chapter 23. Metabolic Effects of Insulin and Glucagon
I. OVERVIEW
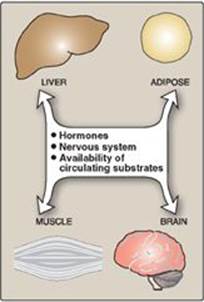
Four major tissues play a dominant role in fuel metabolism: liver, adipose, muscle, and brain. These tissues contain unique sets of enzymes, such that each tissue is specialized for the storage, use, or generation of specific fuels. These tissues do not function in isolation, but rather form part of a network in which one tissue may provide substrates to another or process compounds produced by other organs. Communication between tissues is mediated by the nervous system, by the availability of circulating substrates, and by variation in the levels of plasma hormones (Figure 23.1). The integration of energy metabolism is controlled primarily by the actions of two peptide hormones, insulin and glucagon (secreted in response to changing substrate levels in the blood), with the catecholamines epinephrine and norepinephrine (secreted in response to neural signals) playing a supporting role. Changes in the circulating levels of these hormones allow the body to store energy when food is abundant or to make stored energy available such as during “survival crises” (for example, famine, severe injury, and “fight-or-flight” situations). This chapter describes the structure, secretion, and metabolic effects of the two hormones that most profoundly affect energy metabolism.
Figure 23.1 Mechanisms of communication between four major tissues.
II. INSULIN
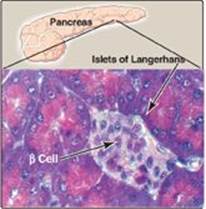
Insulin is a peptide hormone produced by the β cells of the islets of Langerhans, which are clusters of cells that are embedded in the endocrine portion of the pancreas (Figure 23.2). [Note: “Insulin” is from the Latin for island.] The islets make up only about 1%–2% of the total cells of the pancreas. Insulin is the most important hormone coordinating the use of fuels by tissues. Its metabolic effects are anabolic, favoring, for example, synthesis of glycogen, triacylglycerols (TAGs), and protein.
A. Structure of insulin
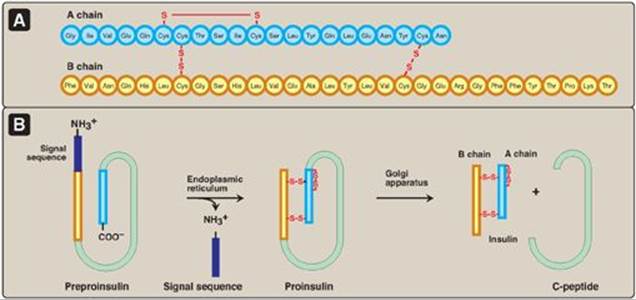
Insulin is composed of 51 amino acids arranged in two polypeptide chains, designated A (21 amino acids) and B, which are linked together by two disulfide bridges (Figure 23.3A). The insulin molecule also contains an intramolecular disulfide bridge between amino acid residues of the A chain. [Note: Insulin was the first peptide for which the primary structure was determined, and the first therapeutic molecule made by recombinant DNA technology (see p. 470).]
Figure 23.2 Islets of Langerhans.
B. Synthesis of insulin
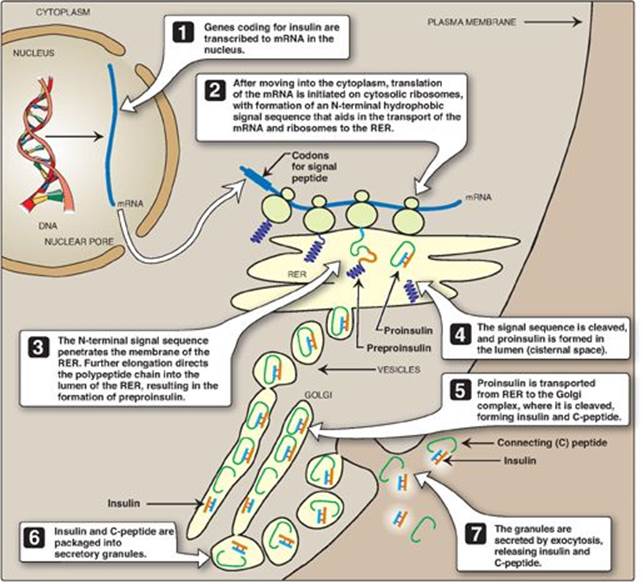
The processing and transport of intermediates that occur during the synthesis of insulin are shown in Figures 23.3B and 23.4. Biosynthesis involves production of two inactive precursors, preproinsulin and proinsulin, that are sequentially cleaved to form the active hormone plus the connecting or C-peptide (see Figure 23.4). [Note: The C-peptide is essential for proper insulin folding. Also, because its half-life in the plasma is longer than that of insulin, the C-peptide is a good indicator of insulin production and secretion.] Insulin is stored in the cytosol in granules that, given the proper stimulus (see below), are released by exocytosis. (See p. 166 for a discussion of the synthesis of secreted proteins.) Insulin is degraded by insulin-degrading enzyme, which is present in the liver and, to a lesser extent, in the kidneys. Insulin has a plasma half-life of approximately 6 minutes. This short duration of action permits rapid changes in circulating levels of the hormone.
