Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT II: Bioenergetics and Carbohydrate Metabolism
Chapter 7. Introduction to Carbohydrates
I. OVERVIEW
Carbohydrates (saccharides) are the most abundant organic molecules in nature. They have a wide range of functions, including providing a significant fraction of the dietary calories for most organisms, acting as a storage form of energy in the body, and serving as cell membrane components that mediate some forms of intercellular communication. Carbohydrates also serve as a structural component of many organisms, including the cell walls of bacteria, the exoskeleton of many insects, and the fibrous cellulose of plants. The empiric formula for many of the simpler carbohydrates is (CH2O)n, where n ≥ 3, hence the name “hydrate of carbon.”
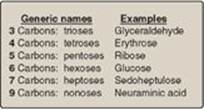
Figure 7.1 Examples of monosaccharides found in humans, classified according to the number of carbons they contain.
II. CLASSIFICATION AND STRUCTURE
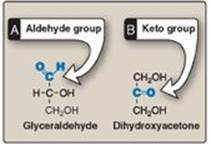
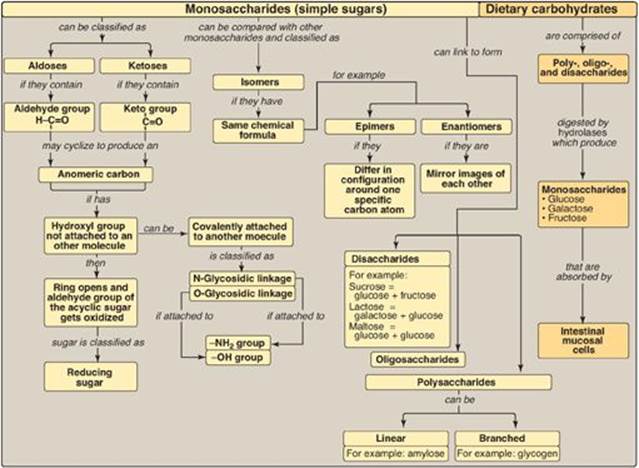
Monosaccharides (simple sugars) can be classified according to the number of carbon atoms they contain. Examples of some monosaccharides commonly found in humans are listed in Figure 7.1. They can also be classified by the type of carbonyl group they contain. Carbohydrates with an aldehyde as their carbonyl group are called aldoses, whereas those with a keto as their carbonyl group are called ketoses (Figure 7.2). For example, glyceraldehyde is an aldose, whereas dihydroxyacetone is a ketose. Carbohydrates that have a free carbonyl group have the suffix –ose. [Note: Ketoses have an additional “ul” in their suffix such as xyulose. There are exceptions, such as fructose, to this rule.] Monosaccharides can be linked by glycosidic bonds to create larger structures (Figure 7.3). Disaccharides contain two monosaccharide units, oligosaccharides contain three to ten monosaccharide units, and polysaccharides contain more than ten monosaccharide units and can be hundreds of sugar units in length.
Figure 7.2 Examples of an aldose (A) and a ketose (B) sugar.
A. Isomers and epimers
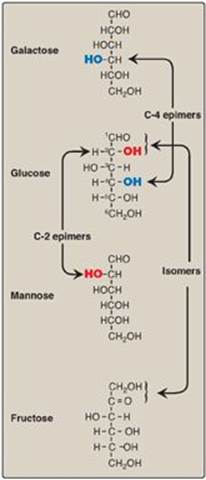
Compounds that have the same chemical formula but have different structures are called isomers. For example, fructose, glucose, mannose, and galactose are all isomers of each other, having the same chemical formula, C6H12O6. Carbohydrate isomers that differ in configuration around only one specific carbon atom (with the exception of the carbonyl carbon; see “anomers” below) are defined as epimers of each other. For example, glucose and galactose are C-4 epimers because their structures differ only in the position of the –OH group at carbon 4. [Note: The carbons in sugars are numbered beginning at the end that contains the carbonyl carbon (that is, the aldehyde or keto group) as shown in Figure 7.4.] Glucose and mannose are C-2 epimers. However, because galactose and mannose differ in the position of –OH groups at two carbons (carbons 2 and 4), they are isomers rather than epimers (see Figure 7.4).
