Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 36
During your medical school training, you spend some time in a pediatric clinic in a third-world country. One of the first patients you encounter is an 8-month-old girl brought to the clinic because of excessive exhaustion and fatigue. On further questioning of the mother, she reports that she was previously breast-feeding but had to stop to return to work. To feed all of her other children, she has had to dilute her formula with water to make the formula last longer for the entire family. After your physical examination is performed, you diagnose the infant with severe malnutrition and aid the mother with resources to increase food intake for their household.
![]() What is this syndrome called (with deficiencies in both calories and protein)?
What is this syndrome called (with deficiencies in both calories and protein)?
![]() What is the difference between this syndrome and kwashiorkor syndrome?
What is the difference between this syndrome and kwashiorkor syndrome?
![]() What physical findings might differentiate the 2 syndromes?
What physical findings might differentiate the 2 syndromes?
ANSWERS TO CASE 36:
Starvation
Summary: An 8-month-old girl presents with exhaustion and excessive starvation secondary to deficient intake of calories and protein.
• Diagnosis: Marasmus
• Differences: Marasmus has inadequate total caloric intake, whereas kwashiorkor is due to a lack of protein intake.
• Physical findings of kwashiorkor and not marasmus: Subcutaneous fat, distended abdomen, hepatomegaly, and fatty liver.
CLINICAL CORRELATION
Protein-energy malnutrition is caused by inadequate food intake or diseases interfering with food absorption or digestion. The 2 major types of malnutrition are marasmus and kwashiorkor. In marasmus, a child usually between the ages of 1 to 3 years has inadequate caloric intake leading to loss of subcutaneous fat, loose wrinkled skin, and either flat or distended abdomen resulting from atrophic abdominal wall muscles. Often, children are susceptible as they go from breast milk to solid food. The affected child usually has the appearance of an “old person’s face” In kwashiorkor, the main issue is lack of protein, leading to edema, sparse hair, enlarged liver, and a distended abdomen. The edema of the face and legs is different from that of marasmus. The therapy for both of these diseases is caloric replacement.
APPROACH TO:
Starvation-Related Diseases
OBJECTIVES
1. Describe the metabolic changes in starvation.
2. Explain the increased formation of ketone bodies in starvation.
3. Describe the oxidation of fatty acids.
4. Contrast the metabolic change in fasting states as compared to starvation.
DEFINITIONS
MARASMUS: Malnutrition resulting from inadequate intake of protein and calories.
KWASHIORKOR: Malnutrition resulting from inadequate intake of protein though the intake of total calories is adequate.
KETONE BODIES: The short chain fatty acid metabolites acetoacetate, β-hydroxybutyrate, and acetone.
TRIGLYCERIDE: A glycerol molecule with each hydroxyl group esterified with a fatty acid moiety.
DIGLYCERIDE: A glycerol molecule with 2 hydroxyl groups esterified with a fatty acid moiety.
MONOGLYCERIDE: A glycerol molecule with one hydroxyl group esterified with a fatty acid moiety.
DISCUSSION
Malnutrition and its ultimate form starvation arise from many different causes and are present even in affluent societies. The case description reveals that the child lives in a third-world country, and the physical findings reveal that the child is experiencing protein-calorie-deficient starvation, or marasmus.
Fasting and starvation represent changes from the baseline metabolic interactions between tissues that exist in the fed state. Each of 3 states—fed, fasting, and starvation—must be considered from the standpoint of the whole body primarily because the constituent tissues have different requirements for their nutritional sources. For example, red blood cells have an absolute requirement for glucose as the exclusive food source from which energy is derived. Although other tissues use fatty acids and amino acids as well, the red blood cell cannot because it lacks mitochondria and therefore the enzymes required for most of the metabolic steps required in β-oxidation of fatty acids and metabolism of the carbon skeletons of amino acids. Brain tissue normally has an exclusive preference for glucose, the exception being in advanced starvation when the brain can use ketone bodies for energy production.
