Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 21
A 29-year-old man presents to the emergency department with complaints of dark-colored urine, generalized fatigue, myalgias, and weakness after completing a marathon. The patient states that this was his first marathon. He has no significant medical history and denies any medications or drug use. On examination, he appears moderately ill and is afebrile with normal vital signs. Physical examination reveals diffuse musculoskeletal tenderness. Urinalysis revealed large amounts of blood (hemoglobin and myoglobin), and serum creatine phosphokinase (CPK) was significantly elevated, as well as the potassium level on his electrolytes. Serum lactate level was markedly elevated.
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What is the most appropriate treatment?
What is the most appropriate treatment?
![]() What is the biochemical basis for the markedly elevated serum lactate level?
What is the biochemical basis for the markedly elevated serum lactate level?
ANSWERS TO CASE 21:
Rhabdomyolysis
Summary: 29-year-old marathon runner with acute episode of generalized myalgias, weakness, fatigue, and dark-colored urine with urine myoglobin/hemoglobin, hyperkalemia, and significantly increased CPK isoenzyme.
• Most likely diagnosis: Rhabdomyolysis (skeletal muscle cell lysis) after strenuous exercise.
• Treatment: Aggressive intravenous hydration to help clear the excess myoglobin from the serum, and correction of electrolyte abnormalities and treatment of kidney failure if present.
• Biochemical basis for elevated lactate: Reduced nicotinamide adenine dinucleotide (NADH) levels increase because of the relative lack of oxygen for muscle, adenosine diphosphate (ADP) and adenosine monophosphate (AMP) concentrations rise in the cytoplasm, leading to an increased flux of glucose through the glycolytic pathway in the muscle, causing pyruvate levels to increase. Pyruvate is reduced by NADH to lactate in a reaction catalyzed by lactate dehydrogenase. Lactate is transported out of the muscle cell to the blood.
CLINICAL CORRELATION
Skeletal muscle has a need for oxygen and fuel (glucose and fatty acids). Short exertion allows for replenishment of these important substrates; however, long, grueling demands on muscle, such as running a marathon, can lead to relative deprivation of oxygen (because of either overexertion or dehydration and insufficient blood flow to the muscles). This lack of oxygen leads to the conversion to the glycolytic pathway versus the tricarboxylic acid (TCA) pathway for adenosine triphosphate (ATP) production. Marathon running has been shown to effect increases in the blood and urinary concentrations of a number of biochemical parameters that result from exertional muscle damage (rhabdomyolysis) and hemolysis. These include increases in serum myoglobin, CPK, as well as an increase in the anionic gap, leading to a metabolic acidosis. In the case of marathon runners, this is usually caused by an increase in the serum lactate concentration. Other causes of rhabdomyolysis include cocaine intoxication, hyperthermia, convulsions, or toxins.
APPROACH TO:
Oxidative Phosphorylation and Lactate
OBJECTIVES
1. Understand how hypoxemia (eg, rhabdomyolysis) leads to reduced oxidative phosphorylation and increased lactic acid production.
2. Be familiar with the pyruvate cycle and the importance of NADH levels.
3. Know about the lactic acid pathway.
DEFINITIONS
ANION GAP: A calculation of the routinely measured cations minus the routinely measured anions. Since in all fluids the sum of the positive charges (cations) must be balanced with the negative charges (anions), the anion gap is an artifact of measurement. Because the [K+] is small, it is usually omitted from the calculation. The equation most frequently used to calculate the anion gap is
AG = [Na+] − ([Cl−] + [HCO3−])
GLUCONEOGENESIS: The series of biochemical reactions in which glucose is synthesized in the liver (and other gluconeogenic tissues) from small organic acids such as lactate, pyruvate, and oxaloacetate.
HEMATIN: Heme in which the coordinated iron is in the ferric (Fe3+) oxidation state.
MYOGLOBIN: A large heme-containing protein that is able to bind oxygen and release it in tissues in which the oxygen tension is low.
β-OXIDATION: The series of biochemical reactions in which fatty acids are degraded to acetyl-CoA, which then enters the tricarboxylic acid cycle for the production of energy in the form of reducing equivalents and guanosine triphosphate (GTP). Each round of β-oxidation shortens the fatty acid by 2 carbons and, in addition to acetyl-CoA, produces NADH and FADH2, which are fed into the electron transport system for the production of ATP.