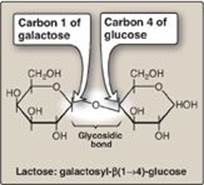
Figure 7.3 A glycosidic bond between two hexoses producing a disaccharide.
B. Enantiomers
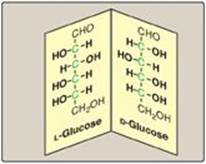
A special type of isomerism is found in the pairs of structures that are mirror images of each other. These mirror images are called enantiomers, and the two members of the pair are designated as a D- and an L-sugar (Figure 7.5). The vast majority of the sugars in humans are D-sugars. In the D isomeric form, the –OH group on the asymmetric carbon (a carbon linked to four different atoms or groups) farthest from the carbonyl carbon is on the right, whereas in the L-isomer, it is on the left. Most enzymes are specific for either the D or the L form, but enzymes known as racemases are able to interconvert D- and L-isomers.
Figure 7.4 C-2 and C-4 epimers and an isomer of glucose.
C. Cyclization of monosaccharides
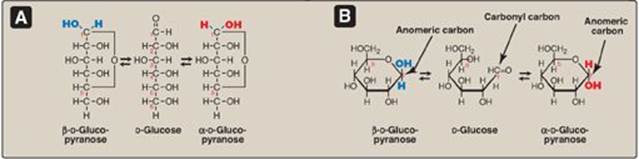
Less than 1% of each of the monosaccharides with five or more carbons exists in the open-chain (acyclic) form in solution. Rather, they are predominantly found in a ring (cyclic) form, in which the aldehyde (or keto) group has reacted with an alcohol group on the same sugar, making the carbonyl carbon (carbon 1 for an aldose, carbon 2 for a ketose) asymmetric. This asymmetric carbon is referred to as the anomeric carbon.
1. Anomers: Creation of an anomeric carbon (the former carbonyl carbon), generates a new pair of isomers, the α and β configurations of the sugar (for example, α-D-glucopyranose and β-D-glucopyranose; see Figure 7.6), that are anomers of each other. [Note: In the α configuration, the –OH group on the anomeric carbon projects to the same side as the ring in a modified Fischer projection formula (Figure 7.6A) and is trans to the CH2OH group in a Haworth projection formula (Figure 7.6B). The α and β forms are not mirror images, and they are referred to as diastereomers.] Enzymes are able to distinguish between these two structures and use one or the other preferentially. For example, glycogen is synthesized from α-D-glucopyranose, whereas cellulose is synthesized from β-D-glucopyranose. The cyclic α and β anomers of a sugar in solution spontaneously (but slowly) form an equilibrium mixture, a process known as mutarotation (see Figure 7.6). [Note: For glucose, the α form makes up 36% of the mixture.]
2. Reducing sugars: If the hydroxyl group on the anomeric carbon of a cyclized sugar is not linked to another compound by a glycosidic bond, the ring can open. The sugar can act as a reducing agent and is termed a reducing sugar. Such sugars can react with chromogenic agents (for example, the Benedict reagent) causing the reagent to be reduced and colored, with the aldehyde group of the acyclic sugar becoming oxidized. All monosaccharides, but not all disaccharides, are reducing sugars. [Note: Glucose can have its terminal hydroxyl group oxidized to a carboxyl group, forming glucuronic acid (see p. 161), or its aldehyde group oxidized to a hydroxyl group, forming a sugar alcohol.]
A colorimetric test can detect a reducing sugar in urine. A positive result is indicative of an underlying pathology, because sugars are not normally present in urine, and can be followed up by more specific tests to identify the reducing sugar.