The metabolic interactions of tissues in the fed state are shown in Figure 36-1. Glucose, fatty acids from triglycerides, and amino acids are provided by the diet and used differentially by the tissues. In the liver, glucose is used for storage as glycogen or converted to fatty acids for formation into triglycerides for storage in adipose tissue. Amino acid carbon skeletons are used for metabolic intermediates for energy production or fatty acid synthesis. Resting muscle takes up glucose and stores it as glycogen and uses amino acids for protein synthesis. Resting muscle prefers fatty acids and ketone bodies rather than glucose to satisfy its energy demands. In adipose tissue, glucose and fatty acids are taken up. Glucose metabolism provides energy and glycerol 3-phosphate for triglyceride formation and storage using fatty acids transported to adipose cells as triglycerides in lipoprotein particles. The brain and red blood cells take up glucose from blood to meet energy demands.
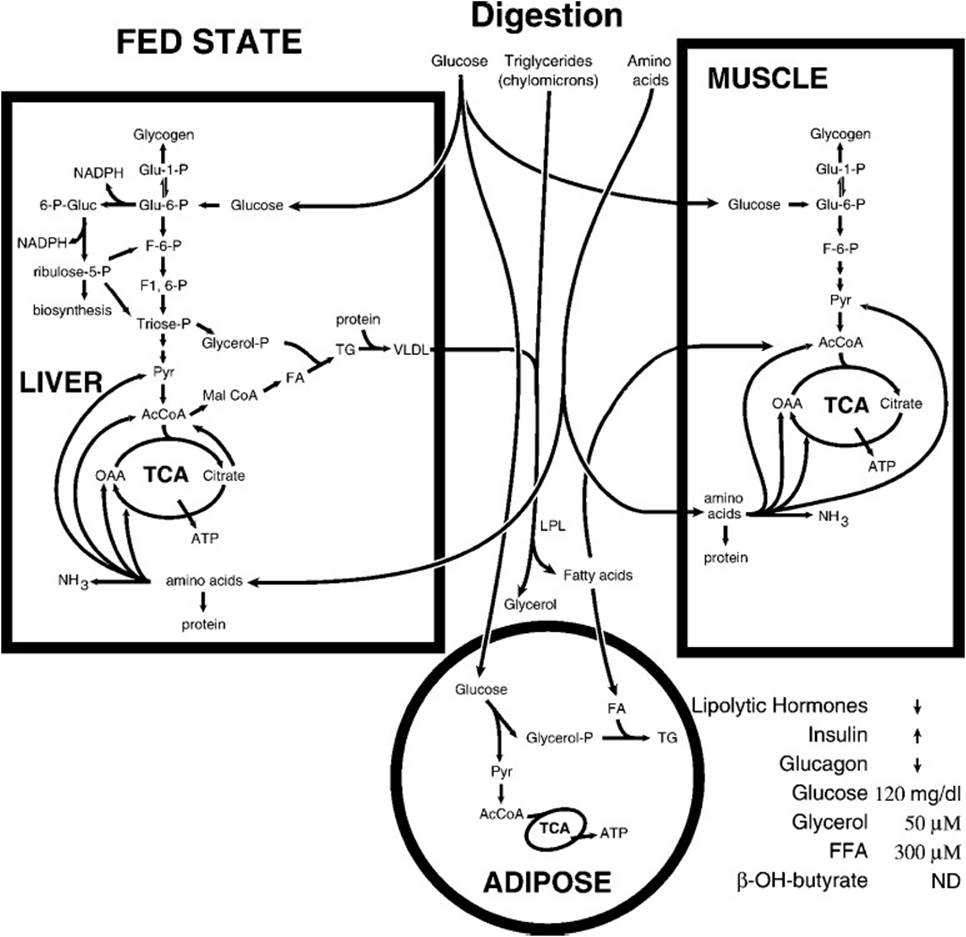
Figure 36-1. The metabolic flow during the fed state. Increased blood glucose triggers release of insulin and a decrease in the release of glucagon and lipolytic hormones.