DISCUSSION
The exercising muscle’s sources of energy are primarily glucose and fatty acids. The muscle obtains glucose from the blood or the breakdown of stored glycogen in the muscle. Fatty acids are acquired as free fatty acids from the blood or from the breakdown of triglycerides that are stored in the muscle. For complete oxidation of these sources of energy, the metabolic intermediate acetyl coenzyme A (acetyl-CoA) must be oxidized through the TCA cycle, and the reducing equivalents produced (NADH and FADH2) must be transferred to oxygen through the mitochondrial electron transport system. This electron transfer process produces a proton gradient across the inner mitochondrial matrix that drives the synthesis of ATP by ATP synthase. For this process to continue, a plentiful supply of oxygen must be supplied to the tissues.
It has been estimated that at the beginning of the marathon, a runner who is running at a reasonable pace consumes energy in a ratio of 75% carbohydrate to 25% fatty acids. This ratio will decrease as glycogen stores in the body diminish. However, as exertion continues and the muscle starts to rely more on the β-oxidation of fatty acids to provide its energy needs, its oxygen demand increases, placing a heavier demand on the heart to provide oxygenated blood. If the runner does not take steps to replace fluids lost through sweat, then dehydration will occur, resulting in decreased perfusion of the muscle with adequately oxygenated blood.
If the muscle uses ATP faster than it can be produced by oxidative phosphorylation either by overexertion or because oxygen uptake is limited, then NADH levels increase in the mitochondria and in the cytoplasm. ADP and AMP concentrations in the cytoplasm will rise because ATP is used by the muscle for contraction. This will increase the flux of glucose through the glycolytic pathway in the muscle, causing pyruvate levels to increase. To regenerate the oxidized cofactor NAD+ that is required in the conversion of glyceraldehyde 3-phosphate to 3-phosphoglycerate, pyruvate is reduced by NADH to lactate in a reaction catalyzed by lactate dehydrogenase. Lactate is transported out of the muscle cell to the blood.
Lactate in the blood is taken up by the liver and used as a carbon source in the synthesis of new glucose by the gluconeogenic pathway. The liver must first reoxidize lactate to pyruvate in a reaction that is catalyzed by lactate dehydrogenase and generates NADH. Pyruvate cannot be directly converted to phosphoenolpyruvate (PEP) by pyruvate kinase because under physiologic conditions; the reaction is thermodynamically irreversible in favor of the formation of pyruvate. Instead, pyruvate must enter the mitochondria and be carboxylated by the enzyme pyruvate carboxylase to form oxaloacetate in a reaction that requires biotin as a cofactor (Figure 21-1). Oxaloacetate is then reduced to malate by malate dehydrogenase and malate then exits the mitochondrion. In the cytosol, malate is reoxidized back to oxaloacetate by cytoplasmic malate dehydrogenase. The cytoplasmic oxaloacetate is then converted to PEP by PEP carboxykinase in a reaction that requires GTP. (In a minor alternate pathway, mitochondrial oxaloacetate can be converted to PEP by the mitochondrial form of the enzyme PEP carboxykinase. PEP then exits the mitochondrion to the cytosol.) The pathway from PEP to glucose is identical to that of glycolysis except for 2 reactions (Figure 21-2). Fructose 1,6-bisphosphate is converted to fructose 6-phosphate by hydrolysis of phosphate by the enzyme fructose-1,6-bisphosphatase. The final reaction is hydrolysis of glucose 6-phosphate to glucose by glucose-6-phosphatase. Hepatic glucose produced via gluconeogenesis is then delivered to the blood for use by the brain and muscle. This process by which extrahepatic lactate is taken back to the liver, converted to glucose by gluconeogenesis, and returned to extrahepatic tissues is called the Cori cycle. When the rate of lactate production by muscle exceeds the rate at which the Cori cycle can operate, lactate accumulates in the blood leading to lactic acidemia.