Figure 7.5 Enantiomers (mirror images) of glucose. Designation of D and L is by comparison to the triose, glyceraldehyde. [Note: The asymmetric carbons are shown in green.]
D. Joining of monosaccharides
Monosaccharides can be joined to form disaccharides, oligosaccharides, and polysaccharides. Important disaccharides include lactose (galactose + glucose), sucrose (glucose + fructose), and maltose (glucose + glucose). Important polysaccharides include branched glycogen (from animal sources) and starch (plant sources) and unbranched cellulose (plant sources). Each is a polymer of glucose. The bonds that link sugars are called glycosidic bonds. These are formed by enzymes known as glycosyltransferases that use nucleotide sugars such as uridine diphosphate glucose as substrates.
1. Naming glycosidic bonds: Glycosidic bonds between sugars are named according to the numbers of the connected carbons and with regard to the position of the anomeric hydroxyl group of the sugar involved in the bond. If this anomeric hydroxyl is in the α configuration, the linkage is an α-bond. If it is in the β configuration, the linkage is a β-bond. Lactose, for example, is synthesized by forming a glycosidic bond between carbon 1 of β-galactose and carbon 4 of glucose. The linkage is, therefore, a β(1→4) glycosidic bond (see Figure 7.3). [Note: Because the anomeric end of the glucose residue is not involved in the glycosidic linkage, it (and, therefore, lactose) remains a reducing sugar.]
E. Complex carbohydrates
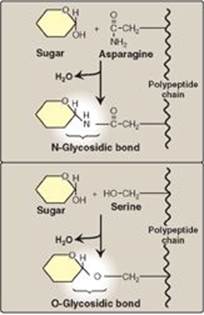
Carbohydrates can be attached by glycosidic bonds to noncarbohydrate structures, including purine and pyrimidine bases (found in nucleic acids), aromatic rings (such as those found in steroids and bilirubin), proteins (found in glycoproteins and proteoglycans), and lipids (found in glycolipids), to form glycosides.
Figure 7.6 A The interconversion (mutarotation) of the α and β anomeric forms of glucose shown as modified Fischer projection formulas. B. The interconversion shown as Haworth projection formulas. [Note: A sugar with a six-membered ring (5C + 1O) is termed a pyranose, whereas one with a five-membered ring (4C + 1O) is a furanose. Virtually all glucose in solution is in the pyranose form.]
1. N- and O-glycosides: If the group on the noncarbohydrate molecule to which the sugar is attached is an –NH2 group, the structure is an N-glycoside, and the bond is called an N-glycosidic link. If the group is an –OH, the structure is an O-glycoside, and the bond is an O-glycosidic link (Figure 7.7). [Note: All sugar–sugar glycosidic bonds are O-type linkages.]
Figure 7.7 Glycosides: examples of N- and O-glycosidic bonds.
III. DIGESTION OF DIETARY CARBOHYDRATES
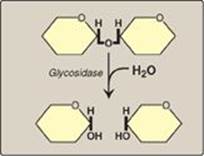
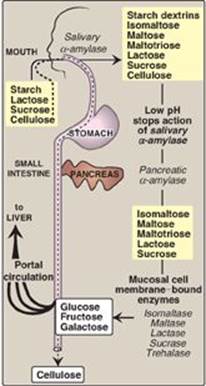
The principal sites of dietary carbohydrate digestion are the mouth and intestinal lumen. This digestion is rapid and is catalyzed by enzymes known as glycoside hydrolases (glycosidases) that hydrolyze glycosidic bonds (Figure 7.8). Because there is little monosaccharide present in diets of mixed animal and plant origin, the enzymes are primarily endoglycosidases that hydrolyze polysaccharides and oliosaccharides, and disaccharidases that hydrolyse tri- and disaccharides into their reducing sugar components. Glycosidases are usually specific for the structure and configuration of the glycosyl residue to be removed as well as for the type of bond to be broken. The final products of carbohydrate digestion are the monosaccharides, glucose, galactose, and fructose that are absorbed by cells of the small intestine.