Figure 23.3 A. Structure of insulin. B. Formation of human insulin from preproinsulin.
C. Regulation of insulin secretion
Secretion of insulin is regulated by bloodborne fuels and hormones.
1. Stimulation of insulin secretion: Insulin secretion by the pancreatic β cells is closely coordinated with the release of glucagon by pancreatic α cells (Figure 23.5). The relative amounts of insulin and glucagon released by the pancreas are regulated so that the rate of hepatic glucose production is kept equal to the use of glucose by peripheral tissues. In view of its coordinating role, it is not surprising that the β cell responds to a variety of stimuli. In particular, insulin secretion is increased by glucose, amino acids, and gastrointestinal peptide hormones.
Figure 23.4 ntracellular movements of insulin and its precursors. mRNA = messenger RNA; RER = rough endoplasmic reticulum.
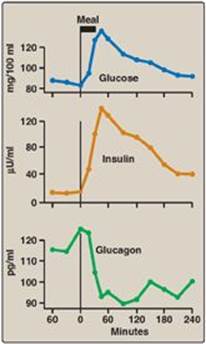
a. Glucose: Ingestion of a carbohydrate-rich meal leads to a rise in blood glucose, the primary stimulus for insulin secretion (see Figure 23.5). The β cells are the most important glucose-sensing cells in the body. Like the liver, β cells contain GLUT-2 transporters and express glucokinase (hexokinase IV; see p. 98). At blood glucose levels above 45 mg/dl, glucokinase phosphorylates glucose in amounts proportional to the glucose concentration. Proportionality results from the lack of direct inhibition of glucokinase by glucose 6-phosphate, its product. Additionally, the sigmoidal relationship between the velocity of the reaction and substrate concentration (see p. 98) maximizes the enzyme’s responsiveness to changes in blood glucose level. Metabolism of glucose 6-phosphate generates adenosine triphosphate (ATP), leading to insulin secretion (see blue box below).
Figure 23.5 Changes in blood levels of glucose, insulin, and glucagon after ingestion of a carbohydrate-rich meal.
b. Amino acids: Ingestion of protein causes a transient rise in plasma amino acid levels (for example, arginine) that enhances the glucose-stimulated secretion of insulin. [Note: Fatty acids have a similar effect.]
c. Gastrointestinal peptide hormones: The intestinal peptides glucagon-like protein-1 (GLP-1) and gastric-inhibitory polypeptide ([GIP]; also called glucose-dependent insulinotropic peptide) increase the sensitivity of β cells to glucose. They are released from the small intestine after the ingestion of food, causing an anticipatory rise in insulin levels and, thus, are referred to as “incretins.” Their action may account for the fact that the same amount of glucose given orally induces a much greater secretion of insulin than if given intravenously (IV).
Glucose-dependent release of insulin into blood is mediated through a rise in calcium (Ca2+) concentration in the β cell. Glucose taken into β cells is phosphorylated and metabolized, with subsequent production of ATP. ATP-sensitive potassium (K+) channels close, causing depolarization of the plasma membrane, opening of voltage-gated Ca2+ channels, and influx of Ca2+ into the cell. Ca2+ causes vesicles containing insulin to be exocytosed from the β cell. Sulfonylureas, oral agents used to treat type 2 diabetes, increase insulin secretion by closing ATP-sensitive K+ channels.
Figure 23.6 Regulation of insulin release from pancreatic β cells. [Note: Gastrointestinal peptide hormones also stimulate insulin release.]
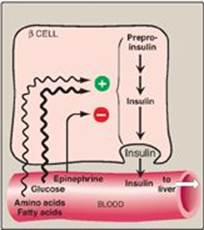
2. Inhibition of insulin secretion: The synthesis and release of insulin are decreased when there is a scarcity of dietary fuels and also during periods of physiologic stress (for example, infection, hypoxia, and vigorous exercise). These effects are mediated primarily by the catecholamines norepinephrine and epinephrine, which are made from tyrosine in the sympathetic nervous system and the adrenal medulla and secreted. Secretion is largely controlled by the nervous system. The catecholamines (primarily epinephrine) have a direct effect on energy metabolism, causing a rapid mobilization of energy-yielding fuels, including glucose from the liver (produced by glycogenolysis or gluconeogenesis; see p. 121) and fatty acids from adipose tissue (produced by lipolysis; see p. 189). In addition, these biogenic amines can override the normal glucose-stimulated release of insulin. Thus, in emergency situations, the sympathetic nervous system largely replaces the plasma glucose concentration as the controlling influence over β-cell secretion. The regulation of insulin secretion is summarized in Figure 23.6.
Figure 23.7 Insulin receptor. P = phosphate; Tyr = tyrosine.
D. Metabolic effects of insulin
Insulin promotes the storage of nutrients as glycogen, TAG, and protein and inhibits their mobilization.