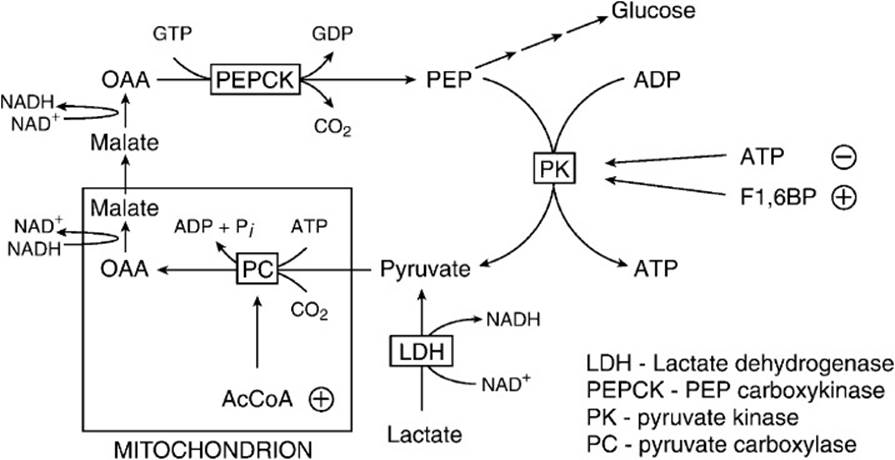
Figure 21-1. Interconversion of phosphoenolpyruvate (PEP) and pyruvate. The conversion of PEP to pyruvate is thermodynamically irreversible in the cell. To convert pyruvate back to PEP for gluconeogenesis, pyruvate must enter the mitochondrion, be carboxylated to oxaloacetate (OAA), and reduced to malate. After exiting the mitochondrion, malate is oxidized back to OAA and converted to PEP by the action of phosphoenolpyruvate carboxykinase.
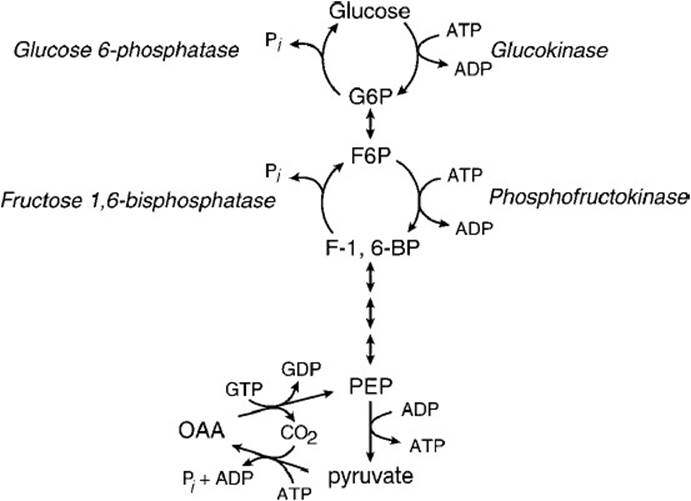
Figure 21-2. The reactions that make up the thermodynamically irreversible steps in glycolysis and gluconeogenesis. These steps make up potential “futile” cycles that must be carefully regulated.
During a marathon run, there is constant stretching and tearing of the leg muscle fibers each time the foot hits the ground. This constant shock causes damage to the muscle cells, resulting in a release of cellular contents to the extracellular matrix and the bloodstream. The concentrations of myoglobin, which is in high concentration in slow twitch (red) muscle fibers, and K+, which is concentrated in all cells, therefore rise in the blood. When the concentration of myoglobin increases above 0.5 to 1.5 mg/dL, it is excreted into the urine. Normally myoglobin is not toxic to the kidney; however, when the pH of the urine drops below 5.6, myoglobin undergoes oxidation to produce hematin (porphyrin bound Fe3+), which is toxic to the kidneys and can lead to acute renal failure. This toxic effect is exacerbated when the urine is concentrated as a result of dehydration.
COMPREHENSION QUESTIONS
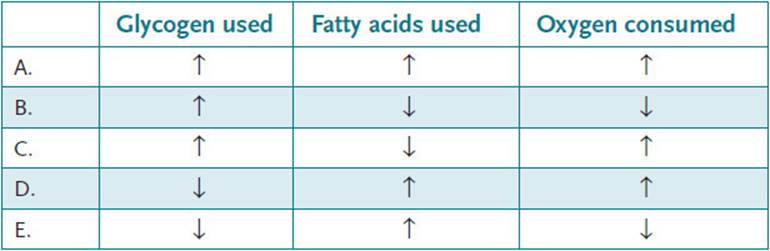
21.1 During the course of a marathon race a runner expends a large amount of energy and must use stored sources of fuel as well as oxygen. Compared with the beginning of the race (first mile), which of the following best describes the utilization of glycogen and fatty acids as fuels and amount of oxygen consumed after running for 26 miles?