During circumstances of no food intake for 16 to 20 hours (the postfeeding state), substantial changes occur in the interactions between tissues (Figure 36-2). The liver shifts from consumption of glucose for glycogen storage to mobilization of its glycogen stores to release glucose to the bloodstream to supply the glucose requirements of the brain and red blood cell. Because the hepatic glycogen supply is depleted fairly quickly, metabolic signals increase liver gluconeogenesis, depleting tricarboxylic acid cycle intermediates and prompting the use of amino acid carbon skeletons from protein breakdown for new glucose formation. The energy required for gluconeogenesis is derived by increasing the β-oxidation of fatty acids mobilized from adipose storage sites. Fatty acid synthesis is simultaneously inhibited to prevent a futile cycle. As the tricarboxylic acid cycle’s supply of 4-carbon intermediates in liver mitochondria is drained for gluconeogenesis, rapid β-oxidation of fatty acids produces acetyl-CoA faster than the tricarboxylic acid cycle can metabolize the carbon atoms of acetyl-CoA to CO2 and free CoA. The result is a high ratio of acetyl-CoA to free CoA and thus a slowing of β-oxidation and compromise of liver mitochondrial ATP formation. The conversion of the free CoA pool to acetyl-CoA is reversed by the formation of the ketone body acetoacetate (and later its reduced product, β-hydroxybutyrate), regenerating free CoA (Figure 36-3). This occurs only in liver mitochondria because of its critical role in gluconeogenesis. The ketone bodies are transported out of the liver mitochondria and the liver into the bloodstream for transport to other tissues where they reenter metabolism by being converted to acetoacetyl-CoA at the expense of succinyl-CoA and then cleaved by β-ketothiolase to produce 2 molecules of acetyl-CoA for metabolism in the TCA cycle.
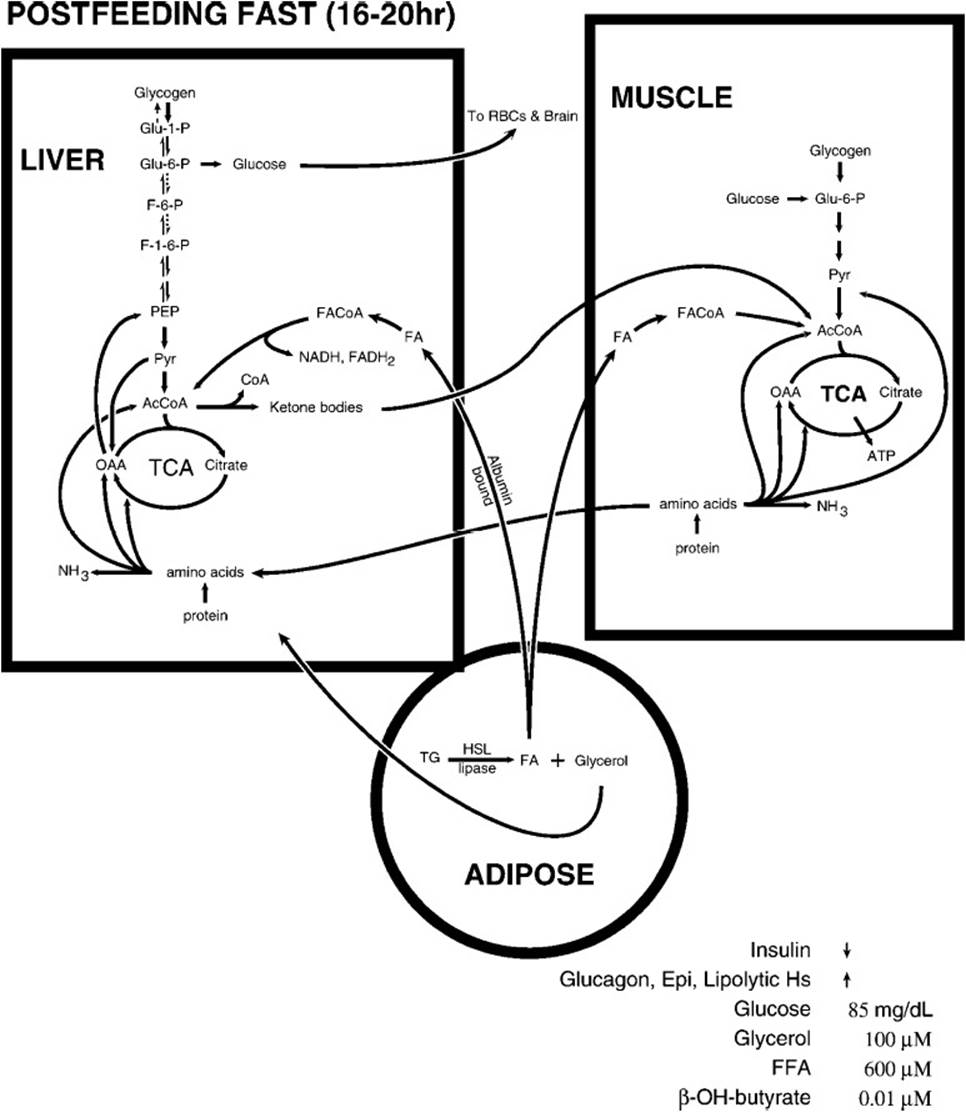
Figure 36-2. The metabolic flow following a postfeeding fast. Blood glucose levels begin to decrease, triggering homeostatic mechanisms to prevent it from dramatically decreasing.