1. Effects on carbohydrate metabolism: The effects of insulin on glucose metabolism promote its storage and are most prominent in three tissues: liver, muscle, and adipose. In the liver and muscle, insulin increases glycogen synthesis. In the muscle and adipose, insulin increases glucose uptake by increasing the number of glucose transporters (GLUT-4; see p. 97) in the cell membrane. The IV administration of insulin, thus, causes an immediate decrease in blood glucose level. In the liver, insulin decreases the production of glucose through the inhibition of glycogenolysis and gluconeogenesis. [Note: The effects of insulin are due not just to changes in enzyme activity, but also in enzyme amount insofar as insulin affects gene transcription.]
2. Effects on lipid metabolism: Adipose tissue responds rapidly to a rise in insulin, which causes a significant reduction in the release of fatty acids by inhibiting the activity of hormone-sensitive lipase, which degrades lipids in adipose tissue. Insulin acts by promoting the dephosphorylation and, hence, inactivation of the enzyme (see p. 190). Insulin also increases the transport and metabolism of glucose into adipocytes, providing the glycerol 3-phosphate substrate for TAG synthesis. Expression of the gene for lipoprotein lipase (see p. 229) is increased by insulin in adipose tissue, thereby providing fatty acids for esterification to the glycerol. [Note: Insulin also promotes the conversion of glucose to TAG in the liver. The TAGs are secreted in very-low-density lipoproteins (VLDLs).]
3. Effects on protein synthesis: In most tissues, insulin stimulates the entry of amino acids into cells and protein synthesis. [Note: Insulin stimulates protein synthesis through activation of factors required for translation initiation.]
E. Mechanism of insulin action
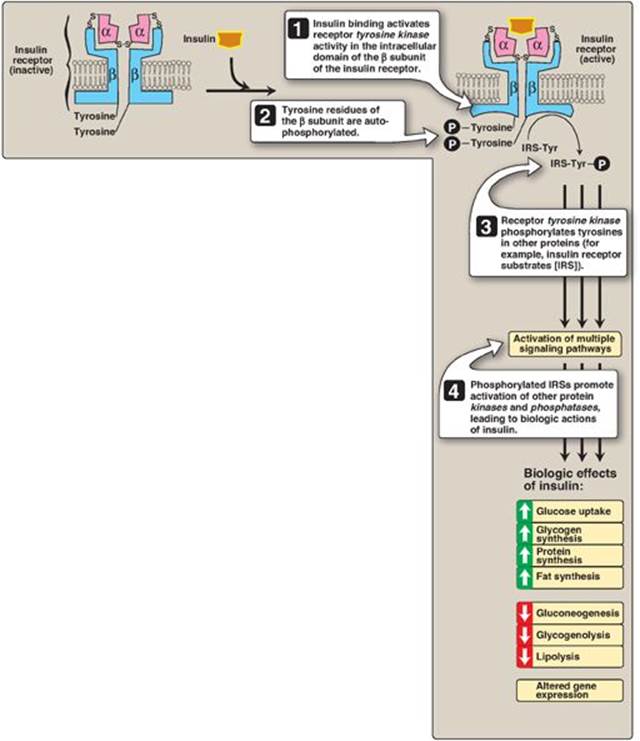
Insulin binds to specific, high-affinity receptors in the cell membrane of most tissues, including liver, muscle, and adipose. This is the first step in a cascade of reactions ultimately leading to a diverse array of biologic actions (Figure 23.7).
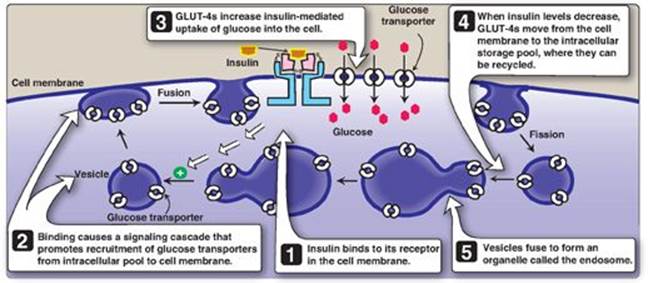
Figure 23.8 Insulin-mediated recruitment of glucose transporters (GLUT-4s) from intracellular stores in skeletal and cardiac muscle and adipose tissue.
1. Insulin receptor: The insulin receptor is synthesized as a single polypeptide that is glycosylated and cleaved into α and β subunits, which are then assembled into a tetramer linked by disulfide bonds (see Figure 23.7). The extracellular α subunit contains the insulin-binding site. A hydrophobic domain in each β subunit spans the plasma membrane. The cytosolic domain of the β subunit is a tyrosine kinase, which is activated by insulin. As a result, the insulin receptor is classified as a tyrosine-kinase receptor.
2. Signal transduction: The binding of insulin to the α subunits of the insulin receptor induces conformational changes that are transmitted to the β subunits. This promotes a rapid autophosphorylation of specific tyrosine residues on each β subunit (see Figure 23.7). Autophosphorylation initiates a cascade of cell-signaling responses, including phosphorylation of a family of proteins called insulin receptor substrates (IRSs). At least four IRSs have been identified that show similar structures but different tissue distributions. Phosphorylated IRS proteins interact with other signaling molecules through specific domains, activating a number of pathways that affect gene expression, cell metabolism, and growth. The actions of insulin are terminated by dephosphorylation of the receptor.
Figure 23.9 Characteristics of glucose transport in various tissues.