A. Salivary a-amylase
The major dietary polysaccharides are of plant (starch, composed of amylose and amylopectin) and animal (glycogen) origin. During mastication, salivary α-amylase acts briefly on dietary starch and glycogen, hydrolyzing random α(1→4) bonds. [Note: There are both α(1→4)- and β(1→4)-endoglucosidases in nature, but humans do not produce the latter. Therefore, we are unable to digest cellulose, a carbohydrate of plant origin containing β(1→4) glycosidic bonds between glucose residues.] Because branched amylopectin and glycogen also contain α(1→6) bonds, which α-amylase cannot hydrolyze, the digest resulting from its action contains a mixture of short, branched and unbranched oligosaccharides known as dextrins (Figure 7.9). [Note: Disaccharides are also present as they, too, are resistant to amylase.] Carbohydrate digestion halts temporarily in the stomach, because the high acidity inactivates salivary α-amylase.
Figure 7.8 Hydrolysis of a glycosidic bond.
B. Pancreatic a-amylase
When the acidic stomach contents reach the small intestine, they are neutralized by bicarbonate secreted by the pancreas, and pancreatic α-amylase continues the process of starch digestion.
C. Intestinal disaccharidases
The final digestive processes occur primarily at the mucosal lining of the upper jejunum and include the action of several disaccharidases (see Figure 7.9). For example, isomaltase cleaves the α(1→6) bond in isomaltose, and maltase cleaves the α(1→4) bond in maltose and maltotriose, each producing glucose. Sucrase cleaves the α(1→2) bond in sucrose, producing glucose and fructose, and lactase (β-galactosidase) cleaves the β(1→4) bond in lactose, producing galactose and glucose. [Note: The substrates for isomaltase are broader than its name suggests, and it hydrolyzes the majority of maltose.] Trehalose, an α(1→1) disaccharide of glucose found in mushrooms and other fungi is cleaved by trehalase. These enzymes are transmembrane proteins of the brush border on the luminal surface of the intestinal mucosal cells.
Sucrase and isomaltase are enzymic activities of a single protein (SI) which is cleaved into two functional subunits that remain associated in the cell membrane, forming the sucrase-isomaltase complex. In contrast, maltase is one of two enzymic activities of a single membrane protein maltase-glucoamylase (MGA) that does not get cleaved. Its second enzymic activity, glucoamylase, cleaves a(1→4) glycosidic bonds in dextrins.
Figure 7.9 Digestion of carbohydrates. [Note: Indigestible cellulose enters the colon and is excreted.]
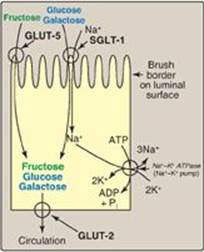
D. Intestinal absorption of monosaccharides
The duodenum and upper jejunum absorb the bulk of the monosaccharide products of digestion. However, different sugars have different mechanisms of absorption (Figure 7.10). For example, galactose and glucose are transported into the mucosal cells by an active, energy-dependent process that requires a concurrent uptake of sodium ions, and the transport protein is the sodium-dependent glucose cotransporter 1 (SGLT-1). Fructose utilizes an energy- and sodium-independent monosaccharide transporter (GLUT-5) for its absorption. All three monosaccharides are transported from the intestinal mucosal cell into the portal circulation by yet another transporter, GLUT-2. (See p. 97 for a discussion of these transporters.)