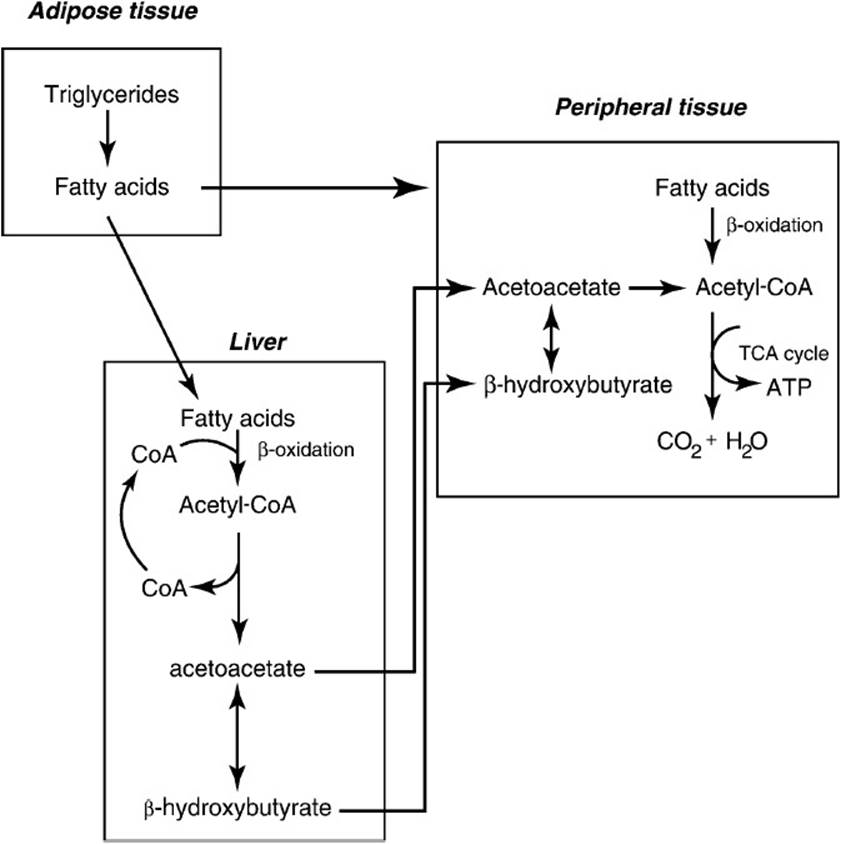
Figure 36-3. Mobilization of fatty acids during times in which the liver is synthesizing glucose via the gluconeogenic pathway. The β-oxidation of fatty acids by the liver produces the energy needed for gluconeogenesis; however, because the TCA cycle is slowed because of depletion of C4 acids (used for glucose synthesis), ketone bodies (acetoacetate and β-hydroxybutyrate) are formed from acetyl-CoA to regenerate CoA for continued β-oxidation. The ketone bodies are exported to extrahepatic tissues where they are used as an energy source.