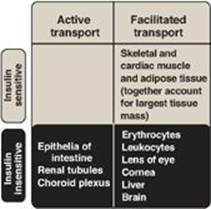
3. Membrane effects of insulin: Glucose transport in some tissues, such as muscle and adipose, increases in the presence of insulin (Figure 23.8). Insulin promotes movement of insulin-sensitive glucose transporters (GLUT-4s) from a pool located in intracellular vesicles to the cell membrane. [Note: Movement is the result of a signaling cascade in which an IRS binds to and activates a kinase (phosphoinositide 3-kinase), leading to phosphorylation of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate to the 3,4,5-trisphosphate form that binds to and activates phosphoinositide kinase 1. This kinase, in turn, activates Akt (protein kinase B), resulting in GLUT-4 movement.] In contrast, other tissues have insulin-insensitive systems for glucose transport (Figure 23.9). For example, hepatocytes; erythrocytes; and cells of the nervous system, intestinal mucosa, renal tubules, and cornea do not require insulin for glucose uptake.
4. Receptor regulation: Binding of insulin is followed by internalization of the hormone–receptor complex. Once inside the cell, insulin is degraded in the lysosomes. The receptors may be degraded, but most are recycled to the cell surface. [Note: Elevated levels of insulin promote the degradation of receptors, therby decreasing the number of surface receptors. This is one type of “downregulation.”]
5. Time course of insulin actions: The binding of insulin provokes a wide range of actions. The most immediate response is an increase in glucose transport into adipocytes and skeletal and cardiac muscle cells that occurs within seconds of insulin binding to its membrane receptor. Insulin-induced changes in enzymic activity in many cell types occur over minutes to hours and reflect changes in the phosphorylation states of existing proteins. Insulin-induced increase in the amount of many enzymes, such as glucokinase, liver pyruvate kinase, acetyl coenzyme A (CoA) carboxylase (ACC), and fatty acid synthase, requires hours to days. These changes reflect an increase in gene expression through increased transcription (mediated by sterol regulatory element–binding protein-1; see p. 184) and translation.
Figure 23.10 Opposing actions of insulin and glucagon plus epinephrine.
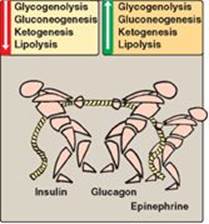
III. GLUCAGON
Glucagon is a peptide hormone secreted by the α cells of the pancreatic islets of Langerhans. Glucagon, along with epinephrine, norepinephrine, cortisol, and growth hormone (the “counterregulatory” hormones), opposes many of the actions of insulin (Figure 23.10). Most importantly, glucagon acts to maintain blood glucose levels by activation of hepatic glycogenolysis and gluconeogenesis. Glucagon is composed of 29 amino acids arranged in a single polypeptide chain. [Note: Unlike insulin, the amino acid sequence of glucagon is the same in all mammalian species examined to date.] Glucagon is synthesized as a large precursor molecule (preproglucagon) that is converted to glucagon through a series of selective proteolytic cleavages, similar to those described for insulin biosynthesis (see Figure 23.3). In contrast to insulin, preproglucagon is processed to different products in different tissues, for example, GLP-1 in intestinal L cells. Like insulin, glucagon has a short half-life.
A. Stimulation of glucagon secretion
The α cell is responsive to a variety of stimuli that signal actual or potential hypoglycemia (Figure 23.11). Specifically, glucagon secretion is increased by low blood glucose, amino acids, and catecholamines.
1. Low blood glucose: A decrease in plasma glucose concentration is the primary stimulus for glucagon release. During an overnight or prolonged fast, elevated glucagon levels prevent hypoglycemia (see below for a discussion of hypoglycemia).
Figure 23.11 Regulation of glucagon release from pancreatic α cells. [Note: Amino acids increase release of insulin and glucagon, whereas glucose increases release of insulin only.]
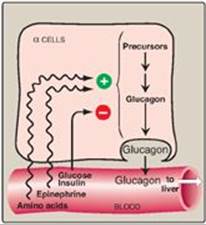
2. Amino acids: Amino acids (for example, arginine) derived from a meal containing protein stimulate the release of glucagon. The glucagon effectively prevents the hypoglycemia that would otherwise occur as a result of the increased insulin secretion that also occurs after a protein meal.
3. Catecholamines: Elevated levels of circulating epinephrine produced by the adrenal medulla, norepinephrine produced by sympathetic innervation of the pancreas, or both stimulate the release of glucagon. Thus, during periods of physiologic stress, the elevated catecholamine levels can override the effect on the α cell of circulating substrates. In these situations, regardless of the concentration of blood glucose, glucagon levels are elevated in anticipation of increased glucose use. In contrast, insulin levels are depressed.
B. Inhibition of glucagon secretion
Glucagon secretion is significantly decreased by elevated blood glucose and by insulin. Both substances are increased following ingestion of glucose or a carbohydrate-rich meal (see Figure 23.5). The regulation of glucagon secretion is summarized in Figure 23.11.
C. Metabolic effects of glucagon
Glucagon is a catabolic hormone that promotes the maintenance of blood glucose levels. Its primary target is the liver.