E. Abnormal degradation of disaccharides
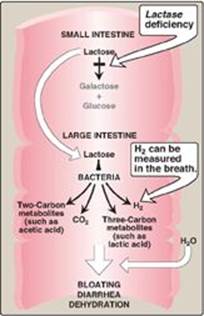
The overall process of carbohydrate digestion and absorption is so efficient in healthy individuals that ordinarily all digestible dietary carbohydrate is absorbed by the time the ingested material reaches the lower jejunum. However, because only monosaccharides are absorbed, any deficiency (genetic or acquired) in a specific disaccharidase activity of the intestinal mucosa causes the passage of undigested carbohydrate into the large intestine. As a consequence of the presence of this osmotically active material, water is drawn from the mucosa into the large intestine, causing osmotic diarrhea. This is reinforced by the bacterial fermentation of the remaining carbohydrate to two- and three-carbon compounds (which are also osmotically active) plus large volumes of CO2 and H2 gas, causing abdominal cramps, diarrhea, and flatulence.
Figure 7.10 Digestion of carbohydrates. [Note: Indigestible cellulose enters the colon and is excreted.]
1. Digestive enzyme deficiencies: Genetic deficiencies of the individual disaccharidases result in disaccharide intolerance. Alterations in disaccharide degradation can also be caused by a variety of intestinal diseases, malnutrition, and drugs that injure the mucosa of the small intestine. For example, brush border enzymes are rapidly lost in normal individuals with severe diarrhea, causing a temporary, acquired enzyme deficiency. Therefore, patients suffering or recovering from such a disorder cannot drink or eat significant amounts of dairy products or sucrose without exacerbating the diarrhea.
2. Lactose intolerance: More than 70% of the world’s adults arelactose intolerant (Figure 7.11). This is particularly manifested in certain populations. For example, up to 90% of adults of African or Asian descent are lactase-deficient and, therefore, are less able to metabolize lactose than individuals of Northern European origin. The age-dependent loss of lactase activity represents a reduction in the amount of enzyme produced. It is thought to be caused by small variations in the DNA sequence of a region on chromosome 2 that controls expression of the gene for lactase, also on chromosome 2. Treatment for this disorder is to reduce consumption of milk and eat yogurts and some cheeses (bacterial action and aging process decrease lactose content) as well as green vegetables, such as broccoli, to ensure adequate calcium intake; to use lactase-treated products; or to take lactase in pill form prior to eating. [Note: Because the loss of lactase is the norm for most of the world’s adults, use of the term “adult hypolactasia” for lactose intolerance is becoming more common.] Rare cases of congenital lactasedeficiency are known.
3. Congenital sucrase-isomaltase deficiency: This autosomal recessive disorder results in an intolerance of ingested sucrose. Congenital sucrase-isomaltase deficiency has a prevalence of 0.02% in individuals of European descent and appears to be much more common in the Inuit people of Greenland and Canada. Treatment includes the dietary restriction of sucrose and enzyme replacement therapy.
4. Diagnosis: Identification of a specific enzyme deficiency can be obtained by performing oral tolerance tests with the individual disaccharides. Measurement of hydrogen gas in the breath is a reliable test for determining the amount of ingested carbohydrate not absorbed by the body, but which is metabolized instead by the intestinal flora (see Figure 7.11).
Figure 7.11 Abnormal lactose metabolism. H2 = hydrogen gas.
IV. CHAPTER SUMMARY
Monosaccharides (Figure 7.12) containing an aldehyde group are called aldoses, and those with a keto group are called ketoses. Disaccharides, oligosaccharides, and polysaccharides consist of monosaccharides linked by glycosidic bonds. Compounds with the same chemical formula but different structures are called isomers. If two monosaccharide isomers differ in configuration around one specific carbon atom (with the exception of the carbonyl carbon), they are defined as epimers of each other. If a pair of sugars are mirror images (enantiomers), the two members of the pair are designated as D- and L-sugars. If the aldehyde group on an acyclic sugar gets oxidized as a chromogenic agent gets reduced, that sugar is a reducing sugar. When a sugar cyclizes, an anomeric carbon is created from the aldehyde group of an aldose or keto group of a ketose. The sugar can have two configurations, α or β. A sugar with its anomeric carbon linked to another structure forms a glycoside. Sugars can be attached either to an –NH2 or an –OH group, producing N- and O-glycosides. Salivary α-amylase acts on dietary polysaccharides (starch, glycogen), producing oligosaccharides. Pancreatic α-amylase continues the process of carbohydrate digestion. The final digestive processes occur at the mucosal lining of the small intestine. Several disaccharidases (for example, lactase [β-galactosidase], sucrase, isomaltase, and maltase) produce monosaccharides (glucose, galactose, and fructose). These enzymes are transmembrane proteins of the luminal brush border of intestinal mucosal cells. Absorption of the monosaccharides requires specific transporters. If carbohydrate degradation is deficient (as a result of heredity, disease, or drugs that injure the intestinal mucosa), undigested carbohydrate will pass into the large intestine, where it can cause osmotic diarrhea. Bacterial fermentation of the material produces large volumes of CO2 and H2, causing abdominal cramps, diarrhea, and flatulence. Lactose intolerance, primarily caused by the age-dependent loss of lactase (adult hypolactasia), is by far the most common of these deficiencies.