The β-oxidation of fatty acids that occurs in the mitochondrial matrix provides the energy for gluconeogenesis in the liver. Fatty acids transported from adipose tissues by blood albumin cross the hepatic plasma membrane and are activated by fatty acid thiokinases, producing fatty acid-CoA and requiring ATP (Figure 36-4). The fatty acids are transported across the mitochondrial inner membrane as carnitine derivatives utilizing the carnitine shuttle. All the rest of the reactions occur in the mitochondrial matrix beginning with the oxidation of the fatty acid by flavin adenine dinucleotide (FAD)-linked fatty acyl-CoA dehydrogenase producing trans-Δ2-enoyl-CoA. This product is hydrated by enoylhydratase producing L-3-hydroxyacyl-CoA. This product undergoes a second oxidation catalyzed by NAD-linked L-3-hydroxy fatty acyl-CoA dehydrogenase, producing 3-ketoacyl-CoA. This product is cleaved by β-ketothiolase, producing one molecule of acetyl-CoA and a new activated fatty acyl-CoA, 2 carbon atoms shorter than at the outset in a reaction that requires another molecule of free CoA. The newly produced fatty acyl-CoA repeats the cycle of steps in β-oxidation releasing another acetyl-CoA and onward until the last cleavage step that hydrolyzes acetoacetyl-CoA to 2 molecules of acetyl-CoA.
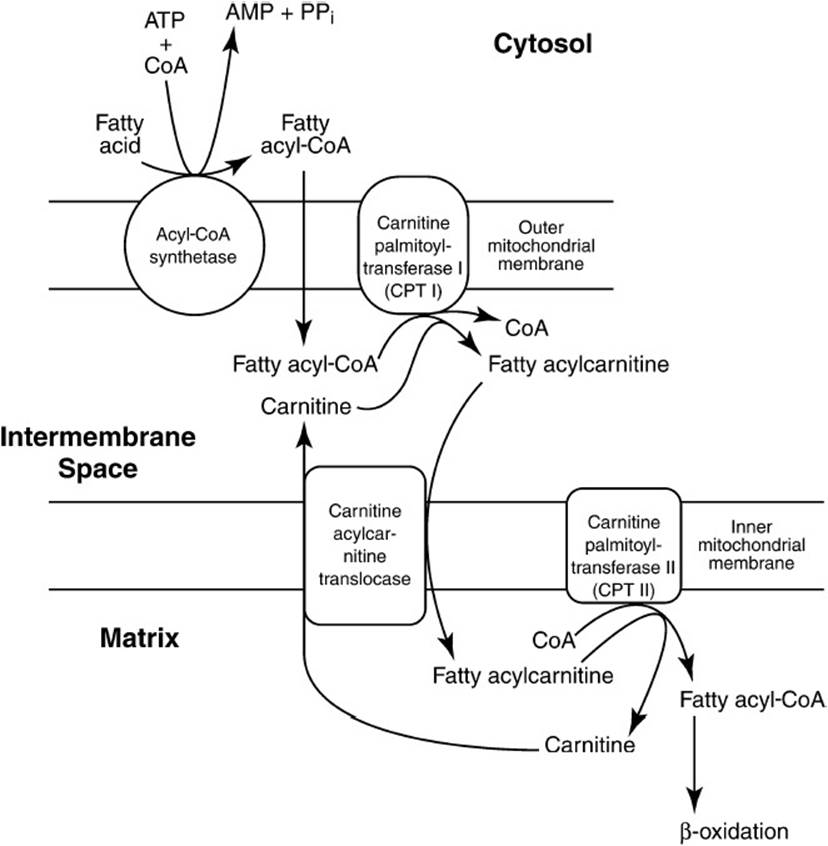
Figure 36-4. The activation of fatty acids and transport into the mitochondrion via the carnitine shuttle. (Reproduced, with permission, from: D.B. Marks, et al, Basic Medical Biochemistry: A Clinical Approach, Philadelphia: Lippincott Williams & Wilkins, 1996:361.)