1. Effects on carbohydrate metabolism: The IV administration of glucagon leads to an immediate rise in blood glucose. This results from an increase in the breakdown of liver glycogen and an increase in hepatic gluconeogenesis.
2. Effects on lipid metabolism: The primary effect of glucagon on lipid metabolism is inhibition of fatty acid synthesis through phosphorylation of ACC (see p. 184). The decrease in malonyl CoA production as a result of ACCinhibition removes the break on long-chain fatty acid β-oxidation. Glucagon also plays a role in lipolysis in adipose, but the major activators of hormone sensitive lipase (via phosphorylation by protein kinase A) are the catecholamines. The free fatty acids released are taken up by liver and oxidized to acetyl CoA, which is used in ketone body synthesis.
3. Effects on protein metabolism: Glucagon increases uptake by the liver of amino acids supplied by muscle, resulting in increased availability of carbon skeletons for gluconeogenesis. As a consequence, plasma levels of amino acids are decreased.
Figure 23.12 Mechanism of action of glucagon. [Note: For clarity, G-protein activation of adenylyl cyclase has been omitted.] R = regulatory subunit; C = catalytic subunit; cAMP = cyclic adenosine monophosphate; ADP = adenosine diphosphate; ![]() = phosphate.
= phosphate.
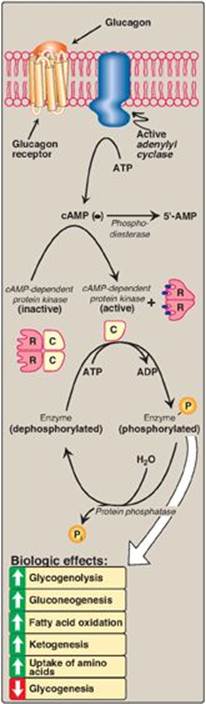
D. Mechanism of action of glucagon
Glucagon binds to high-affinity G protein–coupled receptors on the cell membrane of hepatocytes. The receptors for glucagon are distinct from those that bind insulin or epinephrine. [Note: Glucagon receptors are not found on skeletal muscle.] Glucagon binding results in activation of adenylyl cyclase in the plasma membrane (Figure 23.12; also see p. 94). This causes a rise in cyclic adenosine monophosphate (cAMP), which, in turn, activates cAMP-dependent protein kinase A and increases the phosphorylation of specific enzymes or other proteins. This cascade of increasing enzymic activities results in the phosphorylation-mediated activation or inhibition of key regulatory enzymes involved in carbohydrate and lipid metabolism. An example of such a cascade in glycogen degradation is shown in Figure 11.9 on p. 130. [Note: Glucagon, like insulin, affects gene transcription. For example, glucagon induces expression of phosphoenolpyruvate carboxykinase (see p. 122).]
IV. HYPOGLYCEMIA
Hypoglycemia is characterized by 1) central nervous system (CNS) symptoms, including confusion, aberrant behavior, or coma; 2) a simultaneous blood glucose level equal to or less than 40 mg/dl; and 3) symptoms being resolved within minutes following the administration of glucose (Figure 23.13). Hypoglycemia is a medical emergency because the CNS has an absolute requirement for a continuous supply of bloodborne glucose to serve as fuel for energy metabolism. Transient hypoglycemia can cause cerebral dysfunction, whereas severe, prolonged hypoglycemia causes brain death. Therefore, it is not surprising that the body has multiple overlapping mechanisms to prevent or correct hypoglycemia. The most important hormone changes in combating hypoglycemia are elevated glucagon and the catecholamines, combined with the diminished release of insulin.
Figure 23.13 A. Actions of some of the glucoregulatory hormones in response to low blood glucose. B. Glycemic thresholds for the various responses to hypoglycemia. [Note: Normal fasted blood glucose is 70-99 mg/100 ml.] + = weak stimulation; ++ = moderate stimulation; +++ = strong stimulation; 0 = no effect; ACTH = adrenocorticotropic hormone.
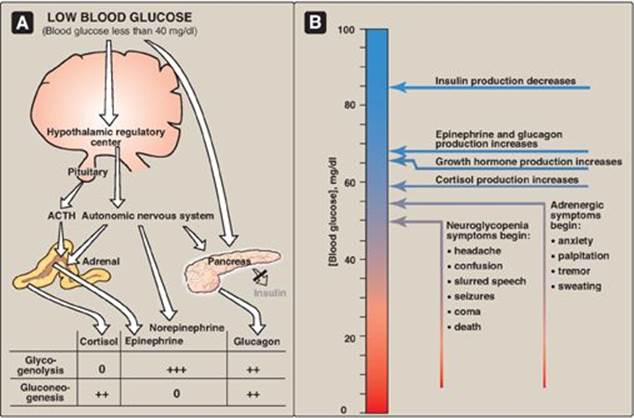
A. Symptoms of hypoglycemia
The symptoms of hypoglycemia can be divided into two categories. Adrenergic symptoms, such as anxiety, palpitation, tremor, and sweating, are mediated by catecholamine release (primarily epinephrine) regulated by the hypothalamus in response to hypoglycemia. Adrenergic symptoms typically occur when blood glucose levels fall abruptly. The second category of hypoglycemic symptoms is neuroglycopenic. Neuroglycopenia (that is, the impaired delivery of glucose to the brain) results in impairment of brain function, causing headache, confusion, slurred speech, seizures, coma, and death. Neuroglycopenic symptoms often result from a gradual decline in blood glucose, often to levels below 40 mg/dl. The slow decline in glucose deprives the CNS of fuel, but fails to trigger an adequate adrenergic response.