21.2 During an extended period of exercise, the enzymes involved in the glycolytic pathway in muscle tissue are actively breaking down glucose to provide the muscle energy. The liver, to maintain blood glucose levels, is synthesizing glucose via the gluconeogenic pathway. Which of the following enzymes involved in these pathways would be most likely to exhibit Michaelis–Menten kinetics (ie, have a hyperbolic curve when plotting substrate concentration versus velocity of the reaction)?
A. Fructose-1,6-bisphosphatase
B. Hexokinase
C. Lactate dehydrogenase
D. Phosphofructokinase 1
E. Pyruvate kinase
21.3 A known person who abuses alcohol is found lying semiconscious at the bottom of a stairwell with a broken arm by his landlady, who called an ambulance to take him to the emergency room. Initial laboratory studies showed a relatively large anion gap of 34 (normal range: 9-15). His blood alcohol was elevated at 245 mg/dL (intoxication level: 150-300 mg/dL), and his blood glucose was 38 mg/dL (low normal). The patient’s large anion gap and hypoglycemia can best be explained by which of the following?
A. Decreased secretion of glucagon
B. Increased secretion of insulin
C. Increased urination resulting from the diuretic effect of alcohol
D. Inhibition of dehydrogenase enzymes by NADH
E. Inhibition of glycogenolysis by ethanol
ANSWERS
21.1 D. At the beginning of the race, a runner running at a reasonable pace consumes energy at a ratio of approximately 75% carbohydrate: 25% fatty acids. However, by the end of the race, glycogen stores are for the most part depleted, and the generation of ATP must come from the β-oxidation of fatty acids, which produces reducing equivalents for oxidative phosphorylation. This requires an increase in the amount of oxygen consumed.
21.2 C. The activity of regulatory enzymes such as fructose-1,6-bisphosphatase, hexokinase, phosphofructokinase 1, and pyruvate kinase are frequently controlled by binding allosteric effectors. These allosteric enzymes usually exhibit sigmoidal kinetics. Lactate dehydrogenase is not controlled by allosteric effectors and therefore would be expected to exhibit Michaelis–Menten kinetics.
21.3 D. People who abuse alcohol frequently do not eat while binge drinking, so it is most likely that his liver glycogen stores became depleted and could not increase his blood glucose levels. The metabolic stress leads to the increase in secretion of epinephrine and other hormones that mobilize fatty acids from stored triglycerides in adipose cells. These fatty acids undergo β-oxidation in the liver but are converted to ketone bodies because of the inhibition of the TCA cycle by high levels of NADH produced by the oxidation of ethanol first to acetaldehyde and acetate. Key gluconeogenic dehydrogenases are also inhibited by the elevated levels of NADH, including lactate dehydrogenase, glycerol 3-phosphate dehydrogenase, and malate dehydrogenase.
BIOCHEMISTRY PEARLS
![]() The exercising muscle’s sources of energy are primarily glucose and fatty acids.
The exercising muscle’s sources of energy are primarily glucose and fatty acids.
![]() With insufficient oxygen to meet the muscle demands, NADH levels increase in the mitochondria and in the cytoplasm, ADP and AMP concentrations in the cytoplasm will rise, and glucose will be shunted to the glycolytic pathway in the muscle. The pyruvate is converted to lactate, which causes the metabolic acidosis.
With insufficient oxygen to meet the muscle demands, NADH levels increase in the mitochondria and in the cytoplasm, ADP and AMP concentrations in the cytoplasm will rise, and glucose will be shunted to the glycolytic pathway in the muscle. The pyruvate is converted to lactate, which causes the metabolic acidosis.
![]() Muscle injury can lead to myoglobinemia and myoglobinuria (red urine) and may crystallize in the renal tubules, leading to renal insufficiency.
Muscle injury can lead to myoglobinemia and myoglobinuria (red urine) and may crystallize in the renal tubules, leading to renal insufficiency.
REFERENCES
Brady HR, Brenner BM. Acute renal failure. In: Longo D, Fauci AS, Kaspar D, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011.
Kratz A, Lewandrowski KB, Siegel AJ, et al. Effect of marathon running on hematologic and biochemical laboratory parameters, including cardiac markers. Am J Clin Pathol. 2002;118(12):856-863.
Murakami K. Rhabdomyolysis and acute renal failure. In: Glew RH, Ninomiya Y, eds. Clinical Studies in Medical Biochemistry. 2nd ed. New York: Oxford University Press; 1997.