Although the postfeeding fast represents a normal state reflective of the alternation of feeding and not feeding, the starvation state shown in Figure 36-5 represents an abnormal state and reflects a dramatic increase in the metabolic changes observed in the postfeeding state, illustrated in Figure 36-3. Thus, starvation represents an intensification of the metabolic adjustments of the fasting state with some significant differences seen only in prolonged starvation. Two marked changes in plasma concentrations occur, a decrease in glucose concentration and a dramatic increase in the concentration of ketone bodies, reflecting altered metabolic poise. In the liver the tricarboxylic acid (TCA) cycle is slowed by the drainage of 4-carbon intermediates to gluconeogenesis, fatty acid breakdown continues apace, and body proteins continue to be broken down to replenish the tricarboxylic acid cycle intermediates. In muscle the fuels used for energy generation are fatty acids and ketone bodies. Muscle activity decreases as result of the mobilization of muscle protein, which itself slows as the period of starvation increases. In adipose tissue, the breakdown of triglycerides to fatty acids is accelerated. In the brain and the central nervous system (CNS) an adaptive change occurs, allowing this tissue to use ketone bodies as an energy source relieving both the total body demand for glucose and the use of muscle protein as a carbon source for gluconeogenesis in the liver. Ketone body concentration increases in the blood, reflecting the accelerated breakdown of triglycerides in adipose tissue and the slower hepatic TCA cycle. In spite of the utilization of ketone bodies by peripheral tissues and the brain and CNS tissues after 5 to 6 weeks of starvation, ketone body blood levels rise, spill over into the urine and are excreted in significant quantity, wasting material that could be used for energy generation because the tissues capable of using ketone bodies as fuel are already using the maximum possible amount. Thus, the major differences between starvation and the postfeeding fast are the adaptive ability of the brain and central nervous system to use ketone bodies to satisfy some of their energy demand and in the levels of circulating ketone bodies that are high enough to spill over into the urine in significant quantities.
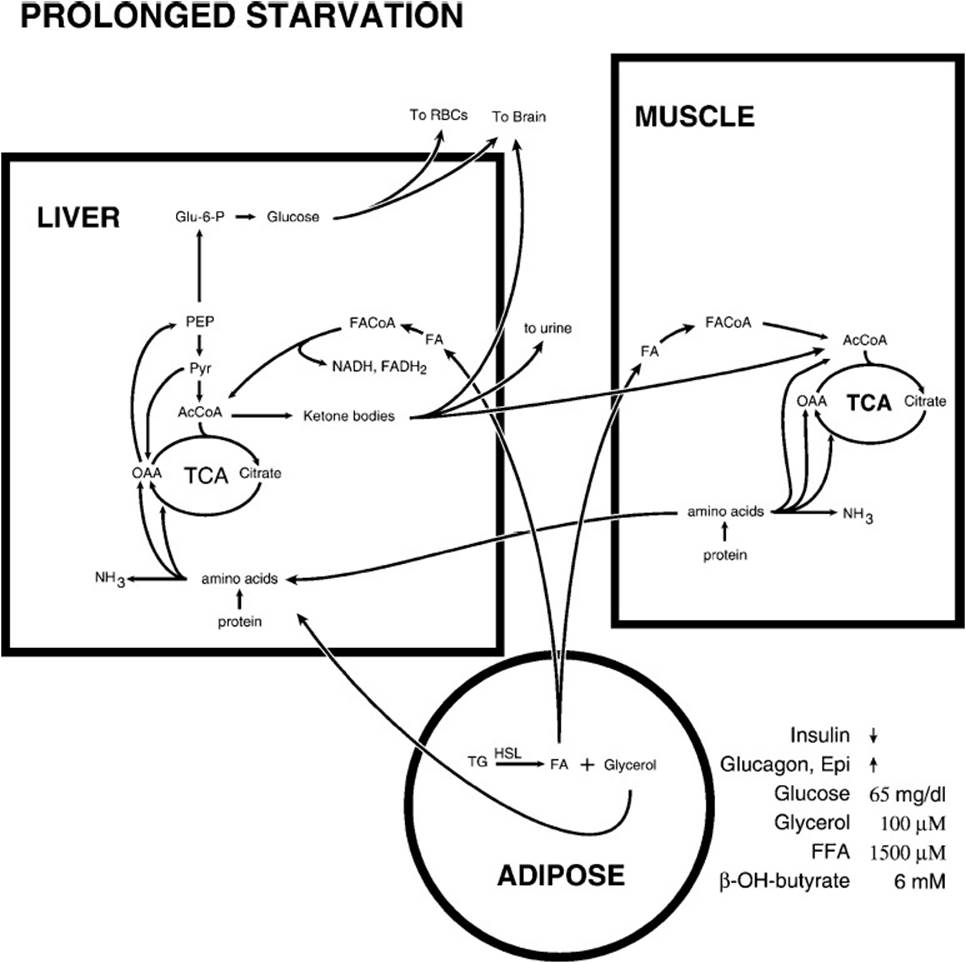
Figure 36-5. The metabolic flow during prolonged starvation. The brain adapts to use ketone bodies as a source of energy, thus decreasing its utilization of glucose.