B. Glucoregulatory systems
Humans have two overlapping glucose-regulating systems that are activated by hypoglycemia: 1) the pancreatic α cells, which release glucagon, and 2) receptors in the hypothalamus, which respond to abnormally low concentrations of blood glucose. The hypothalamic glucoreceptors can trigger both the secretion of catecholamines (mediated by the autonomic nervous system) and release of adrenocorticotropic hormone (ACTH) and growth hormone by the anterior pituitary (see Figure 23.13). [Note: ACTH increases cortisol synthesis and secretion in the adrenal cortex (see p. 239.] Glucagon, the catecholamines, cortisol, and growth hormones are sometimes called the “counterregulatory” hormones because each opposes the action of insulin on glucose use.
1. Glucagon and epinephrine: Secretion of these hormones is most important in the acute, short-term regulation of blood glucose levels. Glucagon stimulates hepatic glycogenolysis and gluconeogenesis. Epinephrine promotes glycogenolysis and lipolysis, inhibits insulin secretion, and inhibits the insulin-mediated uptake of glucose by peripheral tissues. Epinephrine assumes a critical role in hypoglycemia when glucagon secretion is deficient, for example, in the late stages of type 1 diabetes mellitus (see p. 340). The prevention or correction of hypoglycemia fails when the secretion of both glucagon and epinephrine is deficient.
2. Cortisol and growth hormone: These hormones are less important in the short-term maintenance of blood glucose concentrations. They do, however, play a role in the long-term (transcriptional) management of glucose metabolism.
Figure 23.14 Reversal of insulin-induced hypoglycemia by administration of subcutaneous glucagon.
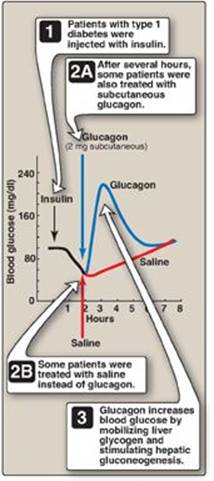
C. Types of hypoglycemia
Hypoglycemia may be divided into four types: 1) insulin-induced, 2) postprandial (sometimes called reactive hypoglycemia), 3) fasting hypoglycemia, and 4) alcohol-related.
1. Insulin-induced hypoglycemia: Hypoglycemia occurs frequently in patients with diabetes who are receiving insulin treatment, particularly those striving to achieve tight control of blood glucose levels. Mild hypoglycemia in fully conscious patients is treated by oral administration of carbohydrate. Unconscious patients are typically given glucagon subcutaneously or intramuscularly (Figure 23.14).
2. Postprandial hypoglycemia: This is the second most common form of hypoglycemia. It is caused by an exaggerated insulin release following a meal, prompting transient hypoglycemia with mild adrenergic symptoms. The plasma glucose level returns to normal even if the patient is not fed. The only treatment usually required is that the patient eats frequent small meals rather than the usual three large meals.
3. Fasting hypoglycemia: Low blood glucose during fasting is rare but is more likely to present as a serious medical problem. Fasting hypoglycemia, which tends to produce neuroglycopenic symptoms, may result from a reduction in the rate of glucose production by hepatic glycogenolysis or gluconeogenesis. Thus, low blood glucose levels are often seen in patients with hepatocellular damage or adrenal insufficiency or in fasting individuals who have consumed large quantities of ethanol (see below). Alternately, fasting hypoglycemia may be the result of an increased rate of glucose use by the peripheral tissues due to overproduction of insulin by rare pancreatic tumors. If left untreated, a patient with fasting hypoglycemia may lose consciousness and experience convulsions and coma. [Note: Certain inborn errors of metabolism, for example, defects in fatty acid oxidation, result in fasting hypoglycemia.]
Figure 23.15 A. Normal gluconeogenesis in the absence of ethanol consumption. B. Inhibition of gluconeogenesis resulting from hepatic metabolism of ethanol. NAD(H) = nicotinamide adenine dinucleotide.