Figure 7.12 Key concept map for the classification and structure of monosaccharides and the digestion of dietary carbohydrates.
Study Question
Choose the ONE best answer.
7.1 Which of the following statements best describes glucose?
A. It is a C-4 epimer of galactose.
B. It is a ketose and usually exists as a furanose ring in solution.
C. It is produced from dietary starch by the action of α-amylase.
D. It is utilized in biological systems only in the L-isomeric form.
Correct answer = A. Glucose and galactose differ only in configuration around carbon 4 and so are C-4 epimers that are interconvertible by the action of an epimerase. Glucose is an aldose sugar that typically exists as a pyranose ring in solution. Fructose, however, is a ketose with a furanose ring. α-Amylase does not produce monosaccharides. The D-isomeric form of carbohydrates is most typically the form found in biologic systems, in contrast to amino acids.
7.2 A young man entered his physician’s office complaining of bloating and diarrhea. His eyes were sunken, and the physician noted additional signs of dehydration. The patient’s temperature was normal. He explained that the episode had occurred following a birthday party at which he had participated in an ice cream–eating contest. The patient reported prior episodes of a similar nature following ingestion of a significant amount of dairy products. This clinical picture is most probably due to a deficiency in the activity of:
A. isomaltase.
B. lactase.
C. pancreatic α-amylase.
D. salivary α-amylase.
E. sucrase.
Correct answer = B. The physical symptoms suggest a deficiency in an enzyme responsible for carbohydrate degradation. The symptoms observed following the ingestion of dairy products suggest that the patient is deficient in lactase.
7.3 Routine examination of the urine of an asymptomatic pediatric patient showed a positive reaction with Clinitest (a copper reduction method of detecting reducing sugars) but a negative reaction with the glucose oxidase test for detecting glucose. Using these data, show on the chart below which of the sugars could (YES) or could not (NO) be present in the urine of this individual.
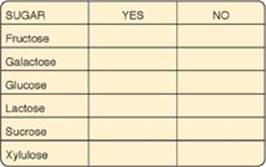
Each of the listed sugars, except for sucrose and glucose, could be present in the urine of this individual. Clinitest is a nonspecific test that produces a change in color if urine is positive for reducing substances such as reducing sugars (fructose, galactose, glucose, lactose, xylulose). Because sucrose is not a reducing sugar, it is not detected by Clinitest. The glucose oxidase test will detect only glucose, and it cannot detect other sugars. The negative glucose oxidase test in the face of a positive reducing sugar test means that glucose cannot be the reducing sugar in the patient’s urine.
7.4 Why are α-glucosidase inhibitors that are taken with meals, such as acarbose and miglitol, used in the treatment of diabetes? What effect should these drugs have on the digestion of lactose?
α-Glucosidase inhibitors slow the production of glucose from dietary carbohydrates, thereby reducing the postprandial rise in blood glucose and facilitating better blood glucose control in diabetics. These drugs have no effect on lactose digestion because the disaccharide lactose contains a β-glycosidic bond, not an α-glycosidic bond.