COMPREHENSION QUESTIONS
36.1 In cases of starvation many metabolic changes take place to meet the metabolic demands of the body. Which of the following illustrates starvation-triggered changes in intermediary metabolism?
A. Increased dependence of liver on glucose for its energy supply
B. Increased synthesis of proteins in muscle tissue
C. Increased use of ketone bodies for energy source in brain
D. Decreased mobilization of triglycerides by adipose tissue
E. Adaptation of red blood cells to use ketone bodies for energy
Use the reactions below for question 36.2:
1. Acetoacetyl-CoA + acetyl-CoA → β-hydroxy-β-methylglutaryl-CoA + CoA
2. Acetoacetate + NADH → β-hydroxybutyrate + NAD+
3. β-hydroxy-β-methylglutaryl-CoA + H2O → acetoacetate + acetyl-CoA
4. Acetyl-CoA + acetyl-CoA → acetoacetyl-CoA + CoA
5. Acetoacetate + succinyl-CoA → acetoacetyl-CoA + succinate
36.2 Using the above reactions, which of the following correctly describes the pathway of ketone body formation?
A. 3 → 2 → 1 → 4
B. 4 → 1 → 3 → 2
C. 4 → 2 → 3 → 1
D. 5 → 1 → 2 → 3
E. 5 → 2 → 3 → 1
36.3 During starvation muscle activity decreases, and muscle protein is broken down to provide a carbon source for the liver production of glucose via gluconeogenesis. Which of the following amino acids remains in the muscle cell to provide a source of energy for the muscle?
A. Alanine
B. Aspartate
C. Leucine
D. Glutamate
E. Threonine
ANSWERS
36.1 C. In starvation a major metabolic adjustment is that the brain activates the ketone body metabolic pathway and uses ketone bodies for energy, thus sparing somewhat the breakdown of body proteins to generate amino acid carbon skeletons for gluconeogenesis in the liver. In cases of starvation, the liver derives most of its energy from β-oxidation of fatty acids. Muscle proteins are broken down to generate carbon skeletons during starvation. Triglyceride stores in adipose tissue are being used to provide fatty acids for β-oxidation in the liver. Red blood cells are not able to use ketone bodies because they have no mitochondria.
36.2 B. The synthesis of ketone bodies begins with the combination of 2 molecules of acetyl-CoA to generate one molecule of free CoA and a molecule of acetoacetyl-CoA, which combines with another molecule of acetyl-CoA to yield another free CoA molecule and β-hydroxy-β-methylglutaryl-CoA (HMG-CoA). HMG-CoA undergoes hydrolysis to produce 1 molecule acetyl-CoA and 1 molecule of acetoacetate, which can be reduced to β-hydroxybutyrate. The reaction of succinyl-CoA and acetoacetate is a reaction in the pathway of ketone body utilization but not in the pathway of ketone body formation.
36.3 C. None of the other amino acids listed, except for leucine, is a branched-chain amino acid. The muscle has a very active branched-chain amino acid metabolic pathway and uses that pathway to provide energy for its own use. The products of leucine metabolism are acetyl-CoA and acetoacetate, both of which are used in the TCA cycle. Acetoacetate is activated by succinyl-CoA and cleaved to 2 molecules of acetyl-CoA in the β-ketothiolase reaction. The other branched-chain amino acids, valine, and isoleucine, yield succinyl-CoA and acetyl-CoA as products of their catabolism.
BIOCHEMISTRY PEARLS
![]() Red blood cells have an absolute requirement for glucose as the exclusive food source from which energy is derived.
Red blood cells have an absolute requirement for glucose as the exclusive food source from which energy is derived.
![]() Brain tissue normally has an exclusive preference for glucose, the exception being in advanced starvation when the brain can use ketone bodies for energy production.
Brain tissue normally has an exclusive preference for glucose, the exception being in advanced starvation when the brain can use ketone bodies for energy production.
REFERENCE
Devlin TM, ed. Textbook of Biochemistry with Clinical Correlations. 7th ed. New York: Wiley-Liss; 2010.