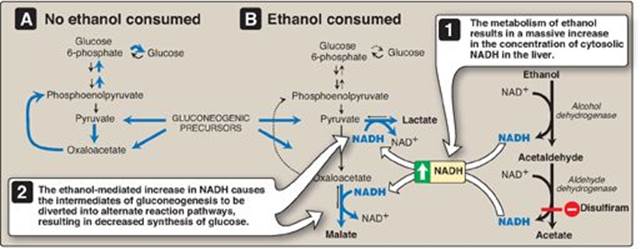
4. Alcohol-related hypoglycemia: Alcohol is metabolized in the liver by two oxidation reactions (Figure 23.15). Ethanol is first converted to acetaldehyde by alcohol dehydrogenase. Acetaldehyde is subsequently oxidized to acetate by aldehyde dehydrogenase (ALDH). [Note: ALDH is inhibited by disulfiram, a drug that is used in the treatment of chronic alcoholism. The resulting rise in acetaldehyde results in flushing, tachycardia, hyperventilation, and nausea.] In each reaction, electrons are transferred to oxidized nicotinamide adenine dinucleotide (NAD+), resulting in an increase in the concentration of cytosolic NADH. The abundance of NADH favors the reduction of pyruvate to lactate and of oxaloacetate (OAA) to malate. Recall from p. 118 that pyruvate and OAA are intermediates in the synthesis of glucose. Thus, the ethanol-mediated increase in NADH causes these intermediates of gluconeogenesis to be diverted into alternate pathways, resulting in the decreased synthesis of glucose. This can precipitate hypoglycemia, particularly in individuals who have depleted their stores of liver glycogen. [Note: Decreased availability of OAA allows acetyl CoA to be diverted to ketone body synthesis in the liver (see p. 195) and can result in alcoholic ketoacidosis.] Hypoglycemia can produce many of the behaviors associated with alcohol intoxication, such as agitation, impaired judgment, and combativeness. Therefore, alcohol consumption in vulnerable individuals (such as those who are fasted or have engaged in prolonged, strenuous exercise) can produce hypoglycemia that may contribute to the behavioral effects of alcohol. Becuase alcohol consumption can also increase the risk for hypoglycemia in patients using insulin, those in an intensive insulin treatment protocol (see p. 340) are counseled about the increased risk of hypoglycemia that generally occurs many hours after alcohol ingestion. [Note: Chronic alcohol consumption can also result in alcoholic fatty liver due to increased hepatic synthesis of TAGs coupled with impaired formation or release of VLDLs. This occurs as a result of decreased fatty acid oxidation due to a fall in the NAD+/NADH ratio and increased lipogenesis due to the increased availability of fatty acids (decreased catabolism) and of glyceraldehyde 3-phosphate (the dehydrogenase is inhibited by the low NAD+/NADH ratio). With continued alcohol consumption, alcoholic fatty liver can progress first to alcoholic hepatitis and then to alcoholic cirrhosis (Figure 23.16).]
Figure 23.16 Effects of chronic alcohol consumption on liver morphology.

V. CHAPTER SUMMARY
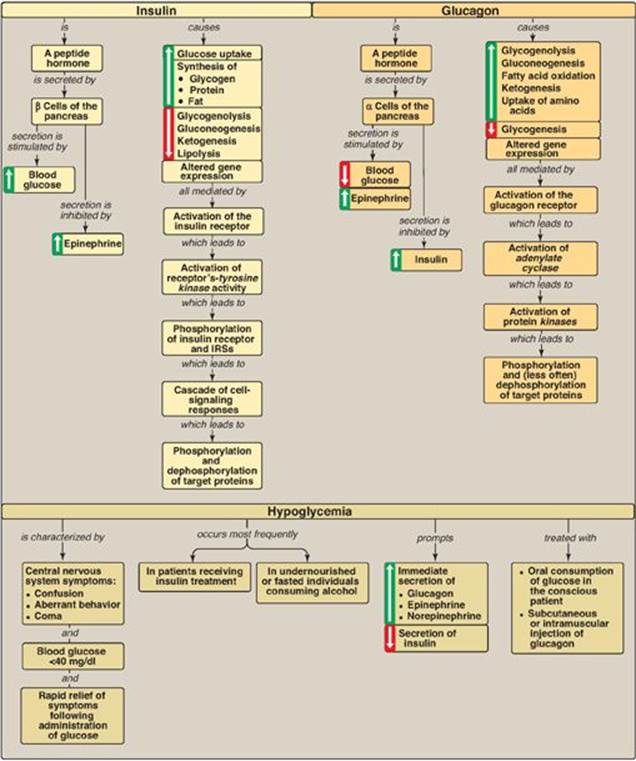
The integration of energy metabolism is controlled primarily by insulin and the opposing actions of glucagon and the catecholamines, particularly epinephrine (Figure 23.17). Changes in the circulating levels of these hormones allow the body to store energy when food is abundant or to make stored energy available in times of physiologic stress (for example, during “survival crises,” such as famine). Insulin is a peptide hormone produced by the β cells of the islets of Langerhans of the pancreas. It consists of disulfide-linked A and B chains. A rise in blood glucose is the most important signal for insulin secretion. The catecholamines, secreted in response to stress, trauma, or extreme exercise, inhibit insulin secretion. Insulin increases glucose uptake (by muscle and adipose) and the synthesis of glycogen, protein, and triacylglycerol: it is an anabolic hormone. These actions are mediated by binding to its tyrosine kinase receptor. Binding initiates a cascade of cell-signaling responses, including phosphorylation of a family of proteins called insulin receptor substrate proteins. Glucagon is a monomeric peptide hormone produced by the α cells of the pancreatic islets (both insulin and glucagon synthesis involves formation of inactive precursors that are cleaved to form the active hormones). Glucagon, along with epinephrine, norepinephrine, cortisol, and growth hormone (the “counterregulatory” hormones), opposes many of the actions of insulin. Glucagon acts to maintain blood glucose during periods of potential hypoglycemia. Glucagon increases glycogenolysis, gluconeogenesis, fatty acid oxidation, ketogenesis, and uptake of amino acids: it is a catabolic hormone. Glucagon secretion is stimulated by low blood glucose, amino acids, and the catecholamines. Its secretion is inhibited by elevated blood glucose and by insulin. Glucagon binds to high-affinity receptors of hepatocytes. Binding results in the activation of adenylyl cyclase, which produces the second messenger cyclic adenosine monophosphate (cAMP). Subsequent activation of cAMP-dependent protein kinase A results in the phosphorylation-mediated activation or inhibition of key regulatory enzymes involved in carbohydrate and lipid metabolism. Both insulin and glucagon affect gene transcription. Hypoglycemia is characterized by low blood glucose accompanied by adrenergic and neuroglycopenic symptoms that are rapidly resolved by the administration of glucose. Insulin-induced, postprandial, and fasting hypoglycemia result in release of glucagon and epinephrine. The rise in nicotinamide adenine dinucleotide (NADH) that accompanies ethanol metabolism inhibits gluconeogenesis, leading to hypoglycemia in individuals with depleted stores. Alcohol consumption also increases the risk for hypoglycemia in patients using insulin. Chronic alcohol consumption can cause fatty liver disease.
Figure 23.17 Key concept map for the metabolic effects of insulin and glucagon, and hypoglycemia. IRSs = insulin receptor substrates.
Study Questions
Choose the ONE best answer.
23.1 Which of the following statements is true for insulin but not for glucagon?
A. It is a peptide hormone secreted by pancreatic cells.
B. Its actions are mediated by binding to a receptor found on the cell membrane of liver cells.
C. Its effects include alterations in gene expression.
D. Its secretion is decreased by the catecholamines.
E. Its secretion is increased by amino acids.
F. Its synthesis involves a nonfunctional precursor that gets cleaved to yield a functional molecule.
Correct answer = D. Secretion of insulin by pancreatic β cells is inhibited by the catecholamines, whereas glucagon secretion by the α cells is stimulated by them. All of the other statements are true for both insulin and glucagon.
23.2 In which one of the following tissues is glucose transport into the cell insulin dependent?
A. Adipose
B. Brain
C. Liver
D. Red blood cells
Correct answer = A. The glucose transporter (GLUT-4) in adipose (and muscle) tissue is dependent on insulin. Insulin results in transport of GLUT-4 from intracellular vesicles to the cell membrane. The other tissues in the list contain GLUTs that are independent of insulin because they are always located on the cell membrane.
23.3 A 39-year-old woman is brought to the emergency room complaining of weakness and dizziness. She recalls getting up early that morning to do her weekly errands and had skipped breakfast. She drank a cup of coffee for lunch and had nothing to eat during the day. She met with friends at 8 p.m. and had a few drinks. As the evening progressed, she soon became weak and dizzy and was taken to the hospital. Laboratory tests revealed her blood glucose was 45 mg/dl (normal = 70–99). She was given orange juice and immediately felt better. The biochemical basis of her alcohol-induced hypoglycemia is an increase in:
A. fatty acid oxidation.
B. the ratio of the reduced-to-oxidized forms of nicotinamide adenine dinucleotide.
C. oxaloacetate and pyruvate.
D. use of acetyl coenzyme A in fatty acid synthesis.
Correct answer = B. The oxidation of ethanol to acetate by dehydrogenases is accompanied by the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. The rise in NADH shifts pyruvate to lactate and oxaloacetate (OAA) to malate, decreasing the availability of substrates for gluconeogenesis and resulting in hypoglycemia. The rise in NADH also reduces the NAD+ needed for fatty acid oxidation. The decrease in OAA shunts any acetyl coenzyme A produced to ketogenesis. Note that the inhibition of fatty acid degradation results in their reesterification into triacylglycerol that can result in fatty liver.
23.4 A patient is diagnosed with an insulinoma, a rare neuroendocrine tumor, the cells of which are derived primarily from pancreatic β cells. Which of the following would logically be characteristic of an insulinoma?
A. Decreased body weight
B. Decreased connecting peptide in the blood
C. Decreased glucose in the blood
D. Decreased insulin in the blood
Correct answer = C. Insulinomas are characterized by constant production of insulin (and, therefore, of C-peptide) by the tumor cells. The increase in insulin drives glucose uptake by tissues such as muscle and adipose that have insulin-dependent glucose transporters, resulting in hypoglycemia. The hypoglycemia is insufficient to suppress insulin production and secretion, however. Insulinomas, then, are characterized by increased blood insulin and decreased blood glucose. Insulin, as an anabolic hormone, results in weight gain.
23.5 In a patient with an even rarer glucagon-secreting tumor derived from the α cells of the pancreas, how would the presentation be expected to differ relative to the patient in Question 23.4?
A glucagon-secreting tumor of the pancreas (glucagonoma) would result in hyperglycemia, not hypoglycemia. The constant production of glucagon would result in constant gluconeogenesis, using amino acids from proteolysis as substrates. This results in loss of body weight.